A Green-emitting Fluorescent Probe Based on a Benzothiazole Derivative for Imaging Biothiols in Living Cells
Abstract
1. Introduction
2. Results and Discussion
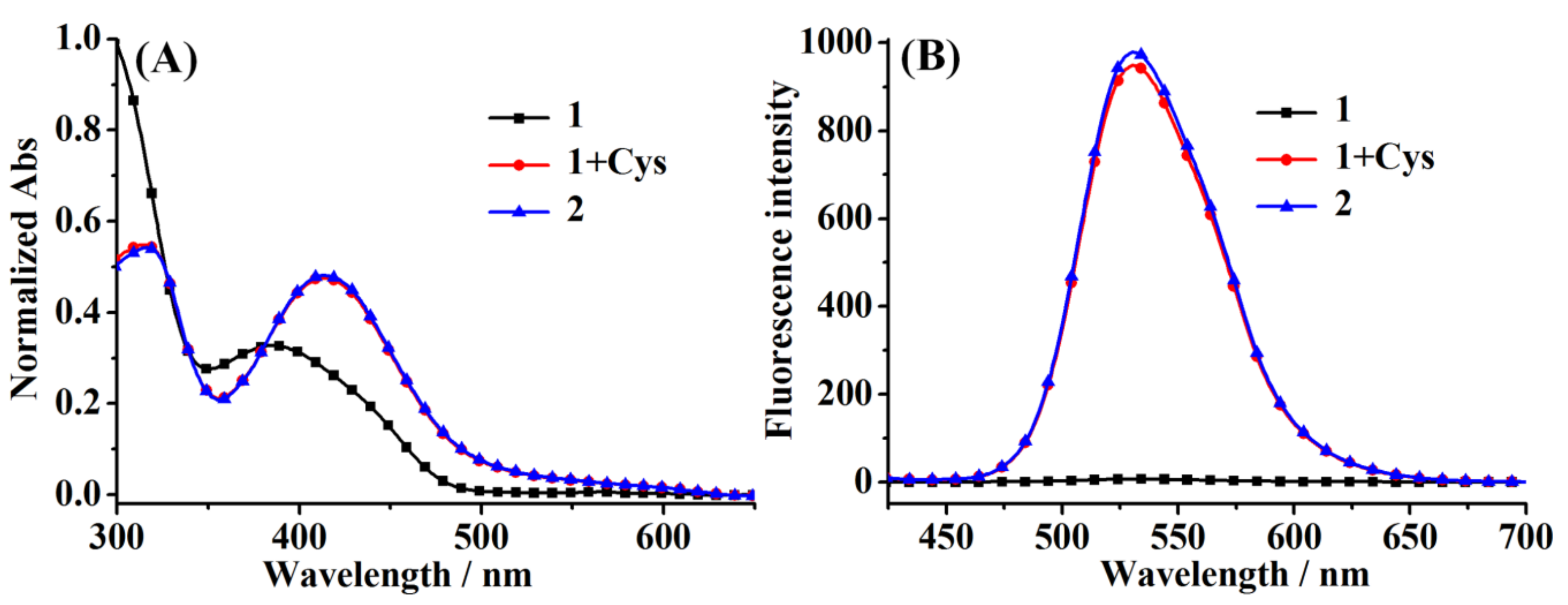
2.1. Spectroscopic Studies
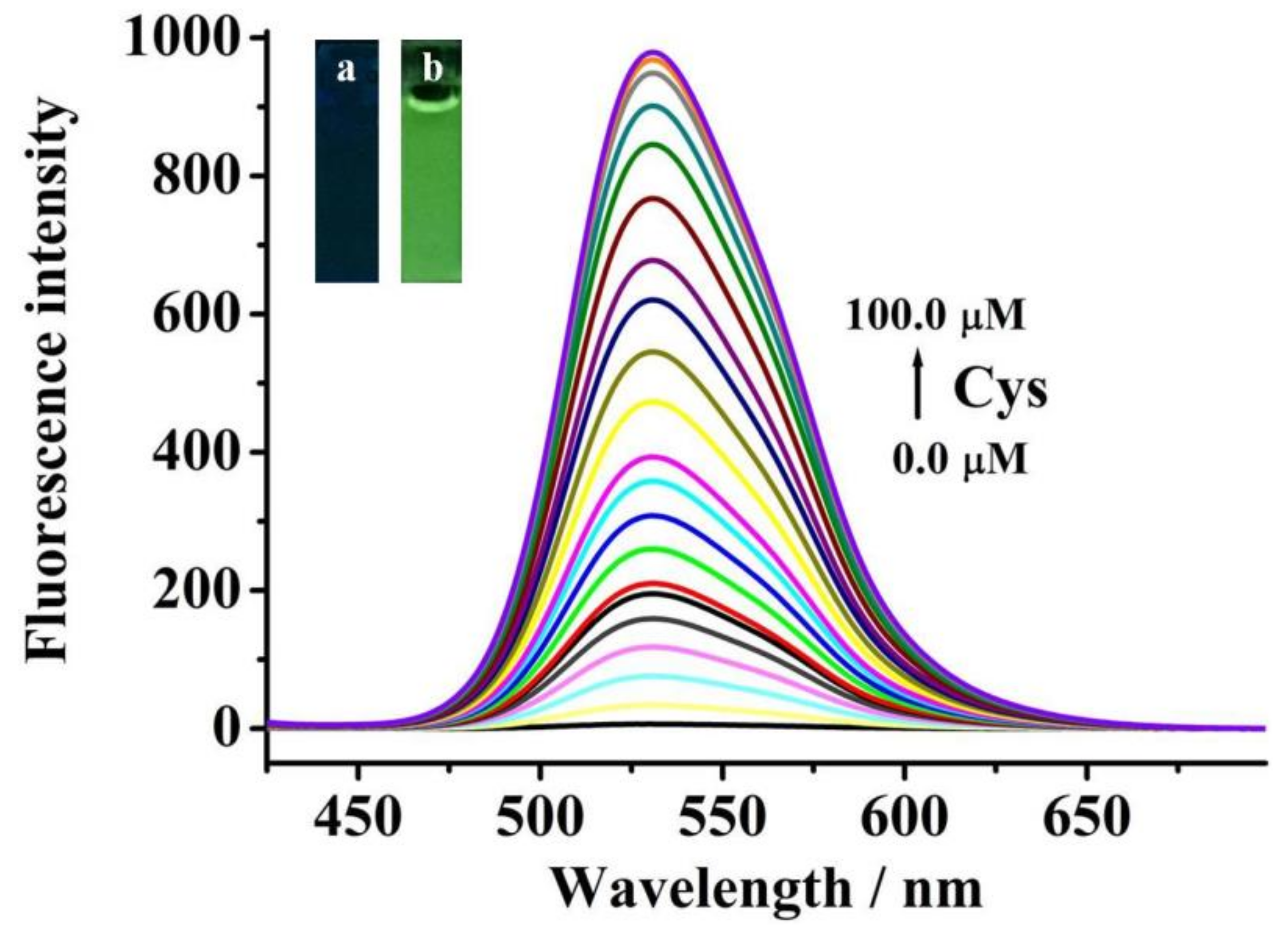
2.2. Sensitivity Studies
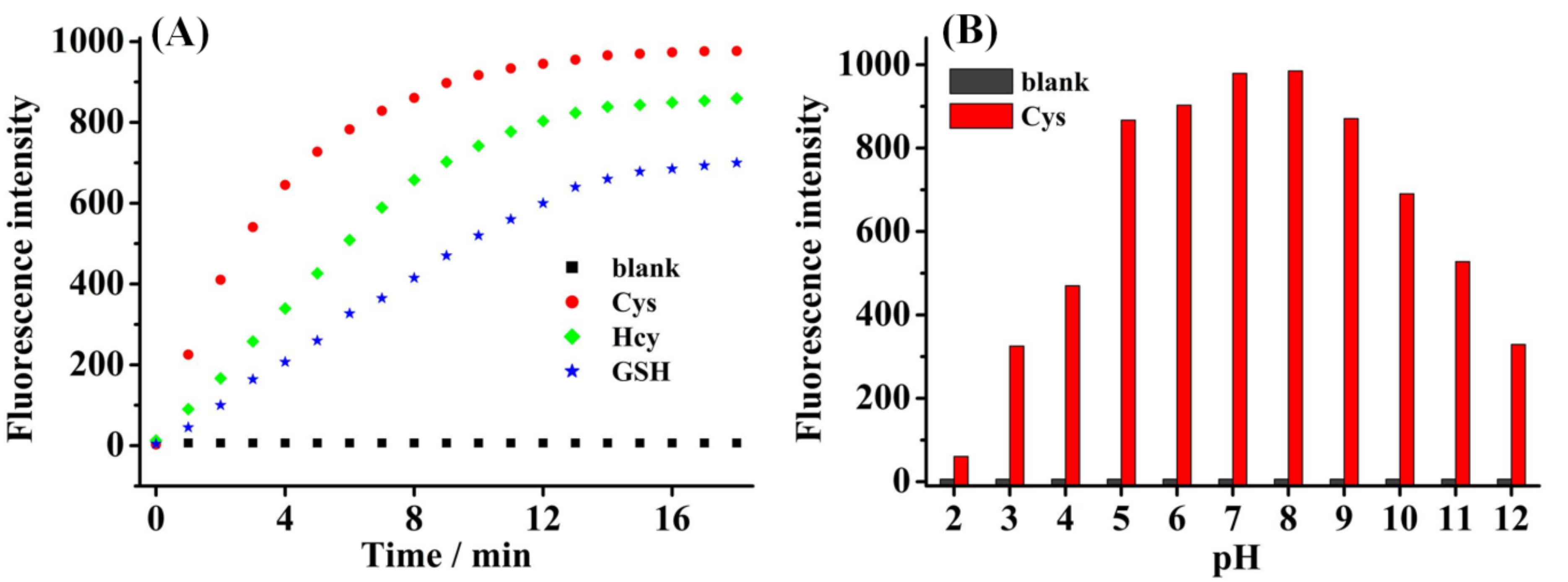
2.3. Selective and Interference Fluorescence Response to Thiols
2.4. Kinetics and the Effects of pH
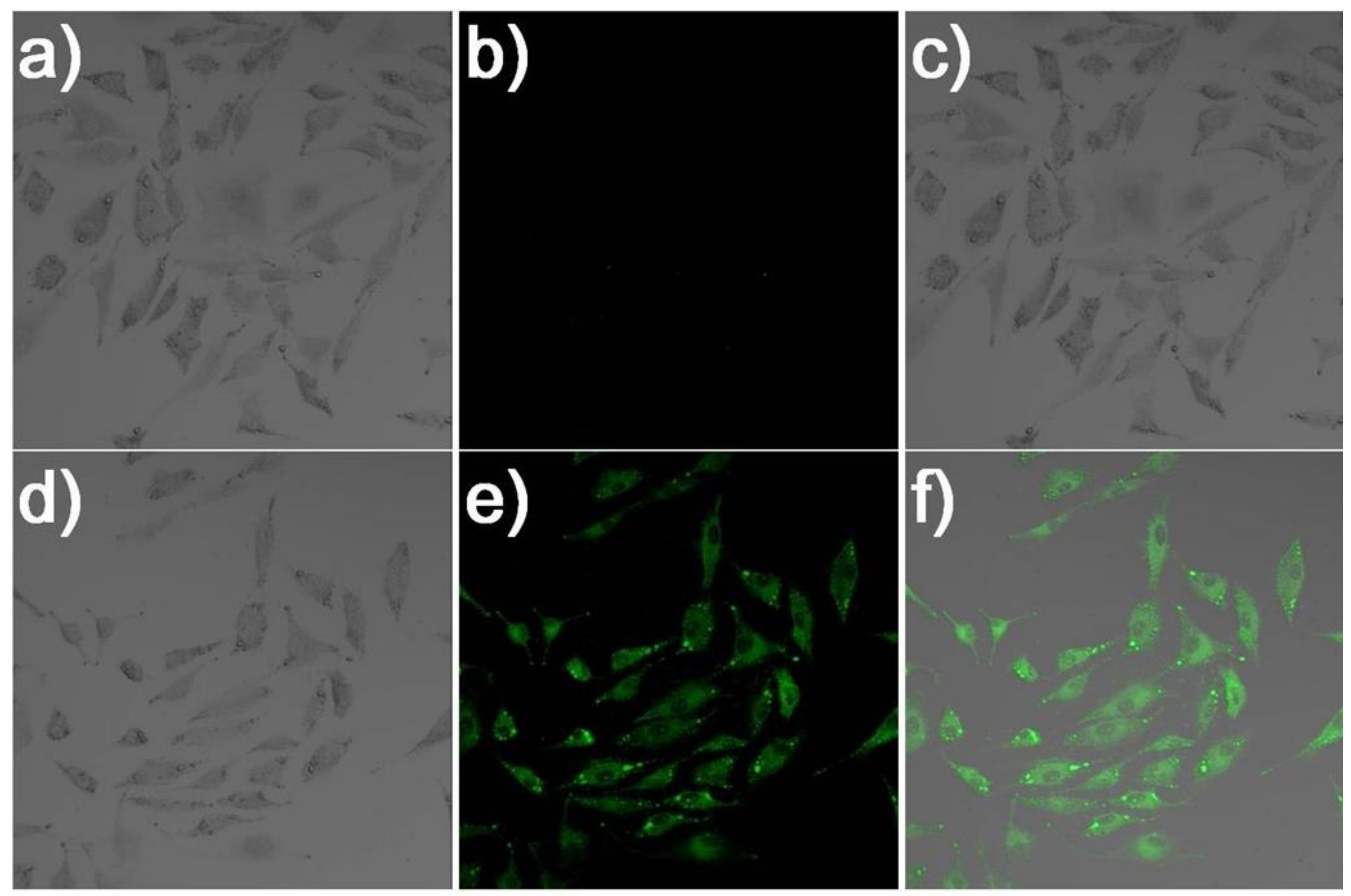
2.5. Bioimaging of Probe 1
3. Materials and Methods
3.1. Instruments and Chemicals
3.2. Preparation of Spectra Measurements
3.3. Cell Assay
3.4. Synthesis of Dye 2
3.5. Synthesis of Probe 1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Zhou, Y.; Peng, X.; Yoon, J. Fluorescent and colorimetric probes for detection of thiols. Chem. Soc. Rev. 2010, 39, 2120–2135. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Ding, H.; Xu, H.J.; Lv, Y.L.; Liu, H.; Wang, H.D.; Tian, Z.Y. Dual-functional probes for sequential thiol and redox homeostasis sensing in live cells. Analyst 2015, 140, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Yu, Y.W.; Wang, B.X.; Jiang, Y.L. A sensitive fluorescent probe based on coumarin for detection of cysteine in living cells. J. Photochem. Photobiol. A 2017, 338, 178–182. [Google Scholar] [CrossRef]
- Gao, J.H.; Tao, Y.F.; Wang, N.N.; He, J.L.; Zhang, J.; Zhao, W.L. BODIPY-based turn-on fluorescent probes for cysteine and homocysteine. Spectrochim. Acta Part A 2018, 203, 77–84. [Google Scholar] [CrossRef]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The importance of glutathione in human disease. Biomed. Pharmacother. 2003, 57, 145–155. [Google Scholar] [CrossRef]
- Zhang, S.; Ong, C.N.; Shen, H.M. Critical roles of intracellular thiols and calcium in parthenolide-induced apoptosis in human colorectal cancer cells. Cancer Lett. 2004, 208, 143–153. [Google Scholar] [CrossRef]
- Weerapana, E.; Wang, C.; Simon, G.M.; Richter, F.; Khare, S.; Dillon, M.B.D.; Bachovchin, D.A.; Mowen, K.; Baker, D.; Cravatt, B.F. Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature 2010, 468, 790–795. [Google Scholar] [CrossRef]
- Chu, P.Y.; Liu, M.Y. Amino acid cysteine induces senescence and decelerates cell growth in melanoma. J. Funct. Foods 2015, 18, 455–462. [Google Scholar] [CrossRef]
- Dorszewska, J.; Prendecki, M.; Oczkowska, A.; Dezor, M.; Kozubski, W. Molecular basis of familial and sporadic Alzheimer’s disease. Curr. Alzheimer. Res. 2016, 13, 952–963. [Google Scholar] [CrossRef]
- Kong, F.P.; Liu, R.P.; Chu, R.R.; Wang, X.; Xu, K.H.; Tang, B. A highly sensitive near-infrared fluorescent probe for cysteine and homocysteine in living cells. Chem. Commun. 2013, 49, 9176–9178. [Google Scholar] [CrossRef]
- Nekrassova, O.; Lawrence, N.S.; Compton, R.G. Analytical determination of homocysteine: A review. Talanta 2003, 60, 1085–1095. [Google Scholar] [CrossRef]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.F.; Wolf, P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef]
- Yin, C.X.; Huo, F.J.; Zhang, J.J.; Martinez-Manez, R.; Yang, Y.T.; Lv, H.G.; Li, S.D. Thiol-addition reactions and their applications in thiol recognition. Chem. Soc. Rev. 2013, 42, 6032–6059. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.Q.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Atkuri, K.R.; Mantovani, J.J.; Herzenberg, L.A.; Herzenberg, L.A. N-Acetylcysteine—A safe antidote for cysteine/glutathione deficiency. Curr. Opin. Pharmacol. 2007, 7, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.W.; Yu, J.J.; Xu, X.H.; Jiang, Y.L.; Wang, B.X. A novel fluorescent probe for highly sensitive and selective detection of cysteine and its application in cell imaging. Sensor. Actuat B Chem. 2017, 251, 902–908. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, H.R.; Sun, H.B.; Liu, S.J.; Wang, J.X.; Zhao, Q.; Liu, X.M.; Xu, W.J.; Li, S.B.; Huang, W. Rational design of an “off-on” phosphorescent chemodosimeter based on an Iridium (III) complex and its application for time-resolved luminescent detection and bioimaging of cysteine and homocysteine. Chem. Eur. J. 2013, 19, 1311–1319. [Google Scholar] [CrossRef]
- Wei, M.J.; Yin, P.; Shen, Y.M.; Zhang, L.L.; Deng, J.H.; Xue, S.Y.; Li, H.T.; Guo, B.; Zhang, Y.Y.; Yao, S.Z. A new turn-on fluorescent probe for selective detection of glutathione and cysteine in living cells. Chem. Common. 2013, 49, 4640–4642. [Google Scholar] [CrossRef]
- Cheng, D.; Xu, W.; Yuan, L.; Zhang, X. Investigation of drug-induced hepatotoxicity and its remediation pathway with reaction-based fluorescent probes. Anal. Chem. 2017, 89, 7693–7700. [Google Scholar] [CrossRef]
- Chen, S.; Li, H.M.; Hou, P. A novel cyanobiphenyl benzothiazole-based fluorescent probe for detection of biothiols with a large Stokes shift and its application in cell imaging. Tetrahedron 2017, 73, 589–593. [Google Scholar] [CrossRef]
- Cheng, D.; Pan, Y.; Yin, B.C.; Yuan, L.; Zhang, X.B. A new fluorescent probe with ultralow background fluorescence for imaging of endogenous cellular selenol under oxidative stress. Chin. Chem. Lett. 2017, 28, 1987–1990. [Google Scholar] [CrossRef]
- Ren, T.B.; Xu, W.; Jin, F.P.; Cheng, D.; Zhang, L.L.; Yuan, L.; Zhang, X.B. rational engineering of bioinspired anthocyanidin fluorophores with excellent two-photon properties for sensing and imaging. Anal. Chem. 2017, 89, 11427–11434. [Google Scholar] [CrossRef]
- Chen, S.; Li, H.M.; Hou, P. Imidazo[1,5-α]pyridine-derived fluorescent turn-on probe for cellular thiols imaging with a large Stokes shift. Tetrahedron Lett. 2017, 58, 2654–2657. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Zhao, W.; Zhang, X.; Luo, X.; Corkins, M.E.; Cole, S.L.; Wang, C.; Xiao, Y.; Bi, X.; et al. Two-photon near infrared fluorescent turn-on probe toward cysteine and its imaging applications. ACS Sensors 2016, 1, 882–887. [Google Scholar] [CrossRef]
- Liu, X.J.; Gao, L.; Yang, L.; Zou, L.F.; Chen, W.Q.; Song, X.Z. A phthalimide-based fluorescent probe for thiol detection with a large Stokes shift. RSC Adv. 2015, 5, 18177–18182. [Google Scholar] [CrossRef]
- Dai, X.; Wu, Q.H.; Wang, P.C.; Tian, J.; Xu, Y.; Wang, S.Q.; Miao, J.Y.; Zhao, B.X. A simple and effective coumarin-based fluorescent probe for cysteine. Biosens. Bioelectron. 2014, 59, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, X.; Zhang, Y.; Zhang, C.; Jin, J.; Li, H.; Yao, S. A novel colorimetric/fluorescence dual-channel sensor based on NBD for the rapid and highly sensitive detection of cysteine and homocysteine in living cells. Anal. Methods 2016, 8, 2420–2426. [Google Scholar] [CrossRef]
- Zhou, X.; Jin, X.; Sun, G.; Wu, X. A Sensitive and selective fluorescent probe for cysteine based on a new response-assisted electrostatic attraction strategy: The role of spatial charge configuration. Chem. Eur. J. 2013, 19, 7817–7824. [Google Scholar] [CrossRef]
- Wang, P.; Liu, J.; Lv, X.; Liu, Y.; Zhao, Y.; Guo, W. A naphthalimide-based glyoxal hydrazone for selective fluorescence turn-on sensing of Cys and Hcy. Org. Lett. 2012, 14, 520–523. [Google Scholar] [CrossRef]
- Jiang, W.N.; Yang, S.L.; Lu, W.; Gao, B.H.; Xu, L.; Sun, X.; Jiang, D.; Xu, H.J.; Ma, M.T.; Cao, F.L. A novel fluorescence “turn off-on” nano-sensor for detecting Cu2+ and cysteine in living cells. J. Photochem. Photobiol. A 2018, 362, 14–20. [Google Scholar] [CrossRef]
- Chen, C.Y.; Liu, W.; Xu, C.; Liu, W.S. A colorimetric and fluorescent probe for detecting intracellular biothiols. Biosens. Bioelectron. 2016, 85, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hou, P.; Wang, J.; Fu, S.; Liu, L. A highly sensitive fluorescent probe based on the Michael addition mechanism with a large Stokes shift for cellular thiols imaging. Anal. Bioanal. Chem. 2018, 410, 4323–4330. [Google Scholar] [CrossRef]
- Chen, S.; Hou, P.; Wang, J.; Fu, S.; Liu, L. A simple but effective fluorescent probe with large stokes shift for specific detection of cysteine in living cells. J. Photochem. Photobiol A 2018, 363, 7–12. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, N.; Fang, Z. A novel dicyanoisophorone-based ratiometric fluorescent probe for selective detection of cysteine and its bioimaging application in living cells. Molecules 2018, 23, 475. [Google Scholar] [CrossRef]
- Zeng, R.-F.; Lan, J.-S.; Li, X.-D.; Liang, H.-F.; Liao, Y.; Lu, Y.-J.; Zhang, T.; Ding, Y. A fluorescent coumarin-based probe for the fast detection of cysteine with live cell application. Molecules 2017, 22, 1618. [Google Scholar] [CrossRef]
- Jiang, G.Y.; Liu, X.; Chen, Q.Q.; Zeng, G.J.; Wu, Y.Q.; Dong, X.B.; Zhang, G.X.; Li, Y.D.; Fan, X.L.; Wang, J.G. A new tetraphenylethylene based AIE probe for light-up and disxriminatory detection of Cys over Hcy and GSH. Sensor. Actuat B Chem. 2017, 252, 712–716. [Google Scholar] [CrossRef]
- Ding, S.Y.; Liu, M.J.; Hong, Y.N. Biothiol-specific fluorescent probes with aggregation-induced emission characteristics. Sci. China Chem. 2018, 61, 882–891. [Google Scholar] [CrossRef]
- Tang, Y.H.; Tang, B.Z. Principles and Applications of Aggregation-Induced Emission; Springer: Gewerbestrasse, Switzerland, 2019; pp. 391–407. [Google Scholar]
- Chen, S.; Li, H.M.; Hou, P. A novel imidazo[1,5-α]pyridine-based fluorescent probe with a large Stokes shift for imaging hydrogen sulfide. Sensor. Actuat B-Chem. 2018, 256, 1086–1092. [Google Scholar] [CrossRef]
- Chen, S.; Li, H.M.; Hou, P. A large Stokes shift fluorescent probe for sensing of thiophenols based on imidazo[1,5-α]pyridine in both aqueous medium and living cells. Anal. Chim. Acta 2017, 993, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.H.; Hao, Y.Q.; Zeng, K.; Fan, S.N.; Li, F.; Yuan, S.K.; Ding, X.J.; Xu, M.T.; Liu, Y.N. A benzothiazole-based fluorescent probe for hypochlorous acid detection and imaging in living cells. Spectrochim. Acta Part A 2018, 199, 189–193. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yang, X.; Xu, K.; Kong, X.; Lin, W. A multi-signal fluorescent probe for simultaneously distinguishing and sequentially sensing cysteine/homocysteine, glutathione, and hydrogen sulfide in living cells. Chem. Sci. 2017, 8, 6257–6265. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hou, P.; Wang, J.X.; Song, X.Z. A highly sulfite-selective ratiometric fluorescent probe based on ESIPT. RSC Adv. 2012, 2, 10869–10873. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, T.; Miao, J.Y.; Zhao, B.X. A ratiometric fluorescent probe with DNBS group for biothiols in aqueous solution. Sensor. Actuat B Chem. 2016, 223, 274–279. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Hao, Y.; Liu, J.; Wu, G.; Liu, L. A Green-emitting Fluorescent Probe Based on a Benzothiazole Derivative for Imaging Biothiols in Living Cells. Molecules 2019, 24, 411. https://doi.org/10.3390/molecules24030411
Ma X, Hao Y, Liu J, Wu G, Liu L. A Green-emitting Fluorescent Probe Based on a Benzothiazole Derivative for Imaging Biothiols in Living Cells. Molecules. 2019; 24(3):411. https://doi.org/10.3390/molecules24030411
Chicago/Turabian StyleMa, Xiaohua, Yuanqiang Hao, Jiaxiang Liu, Guoguang Wu, and Lin Liu. 2019. "A Green-emitting Fluorescent Probe Based on a Benzothiazole Derivative for Imaging Biothiols in Living Cells" Molecules 24, no. 3: 411. https://doi.org/10.3390/molecules24030411
APA StyleMa, X., Hao, Y., Liu, J., Wu, G., & Liu, L. (2019). A Green-emitting Fluorescent Probe Based on a Benzothiazole Derivative for Imaging Biothiols in Living Cells. Molecules, 24(3), 411. https://doi.org/10.3390/molecules24030411






