Comparison between Exhaustive and Equilibrium Extraction Using Different SPE Sorbents and Sol-Gel Carbowax 20M Coated FPSE Media
Abstract
:1. Introduction
2. Results and Discussion
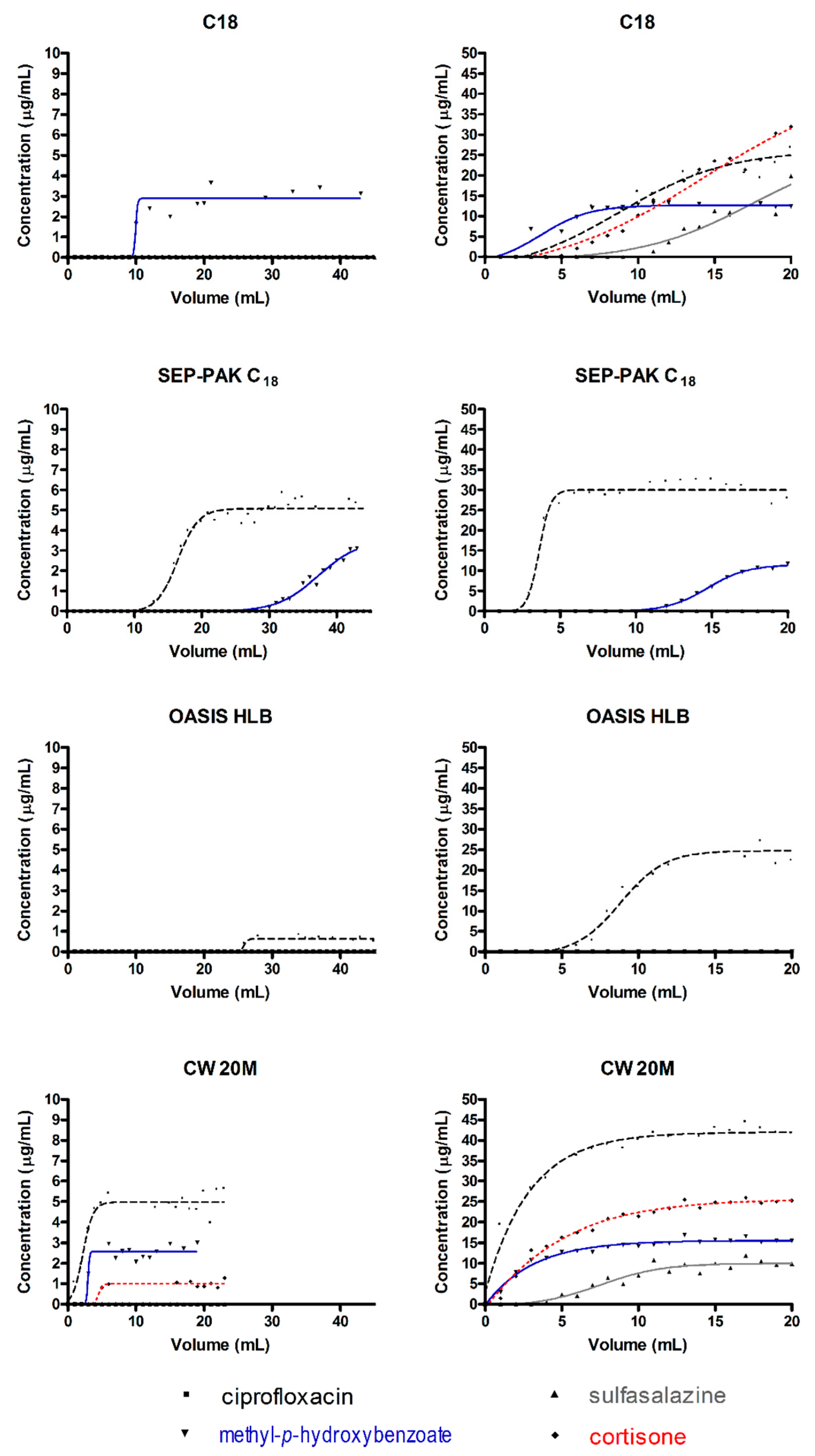
2.1. Determination of SPE Parameters: Breakthrough Volume, Retention Volume, Hold-Up Volume, Retention Factor, and Theoretical Plate Number for SPE Devices
2.2. Mechanism of Extraction in FPSE
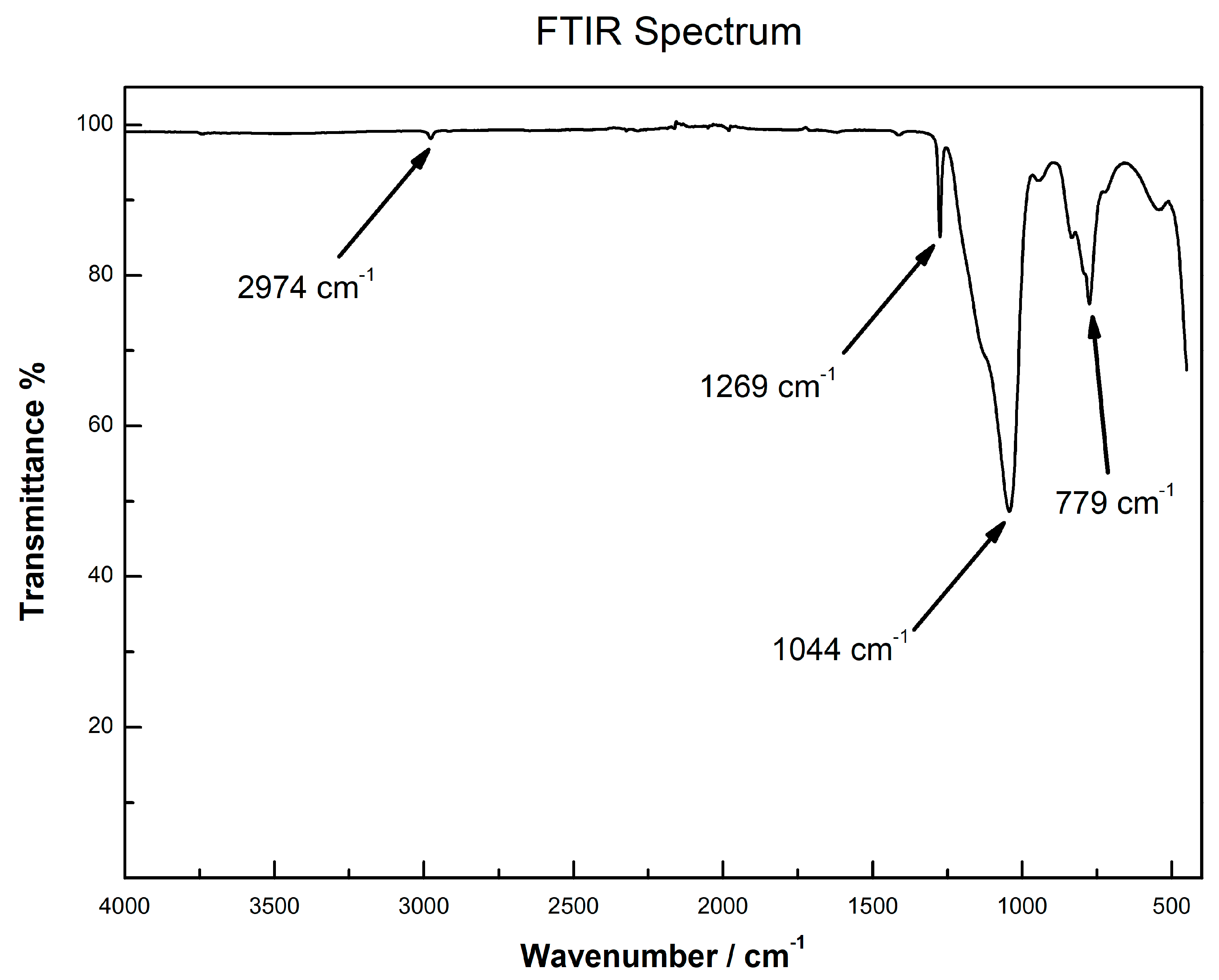
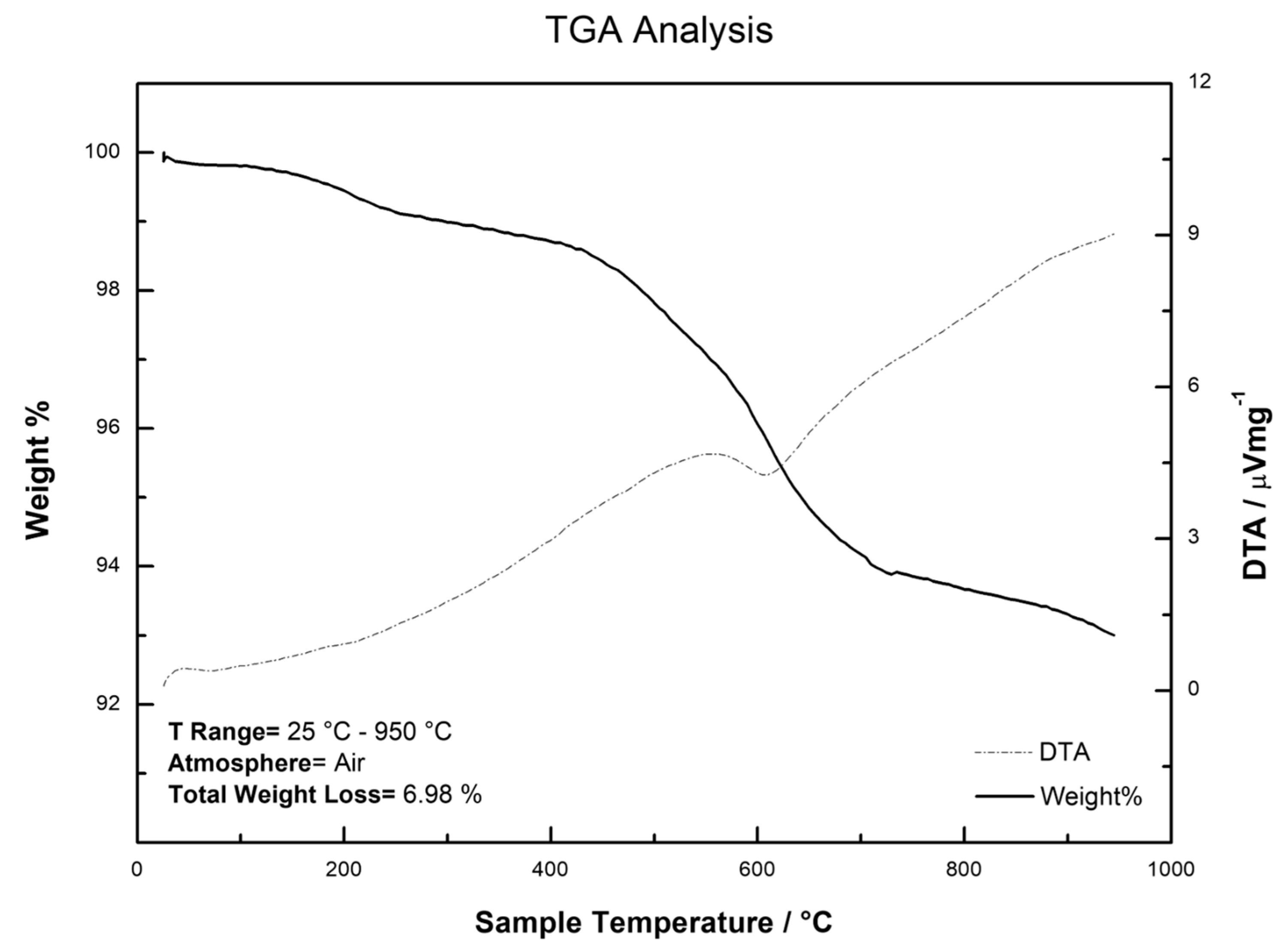
2.3. Characterization of Sol-Gel CW 20M SPE Sorbent Using FTIR and TGA Analysis
2.4. Enrichment Factors Determination
3. Material and Methods
3.1. Chemicals, Solvents and Devices
3.2. Stock Solution and Quality Control Samples
3.3. Apparatus and Chromatographic Conditions
3.4. Preparation of Fabric Phase Sorptive Extraction (FPSE) Media
3.5. Synthesis of Sol-Gel CW 20M SPE Sorbent
3.6. Preparation of the SPE Media
3.7. SPE Sorbents Characterization Using FTIR and TGA
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Samanidou, V.; Kaltzi, I.; Kabir, A.; Furton, K.G. Simplifying sample preparation using fabric phase sorptive extraction technique for the determination of benzodiazepines in blood serum by high-performance liquid chromatography. Biomed. Chromatogr. 2016, 30, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.J.M. Handling large volume samples: applications of SPE to environmental matrices. In Solid-Phase Extraction: Principles, Techniques, and Application, 1st ed.; Simpson, N.K.J., Ed.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Kabir, A.; Furton, K.G. Fabric Phase Sorptive Extractors. United States Patents 9557252, 31 January 2017. [Google Scholar]
- Locatelli, M.; Kabir, A.; Innosa, D.; Lopatriello, T.; Furton, K.G. A Fabric Phase Sorptive Extraction-High Performance Liquid Chromatography-Photo Diode Array Detection Method for the Determination of Twelve Azole Antimicrobial Drug Residues in Human Plasma and Urine. J. Chromatogr. B 2017, 1040, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Aznar, M.; Alfaro, P.; Nerin, C.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction: An innovative sample preparation approach applied to the analysis of specific migration from food packaging. Anal. Chim. Acta 2016, 936, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Furton, K.G.; Tinari, N.; Grossi, L.; Innosa, D.; Macerola, D.; Tartaglia, A.; Di Donato, V.; D’Ovidio, C.; Locatelli, M. Fabric Phase Sorptive Extraction-High Performance Liquid Chromatography-Photo Diode Array Detection Method for Simultaneous Monitoring of Three Inflammatory Bowel Disease Treatment Drugs in Whole Blood, Plasma and Urine. J. Chromatogr. B 2018, 1084, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, M.; Tinari, N.; Grassadonia, A.; Tartaglia, A.; Macerola, D.; Piccolantonio, S.; Sperandio, E.; D’Ovidio, C.; Carradori, S.; Ulusoy, H.I.; et al. FPSE-HPLC-DAD method for the quantification of anticancer drugs in human whole blood, plasma and urine. J. Chromatogr. B 2018, 1095, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Bacalum, E.; Tanase, A.; David, V. Retention mechanisms applied in solid phase extraction for some polar compounds. Analele Universitatii Bucaresti–Chimie 2010, 19, 61–68. [Google Scholar]
- Bielica-Daszkiewicz, K.; Voelkel, A. Theoretical and experimental methods of determination of the breakthrough volume of SPE sorbents. Talanta 2009, 80, 614–621. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, V.; Tessari, F.; Bellagamba, G.; De Luca, E.; Cifelli, R.; Celia, C.; Primavera, R.; Di Francesco, M.; Paolino, D.; Di Marzio, L.; et al. MicroExtraction by Packed Sorbent and HPLC-PDA quantification of multiple anti-inflammatory drugs and fluoroquinolones in human plasma and urine. J. Enz. Inhibit. Med. Chem. 2016, 31, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, M.; Ciavarella, M.T.; Paolino, D.; Celia, C.; Fiscarelli, E.; Ricciotti, G.; Pompilio, A.; Di Bonaventura, G.; Grande, R.; Zengin, G.; et al. Determination of Ciprofloxacin and Levofloxacin in Human Sputum Collected from Cystic Fibrosis Patients using Microextraction by Packed Sorbent-High Performance Liquid Chromatography Photo Diode Array Detector. J. Chromatogr. A 2015, 1419, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, M.; Cifelli, R.; Di Legge, C.; Barbacane, R.C.; Costa, N.; Fresta, M.; Celia, C.; Capolupo, C.; Di Marzio, L. Simultaneous determination of Eperisone Hydrochloride and Paracetamol in mouse plasma by High Performance Liquid Chromatography-Photo Diode Array Detector. J. Chromatogr. A 2015, 1388, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Campestre, C.; Locatelli, M.; Guglielmi, P.; De Luca, E.; Bellagamba, G.; Menta, S.; Zengin, G.; Celia, C.; Di Marzio, L.; Carradori, S. Analysis of imidazoles and triazoles in biological samples after MicroExtraction by Packed Sorbent. J. Enz. Inhibit. Med. Chem. 2017, 32, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Bielica-Daszkiewicz, K.; Voelkel, A.; Rusińska-Roszak, D.; Zarzycki, P.K. Estimation of the breakthrough volume of selected steroids for C-18 solid-phase extraction sorbent using retention data from micro-thin layer chromatography. J. Sep. Sci. 2013, 36, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Mesa, R.; Jurmain, J.; Furton, K. Fabric Phase Sorptive Extraction Explained. Separations 2017, 4, 21. [Google Scholar] [CrossRef]
- Smith, A.L. Infrared spectra-structure correlations for organosilicon compounds. Spectrochim. Acta 1960, 16, 87–105. [Google Scholar] [CrossRef]
- Kabir, A.; Furton, K.G. Sol-Gel Polymeric Stationary Phases for High-Performance Liquid Chromatography and Solid Phase Extraction: Their Method of Making. U.S. Patent 9925518, 27 March 2018. [Google Scholar]
- Raza, N.; Kim, K.-H.; Abdullah, M.; Raza, W.R.; Brown, J.C. Recent developments in analytical quantitation approaches for parabens in human-associated samples. TrAC 2018, 98, 161–173. [Google Scholar] [CrossRef]
- Pavlović, D.M.; Babić, S.; Horvat, A.J.M.; Kaštelan-Macan, M. Sample preparation in analysis of pharmaceuticals. TrAC 2007, 26, 1062–1075. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors under request. |
| Drug Name | CAS No. | Molecular Weight (g/mole) | Molecular Structure | LogP | pKa |
|---|---|---|---|---|---|
| Cortisone | 53-06-5 | 360.44 | 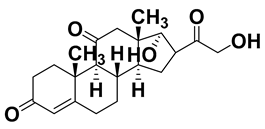 | 1.66 | 12.6 |
| Methyl-p-hydroxy benzoate | 99-76-3 | 152.15 |  | 1.96 | 8.4 |
| Ciprofloxacin | 85721-33-1 | 331.34 |  | 2.30 | 5.76 |
| Sulfasalazine | 599-79-1 | 398.39 | 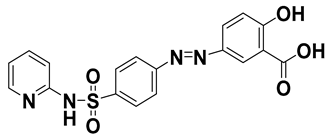 | 2.92 | 3.3 |
| Sorbent | Analyte | Breakthrough Volume (mL) | Retention Volume (mL) | Hold-Up Volume (mL) | Retention Factor (k) | Number of Theoretical Plates (N) |
|---|---|---|---|---|---|---|
| Avantor C18 | Ciprofloxacin | >50 | - | - | - | - |
| Sulfasalazine | >50 | - | - | - | - | |
| Methyl-p-hydroxybenzoate | 11.3 | 9.9 | 11.9 | 0.20 | 2000 | |
| Cortisone | >50 | - | - | - | - | |
| Sep-Pac Vac C18 | Ciprofloxacin | 23.6 | 16.4 | 30.6 | 0.87 | 16 |
| Sulfasalazine | >50 | - | - | - | - | |
| Methyl-p-hydroxybenzoate | 52.8 | 37.3 | 66.8 | 0.79 | 18 | |
| Cortisone | >50 | - | - | - | - | |
| Oasis HLB | Ciprofloxacin | 27.6 | 25.9 | 28.8 | 0.11 | 845 |
| Sulfasalazine | >50 | - | - | - | - | |
| Methyl-p-hydroxybenzoate | >50 | - | - | - | - | |
| Cortisone | >50 | - | - | - | - | |
| CW 20M | Ciprofloxacin | 5.26 | 2.2 | 8.7 | 2.95 | 1 |
| Sulfasalazine | >50 | - | - | - | - | |
| Methyl-p-hydroxybenzoate | 4.4 | 2.9 | 4.9 | 0.66 | 13 | |
| Cortisone | 6.5 | 4.5 | 7.9 | 0.75 | 17 |
| Sorbent | Analyte | Breakthrough Volume (mL) | Retention Volume (mL) | Hold-Up Volume (mL) | Retention Factor (k) | Number of Theoretical Plates (N) |
|---|---|---|---|---|---|---|
| Avantor C18 | Ciprofloxacin | 15.1 | 8.8 | 32.2 | 2.68 | 5 |
| Sulfasalazine | 29.7 | 17.4 | 46.5 | 1.67 | 5 | |
| Methyl-p-hydroxybenzoate | 6.7 | 3.7 | 14.3 | 2.91 | 3 | |
| Cortisone | 25.7 | 14.2 | 49.6 | 2.48 | 4 | |
| Sep-Pac Vac C18 | Ciprofloxacin | 5.4 | 3.6 | 7.2 | 0.99 | 12 |
| Sulfasalazine | >20 | - | - | - | - | |
| Methyl-p-hydroxybenzoate | 21.4 | 14.7 | 27.4 | 0.87 | 15 | |
| Cortisone | >20 | - | - | - | - | |
| Oasis HLB | Ciprofloxacin | 13.9 | 8.8 | 20.7 | 1.34 | 8 |
| Sulfasalazine | >20 | - | - | - | - | |
| Methyl-p-hydroxybenzoate | >20 | - | - | - | - | |
| Cortisone | >20 | - | - | - | - | |
| CW 20M | Ciprofloxacin | >20 | - | - | - | - |
| Sulfasalazine | 13.2 | 7.5 | 22.7 | 2.02 | 4 | |
| Methyl-p-hydroxybenzoate | >20 | - | - | - | - | |
| Cortisone | >20 | - | - | - | - |
| Analyte | QC Concentration (μg/mL) | FPSE CW 20M | SPE Format | |||
|---|---|---|---|---|---|---|
| Avantor C18 | Sep-Pac Vac C18 | Oasis HLB | CW 20M | |||
| Ciprofloxacin | 0.8 | 28.4 | 740 | 304 | 67 | 321 |
| 2.5 | 37.0 | 406 | 199 | 607 | 186 | |
| 8 | 13.6 | 200 | 188 | 367 | 113 | |
| Sulfasalazine | 0.8 | 248 | 640 | 100 | 380 | 202 |
| 2.5 | 164 | 325 | 206 | 408 | 217 | |
| 8 | 278 | 344 | 299 | 493 | 225 | |
| Methyl-p-hydroxybenzoate | 0.8 | 190 | 204 | 78 | 314 | 59 |
| 2.5 | 155 | 310 | 133 | 344 | 113 | |
| 8 | 121 | 205 | 216 | 252 | 76 | |
| Cortisone | 0.8 | 136 | 80 | 123 | n.a. | 171 |
| 2.5 | 88 | 160 | 195 | 167 | 199 | |
| 8 | 128 | 203 | 240 | 245 | 161 | |
| Sorbent | Sorbent Mass (mg) | Surface Area (m2/g) | Pore Diameter (Ǻ) | Pore Volume (cm3/g) | Particle Size (µm) |
|---|---|---|---|---|---|
| Oasis HLB | 30 | 800 | 80 | 1–3 | 30 |
| Sep-Pac Vac C18 | 30 | 325 | 125 | - | 55–105 |
| Avantor C18 | 30 * | 320–350 | 60 | - | 40 |
| Carbowax 20M | 30 | 990 | 71 | 1.8 | 40 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tartaglia, A.; Locatelli, M.; Kabir, A.; Furton, K.G.; Macerola, D.; Sperandio, E.; Piccolantonio, S.; Ulusoy, H.I.; Maroni, F.; Bruni, P.; et al. Comparison between Exhaustive and Equilibrium Extraction Using Different SPE Sorbents and Sol-Gel Carbowax 20M Coated FPSE Media. Molecules 2019, 24, 382. https://doi.org/10.3390/molecules24030382
Tartaglia A, Locatelli M, Kabir A, Furton KG, Macerola D, Sperandio E, Piccolantonio S, Ulusoy HI, Maroni F, Bruni P, et al. Comparison between Exhaustive and Equilibrium Extraction Using Different SPE Sorbents and Sol-Gel Carbowax 20M Coated FPSE Media. Molecules. 2019; 24(3):382. https://doi.org/10.3390/molecules24030382
Chicago/Turabian StyleTartaglia, Angela, Marcello Locatelli, Abuzar Kabir, Kenneth G. Furton, Daniela Macerola, Elena Sperandio, Silvia Piccolantonio, Halil I. Ulusoy, Fabio Maroni, Pantaleone Bruni, and et al. 2019. "Comparison between Exhaustive and Equilibrium Extraction Using Different SPE Sorbents and Sol-Gel Carbowax 20M Coated FPSE Media" Molecules 24, no. 3: 382. https://doi.org/10.3390/molecules24030382
APA StyleTartaglia, A., Locatelli, M., Kabir, A., Furton, K. G., Macerola, D., Sperandio, E., Piccolantonio, S., Ulusoy, H. I., Maroni, F., Bruni, P., Croce, F., & Samanidou, V. F. (2019). Comparison between Exhaustive and Equilibrium Extraction Using Different SPE Sorbents and Sol-Gel Carbowax 20M Coated FPSE Media. Molecules, 24(3), 382. https://doi.org/10.3390/molecules24030382










