Heparinoid Complex-Based Heparin-Binding Cytokines and Cell Delivery Carriers
Abstract
:1. Introduction
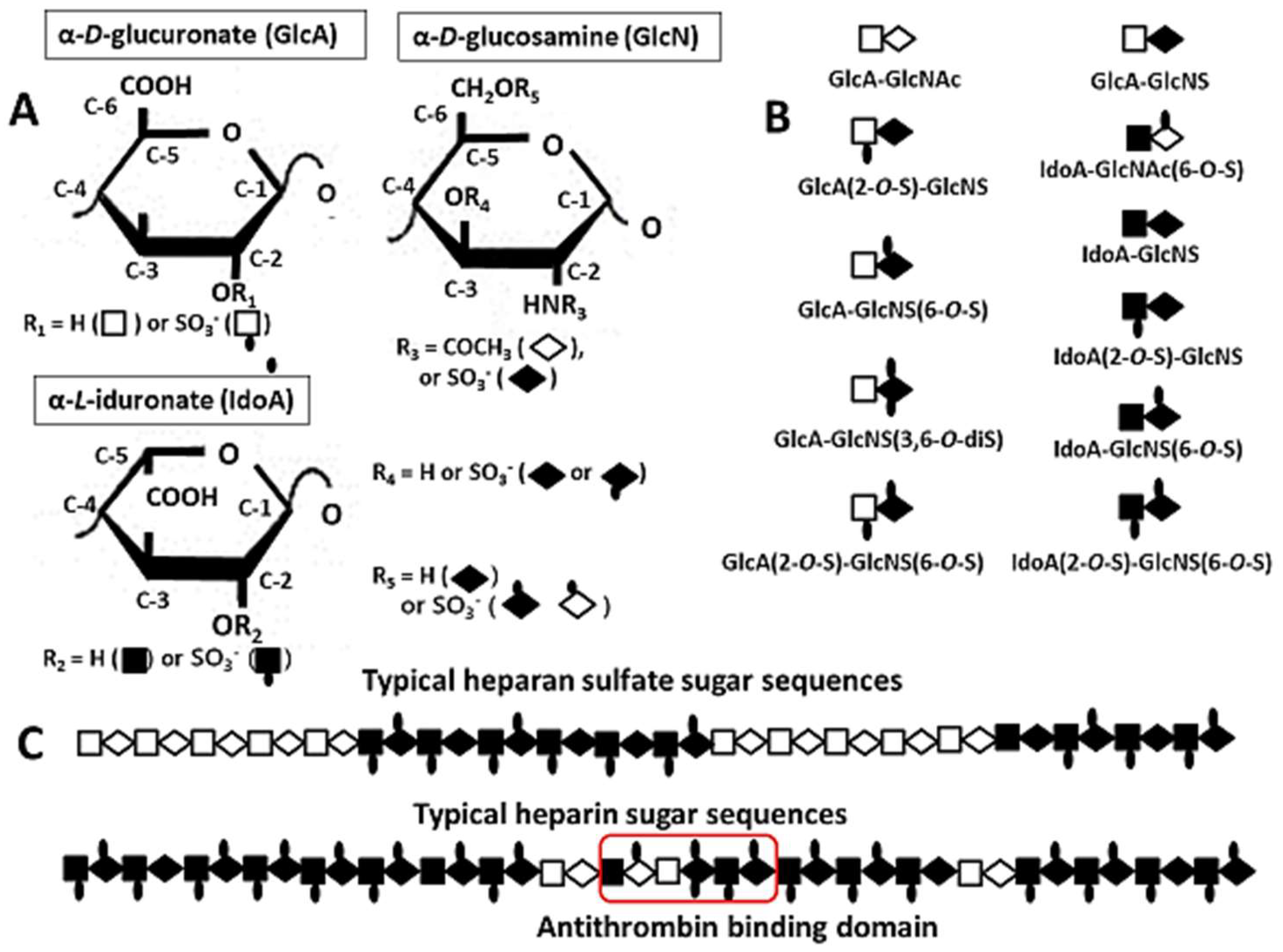
2. Structures of Heparin/HS
2.1. Compositional Structures of Heparin and HS
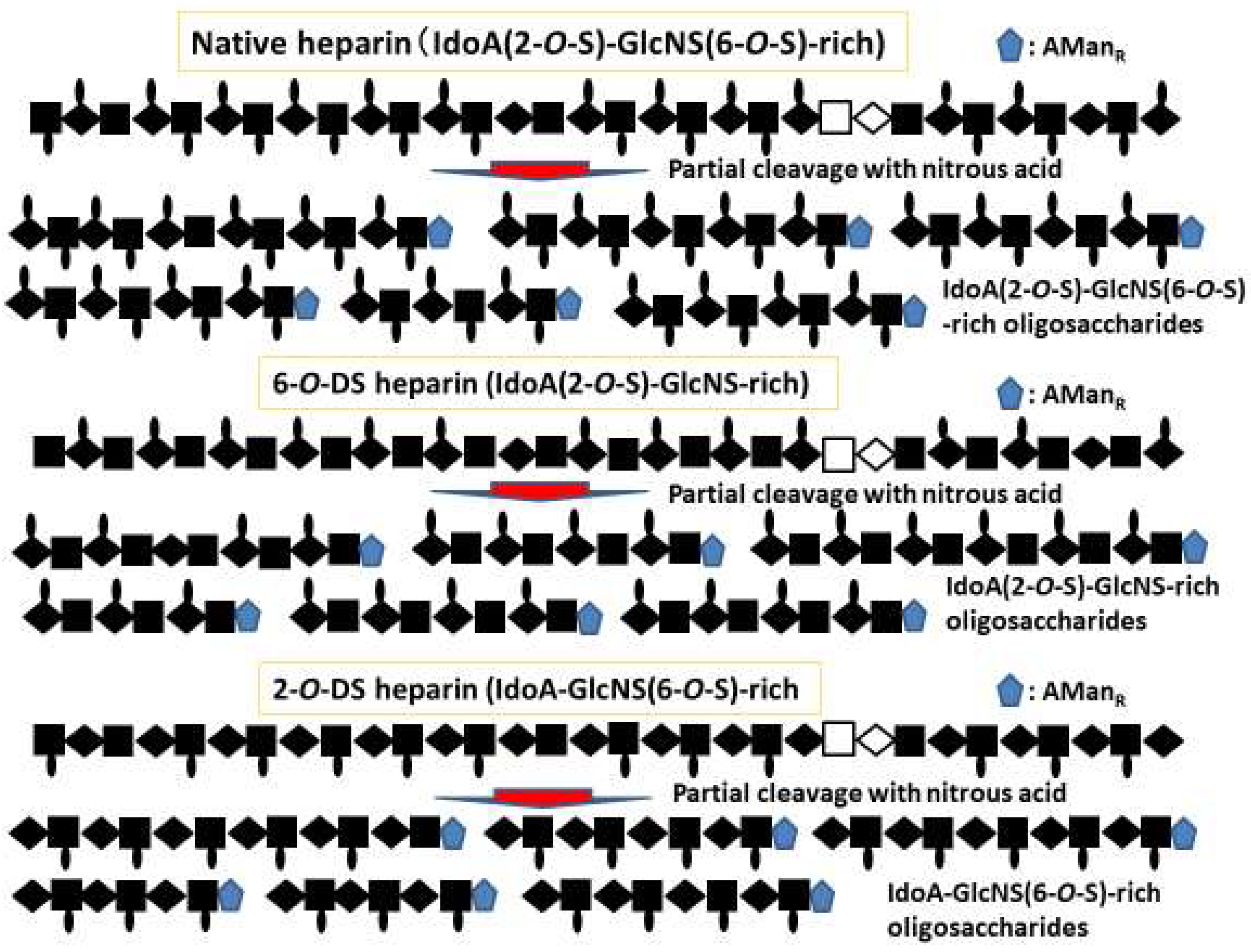
2.2. Heparin-Based Chemically Modified Sulfated Polysaccharides and Oligosaccharides from Heparin
2.3. Size- and Structure-Defined Oligosaccharides from Heparin and their Affinities for and Activation of FGF
3. Interaction of Heparin/HS with Heparin-Binding Cytokines
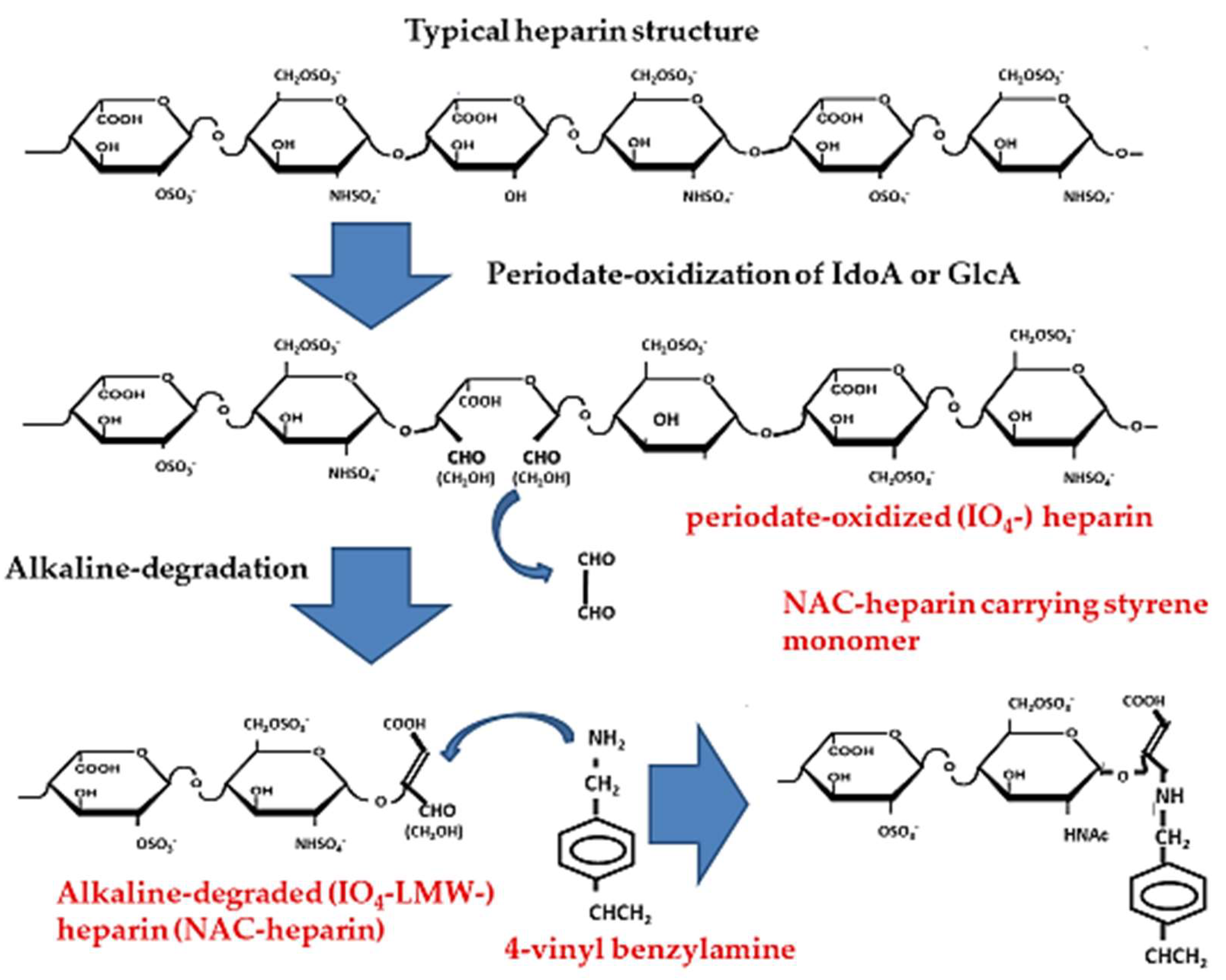
4. Non-Anticoagulant (NAC)-Heparin Carrying Polystyrene (NAC-HCPS)
4.1. Synthesis of NAC-Heparin and its Applications
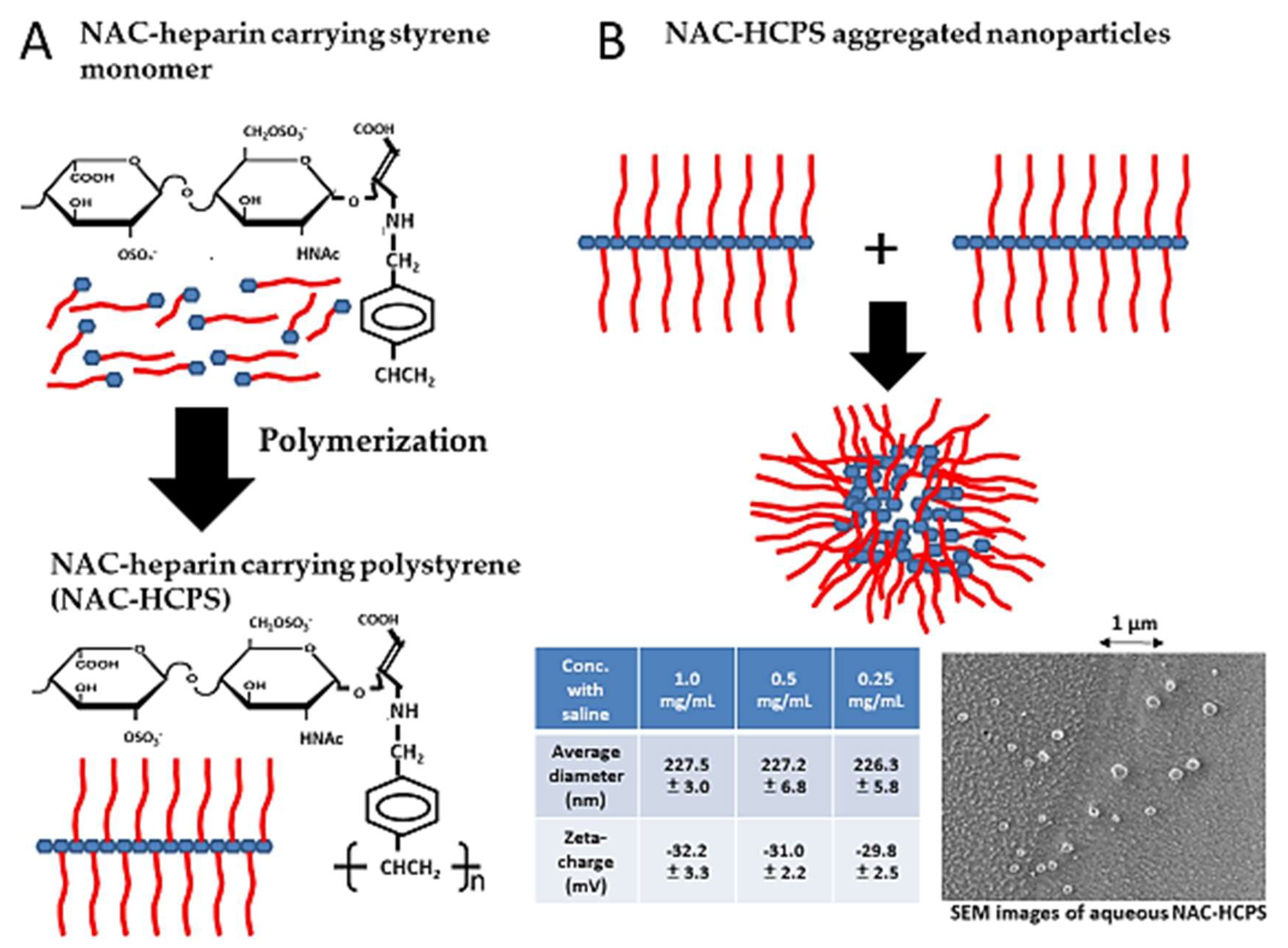
4.2. NAC-HCPS and its Applications
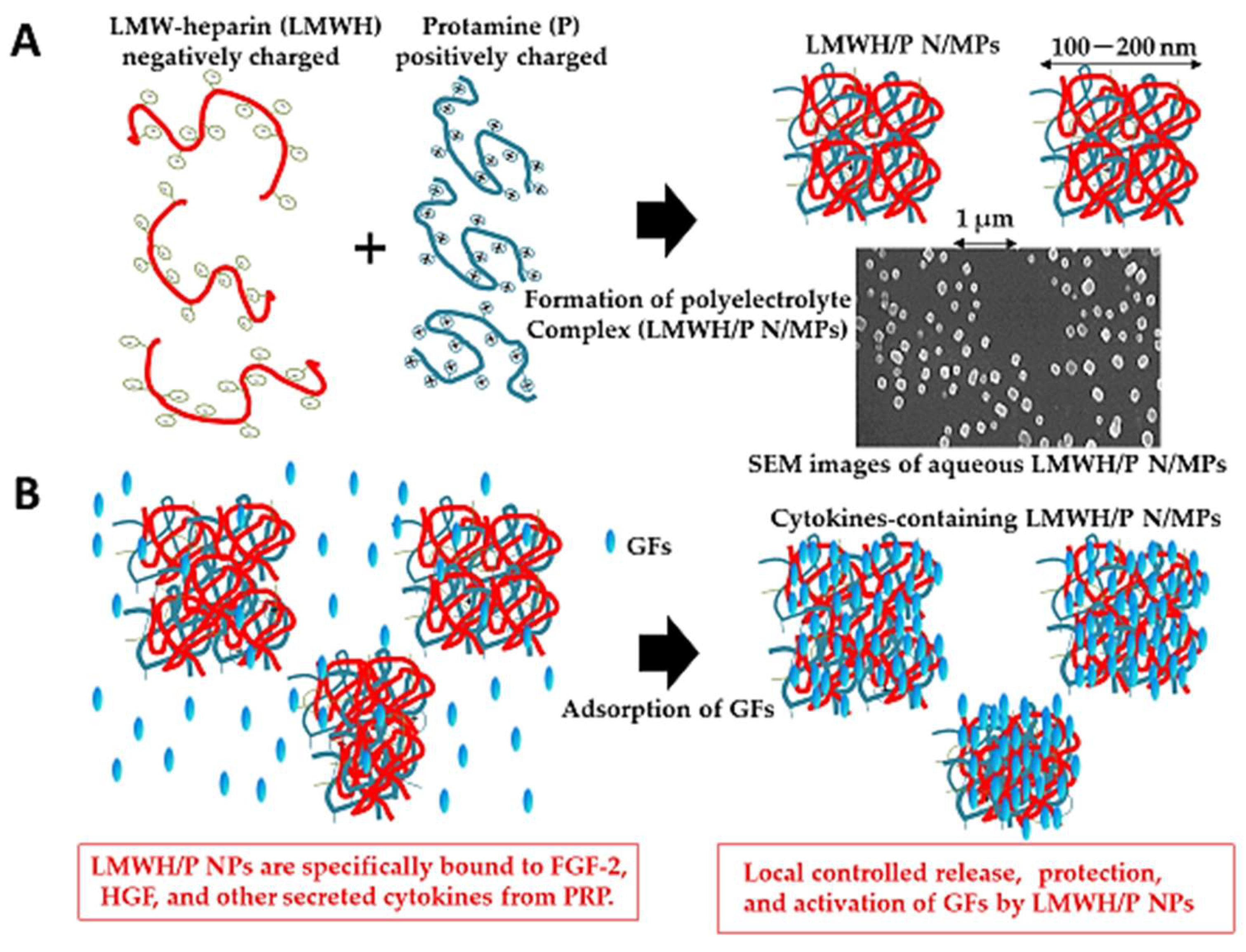
5. Heparin-Based Polyelectrolyte Complex Nano/Micro-Particles (N/MPs) and their Applications
5.1. Low-Molecular-Weight Heparin/Protamine (LMWH/P) N/MPs for Cytokine Carrier
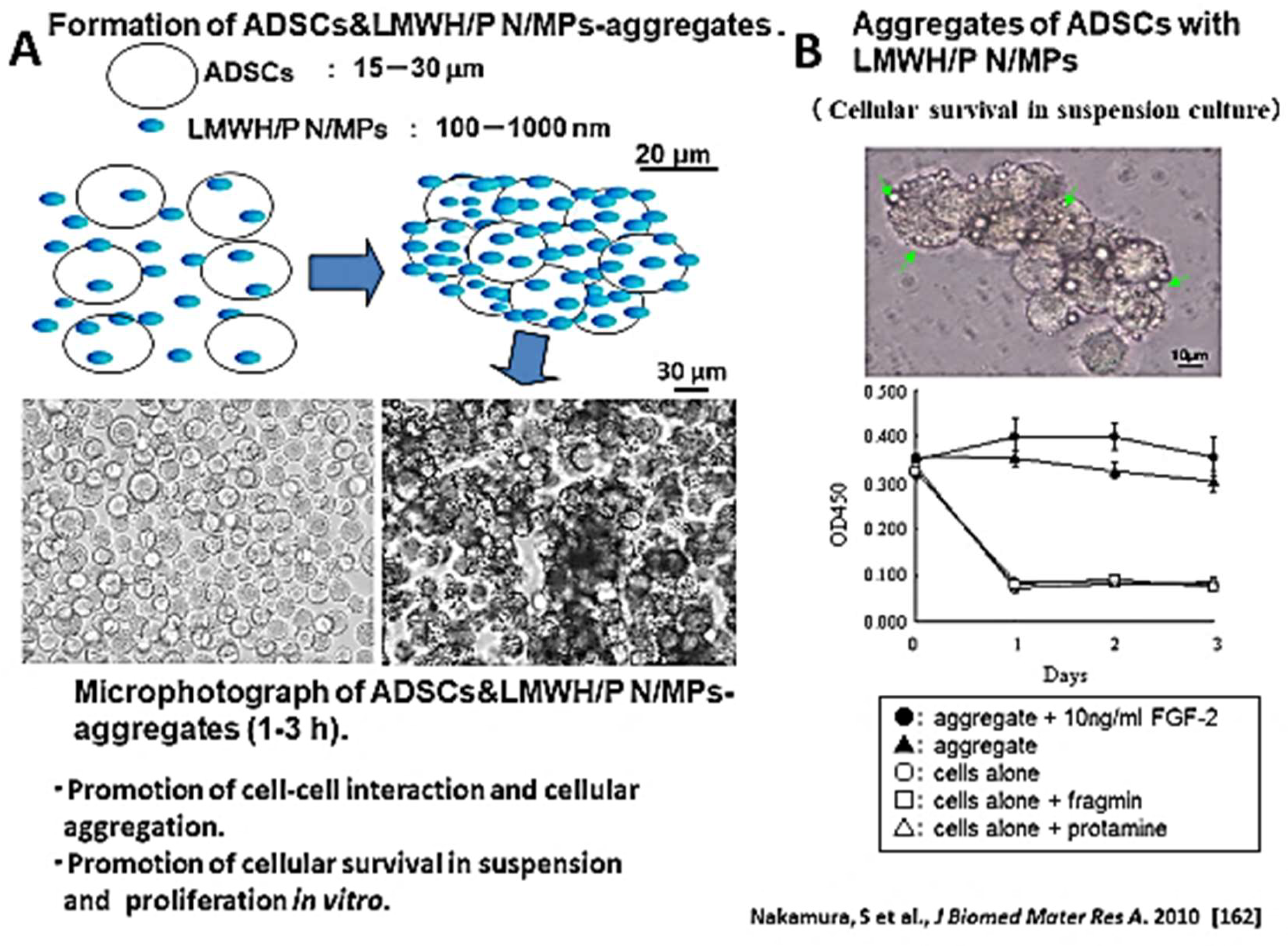
5.2. LMWH/P N/MPs for Cell Carrier
6. Heparinoid-Coated Devices
7. Overview
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kjellen, L.; Lindahl, U. Proteoglycans: Structure and interaction. Annu. Rev. Biochem. 1991, 60, 443–475. [Google Scholar] [CrossRef]
- Gandhi, N.S.; Mancera, R.L. The structure of glycosaminoglycans and their interactions with proteins. Chem. Biol. Drug Design 2008, 72, 455–482. [Google Scholar] [CrossRef]
- Prydz, K. Determinants of glycosaminoglycan (GAG) strucuture. Biomolecules 2015, 5, 2003–2022. [Google Scholar] [CrossRef] [Green Version]
- Mirsra, S.; Hascall, V.C.; Atanelishvili, I.; Rodriguez, R.M.; Markwald, R.R.; Ghatak, S. Utilization of glycosaminoglycan/proteoglycans as carriers for targeted therapy delivery. Int. J. Cell Biol. 2015, 2015, 25. [Google Scholar] [CrossRef] [Green Version]
- Casu, B.; Vlodavsky, I.; Sanderson, R.D. Non-anticoagulant heparins and inhibition of cancer. Pathophysiol. Haematol. Thromb. 2008, 36, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Bernfield, M.; Gotte, M.; Park, P.W.; Reizes, O.; Fitzgerald, M.L.; Lincecum, J.; Zako, M. Function of cell surface heparan sulfate proteoglycans. Ann. Rev. Biochem. 1999, 68, 729–777. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, U.; Lidholt, K.; Spillmann, D.; Kjellen, L. More to “heparin” than anticoagulation. Thromb. Res. 1994, 4, 817–824. [Google Scholar] [CrossRef]
- Lindahl, U.; Kejellen, L. Pathophysiology of heparan sulfate: Many diseases, few drugs. J. Intern. Med. 2013, 6, 555–571. [Google Scholar] [CrossRef] [Green Version]
- Casu, B.; Lindahl, U. Structure and biological interaction of heparin and heparan sulfate. Adv. Carbohydr. Chem. Biochem. 2001, 57, 159–206. [Google Scholar]
- Murdoch, A.D.; Dodge, G.R.; Cohen, I.; Tuan, R.S.; Iosso, R.V. Promery structure of human heparansulfate proteoglycan from basement membrane (HSPG2/perlecan). J. Biol. Chem. 1992, 267, 8544–8557. [Google Scholar]
- Nader, H.B.; Lopes, C.C.; Rocha, H.A.; Santos, E.A.; Dietrich, C.P. Heparins and heparinoids: Occurrence, structure and mechanism of antithrombotic and hemorrhagic activities. Curr. Pharm. Des. 2004, 10, 951–966. [Google Scholar] [CrossRef] [PubMed]
- Lyon, M.; Gallagher, J.T. Bio-specific sequences and domains in heparan sulphate and regulation of cell growth and adhesion. Matrix Biol. 1988, 17, 485–493. [Google Scholar] [CrossRef]
- Coombe, D.R.; Kett, W.C. Heparan sulfate-protein interactions: Therapeutic potential through structure-function insights. Cell. Mol. Life Sci. 2005, 62, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Olsen, S.K.; Ibahimi, O.A. Structural basis for fibroblast growth factor activation. Cytokine Growth Factor Rev. 2005, 16, 107–137. [Google Scholar] [CrossRef]
- Lortat-Jacob, H. The molecular basis and functional implications of chemokine interactions with heparan sulfate. Curr. Opin. Struct. Biol. 2009, 19, 543–548. [Google Scholar] [CrossRef]
- Ishihara, M.; Guo, Y.; Wei, Z.; Yang, Z.; Swiedler, S.J.; Orellana, A.; Hirschberg, C.B. Regulation of biosynthesis of the basic fibroblast growth factor binding domains of heparan sulfate by heparan sulfate-N-deacetylase/N-sulfotransferase expression. J. Biol. Chem. 1993, 268, 20091–20095. [Google Scholar]
- Rapraeger, A.C.; Krufka, A.; Olwin, B.B. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science 1991, 252, 1705–1708. [Google Scholar] [CrossRef] [Green Version]
- Yayon, A.; Klagsbun, M.; Esko, J.D.; Leder, P.; Ornitz, D.M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell 1991, 64, 841–848. [Google Scholar] [CrossRef]
- Ishihara, M.; Shaklee, P.N.; Yang, Z.; Liang, W.; Wei, Z.; Stack, R.J. Structural features in heparin which modulate specific biological activities mediated by basic fibroblast growth factor. Glycobiology 1994, 4, 451–458. [Google Scholar] [CrossRef]
- Ishihara, M. Structural requirements in heparin for binding and activation of FGF-1 and FGF-4 are different from that for FGF-2. Glycobiology 1994, 4, 817–824. [Google Scholar] [CrossRef]
- de Azevedo, T.C.; Bezerra, M.E.; Santos, M.G.L.; Souza, L.A.; Marques, C.T.; Benevides, N.M.; Leite, E.L. Heparinoids algel and anticoagulant, hemorrhage activities and platelet aggregation. Biomed. Pharmacother. 2009, 63, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Kariya, Y.; Watabe, S.; Kyougashima, M.; Ishihara, M.; Ishii, T. Structure of fucose branches fucan in the glycosaminoglycan from the body wall of sea cucumber Stichopus japonicas. Carbohydr. Res. 1997, 297, 273–279. [Google Scholar] [CrossRef]
- Rabenstein, D.L. Heparin and heparan sulfate: Structure and function. Nat. Prod. Rep. 2002, 19, 312–331. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, Q.; Chen, L.; Ren, S.; Xu, P.; Tang, Y.; Luo, D. Higher specificity of the activity of low molecular weight fucoidan for thrombin-induced platelet aggregation. Thromb. Res. 2010, 125, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Manne, B.K.; Getz, T.M.; Hughes, C.E.; Alshehri, O.; Dangelmaier, C.; Naik, U.P.; Watson, S.P.; Kunapuli, S.P. Fucoidan is a novel platelet agonist for the C-type lectin-like receptor 2 (CLEC-2). J. Biol. Chem. 2013, 288, 7717–7726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-based nanomaterials for drug delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef] [Green Version]
- Masuoka, K.; Ishihara, M.; Asazuma, T.; Hattori, H.; Matsui, T.; Takase, B.; Kanatani, Y.; Fujita, M.; Saitoh, Y.; Yura, H.; et al. The interaction of chitosan with fibroblast growth factor-2 and its protection from inactivation. Biomaterials 2005, 26, 3277–3284. [Google Scholar] [CrossRef]
- Schatz, C.; Bionaz, A.; Lucas, M.J.; Pichot, C.; Viton, C.; Domard, A.; Delair, T. Formation of polyelectrolyte complex particles from self-complexation of N-sulfated chitosan. Biomacromolecules 2005, 6, 1642–1647. [Google Scholar] [CrossRef]
- Delair, T. Colloidal polyelectrolyte complexes of chitosan and dextran sulfate towards versatile nanocarriers of bioactive molecules. Eur. J. Pharm. Biopharm. 2011, 78, 10–18. [Google Scholar] [CrossRef]
- Wang, Z.; Ly, M.; Zhong, W.; Suen, A.; Hickey, A.M.; Dordick, J.S.; Linhardt, R.J. E. coli K5 fermentation and the preparation of heparosan, a bioengineered heparin precursor. Biotechnol. Bioeng. 2010, 107, 964–973. [Google Scholar] [CrossRef] [Green Version]
- Higashi, K.; Ly, M.; Wang, Z.; Masuko, S.; Bhaskar, U.; Sterner, E.; Zhang, F.; Toida, T.; Dordick, J.S.; Linhardt, R.J. Controlled photochemical depolymerization of K5 heparosan, a bioengineered heparin precursor. Carbohydr. Polym. 2011, 86, 1365–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joice, A.; Raman, K.; Mencio, C.; Quintero, M.V.; Brown, S.; Nguyen, T.K.; Kuberan, B. Enzymatic synthesis of heparin sulfate and heparin. Methods Mol. Biol. 2015, 1229, 11–19. [Google Scholar] [PubMed]
- Oreste, P.; Zoppetti, G. Semi-synthetic heparinoids. Handb. Exp. Pharmacol. 2012, 207, 403–422. [Google Scholar]
- Schonherr, E.; Hausser, H.-J. Extracellular matrix and cytokines: A functional unit. Dev. Immunol. 2000, 7, 89–101. [Google Scholar] [CrossRef]
- Kemp, M.M.; Linhardt, R.J. Heparin-based nanoparticles. WIREs Nanomed. Nanobiotechnol. 2010, 2, 77–87. [Google Scholar] [CrossRef]
- Jiao, Y.; Ubrich, N.; Marchand-Arvier, M.; Vigneron, C.; Hoffman, M.; Maincent, P. In vitro and in vivo evaluation of oral heparin-loaded polymeric nanoparticles in rabbits. Circulation 2002, 105, 230–235. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, M.; Kishimoto, S.; Takikawa, M.; Hattori, H.; Nakamura, S.; Shimizu, M. Biomedical application of low molecular weight heparin/protamine micro/nanoparticles as cell- and growth factor-carriers and coating matrix. Int. J. Mol. Sci. 2015, 16, 11785–11803. [Google Scholar] [CrossRef] [Green Version]
- Berth, G.; Voigh, A.; Dautzenberg, H.; Donath, E.; Mohwald, H. Polyelectrolyte complex and layer-by layer capsules from chitosan/chitosan sulfate. Biomacromolecules 2002, 3, 579–590. [Google Scholar] [CrossRef]
- Sotiropoulou, M.; Bokias, G.; Staikos, G. Water-soluble complexes through coulombic interactions between bovine serum albumin and anionic polyelectrolytes grafted with hydrophilic nonionic side chains. Biomacromolecules 2005, 6, 1835–1838. [Google Scholar] [CrossRef]
- Kolset, S.O.; Tveit, H. Serglycin-structure and biology. Cell Mol. Life Sci. 2008, 65, 1073–1085. [Google Scholar] [CrossRef]
- Belting, M. Heparan sulfate proteoglycan as a plasma membrane carrier. Trends Biochem. Sci. 2003, 28, 145–151. [Google Scholar] [CrossRef]
- Christianson, H.C.; Belting, M. Heparan sulfate proteoglycan as a cell-surface endocytosis receptor. Matrix Biol. 2014, 35, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, S.; Lamanna, W.C.; Esko, J.D. Heparan sulfate proteoglycans. Cold Spring Harb. Perspect Biol. 2011, 3, a004952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esko, J.D.; Selleck, S.B. Order out of chaos, assembly of ligand binding sites in heparin sulfate. Annu. Rev. Biochem. 2002, 71, 435–471. [Google Scholar] [CrossRef]
- Miller, T.; Goude, M.C.; McDevitt, T.C.; Temenoff, J.S. Molecular engineering of glycosaminoglycan chemistry for biomolecule delivery. Acta Biomater. 2014, 10, 1705–1719. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, J.T.; Turnbull, J.E.; Lyon, M. Patterns of sulfation in heparan sulphate polymorphism based on a common structural theme. Int. J. Biochem. 1992, 24, 553–560. [Google Scholar] [CrossRef]
- Lindahl, U.; Backstrom, G.; Thunberg, L.; Leder, I.G. Evidence for 3-O-sulfated d-glucosamine residue in the antithrombin-binding sequence of heparin. Proc. Natl. Acad. Sci. USA 1980, 77, 6551–6555. [Google Scholar] [CrossRef] [Green Version]
- Meneghetti, M.C.Z.; Hughes, A.J.; Rudd, T.R.; Nader, H.B.; Powell, A.K.; Yates, E.A.; Lima, M.A. Heparan sulfate and heparin interactions with proteins. Interface 2015, 12, 20150589. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, M.; Kariya, Y.; Kikuchi, H.; Minamisawa, T.; Yoshida, K. Importance of 2-O-sulfate groups of uronate residues in heparin for activation of FGF-1 and FGF-2. J. Biochem. 1997, 121, 345–349. [Google Scholar] [CrossRef]
- Imberty, A.; Lortat-Jacob, H.; Perez, S. Structural view of glycosaminoglycan-protein interactions. Carbohydr. Res. 2007, 342, 430–439. [Google Scholar] [CrossRef]
- Ishihara, M.; Takano, R.; Kanda, T.; Hayashi, K.; Hara, S.; Kikuchi, H.; Yoshida, K. Importance of 6-O-sulfate groups of glucosamine residues in heparin for activation of FGF-1 and FGF-2. J. Biochem. 1995, 118, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Kariya, Y.; Kyogashima, M.; Suzuki, K.; Isomura, T.; Sakamoto, T.; Horie, K.; Ishihara, M.; Takano, R.; Kamei, K.; Hara, S. Preparation of completely 6-O-desulfated heparin and its ability to enhance activity of basic fibroblast growth factor. J. Biol. Chem. 2000, 257, 25949–25958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, Y.; Nagasawa, K. Selective N-desulfation of heparin with dimethyl sulfoxide containing water or methanol. Carbohydr. Res. 1976, 46, 87–95. [Google Scholar] [CrossRef]
- Nagasawa, K.; Inoue, Y.; Kamata, T. Solvolytic desulfation of glucosaminoglycuronan sulfates with dimethyl sulfoxide containing water or methanol. Carbohydr. Res. 1977, 58, 47–55. [Google Scholar] [CrossRef]
- Lundin, L.; Larsson, H.; Kreuger, J.; Kanda, S.; Lindahl, U.; Salvimirta, M.; Claesson-Welsh, L. Selectively desulfated heparin inhibits fibroblast growth factor-induced mitogeneity and angiogenesis. J. Biol. Chem. 2009, 275, 24653–24660. [Google Scholar] [CrossRef] [Green Version]
- Raman, K.; Kuberan, B.; Arungundram, S. Chemical modification of heparin and heparosan. Methods Mol. Biol. 2015, 122, 31–36. [Google Scholar]
- Garg, H.G.; Mrabat, H.; Yu, L.; Freeman, C.; Li, B.; Zhang, F.; Linhardt, R.J.; Hales, C.A. Effect of carboxyl-reduced heparin on the growth inhibition of bovine pulmonary artery smooth muscle cells. Carbohydr. Res. 2010, 345, 1084–1087. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, M.; Tyrrell, D.J.; Stauber, G.B.; Brown, S.; Cousens, L.S.; Stack, R.J. Preparation of affinity-fractionated, heparin-derived oligosaccharides and their effects on selected biological activities mediated by basic fibroblast growth factor. J. Biol. Chem. 1993, 268, 4675–4683. [Google Scholar]
- Jastrebova, N.; Vanwildemeersch, M.; Rapraeger, A.C.; Gimenez-Gallego, G.; Lindahl, U.; Spillmann, D. Heparan sulfate-related oligosaccharides in ternary complex formation with fibroblast growth factors 1 and 2 and their receptors. J. Biol. Chem. 2006, 281, 26884–26892. [Google Scholar] [CrossRef] [Green Version]
- Plotnikov, A.N.; Schlessinger, J.; Hubbard, S.R.; Mohammadi, M. Structural basis for FGF receptor dimerization and activation. Cell 1999, 98, 641–650. [Google Scholar] [CrossRef] [Green Version]
- Presta, M.; Dell’Era, P.; Mitola, S.; Moroni, E.; Ronca, R.; Rusnati, M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005, 16, 159–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Zhang, Z.; Lin, X.; Beeken, A.; Eliseenkova, A.V.; Mohammadi, M.; Linhardt, R.J. Compositional analysis on heparin/heparin sulfate interacting with FGF FGFR complexes. Biochemistry 2009, 48, 8379–8386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Zheng, L.; Cheng, S.; Peng, Y.; Fu, L.; Zhang, X.; Lindardt, R.J. Comparison of the interactions of different growth factors and glycosaminoglycans. Molecules 2019, 24, 3360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marianayagam, N.J.; Sunde, M.; Mattews, J.M. The power of two: Protein dimerization in biology. Trends Biochem. Sci. 2004, 29, 618–625. [Google Scholar] [CrossRef]
- Goodger, S.J.; Robinson, C.J.; Murphy, K.J.; Gasiunas, N.; Harmer, N.J.; Blundell, T.L.; Pye, D.A.; Gallagher, J.T. Evidence that heparin saccharides promote FGF2 mitogenesis through two distinct mechanisms. J. Biol. Chem. 2008, 283, 13001–13008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atha, D.H.; Lormeau, J.C.; Petitoum, M.; Rosenberg, R.D.; Choay, J. Contribution of monosaccharide residues in heparin binding to antithrombin III. Biochemistry 1985, 24, 6723–6729. [Google Scholar] [CrossRef]
- Atha, D.H.; Lormeau, J.C.; Petitou, M.; Rosenberg, R.D.; Choay, J. Contribution of 3-O- and 6-O-sulfated glucosamine residues in the heparin-induced conformational change in antithrombin III. Biochemistry 1987, 26, 6454–6461. [Google Scholar] [CrossRef]
- Esko, J.D.; Lindahl, U. Molecular diversity of heparan sulfate. J. Clin. Investig. 2001, 108, 169–173. [Google Scholar] [CrossRef]
- Chavante, S.F.; Brito, A.S.; Lima, M.; Yates, E.; Nader, H.; Guerrini, M.; Torri, G.; Bisio, A. A heparin-like glycosaminoglycan from shrimp containing high levels of 3-O-sulfated d-glucosamine groups in an unusual trisaccharide sequence. Carbohydr. Res. 2014, 390, 59–66. [Google Scholar] [CrossRef]
- Kreuger, A.; Salmivirta, M.; Sturiale, L.; Gimenez-Gallego, G.; Lindahl, U. Sequence analysis of heparan sulfate epitopes with graded affinities for fibroblast growth factors 1 and 2. J. Biol. Chem. 2001, 276, 30744–30752. [Google Scholar] [CrossRef] [Green Version]
- Jemth, P.; Kreuger, J.; Kusche-Gullberg, M.; Sturiale, L.; Gimenez-Gallego, G.; Lindahl, U. Biosynthetic oligosaccharide libraries for identification of protein-binding heparan sulfate motifs. Exploring the structural diversity by screening for fibroblast growth factor (FGF) 1 and FGF -2 binding. J. Biol. Chem. 2002, 277, 30567–30573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlessinger, J.; Plotnikov, A.N.; Ibrahimi, O.A.; Eliseenkova, A.V.; Yeh, B.K.; Yayon, A.; Linhardt, R.J.; Mohammadi, M. Crystal structure of a ternary FGF-FGFR-heparin complex reveals a dual role for heparin in FGFR binding and dimerization. Mol. Cell 2000, 6, 743–750. [Google Scholar] [CrossRef]
- Allen, B.L.; Filla, M.S.; Rapraeger, A.C. Role of heparin sulfate as a tissue-specific regulator of FGF-4 and FGF receptor recognition. J. Cell Biol. 2001, 155, 845–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feyzi, E.; Lustig, F.; Fager, G.; Spillmann, D.; Lindahl, U.; Salmivirta, M. Characterization of heparin and heparan sulfate domains binding to the long splice variant of platelet-derived growth factor A chain. J. Biol. Chem. 1997, 272, 5518–5524. [Google Scholar] [CrossRef] [Green Version]
- Abramsson, A.; Kurup, S.; Busse, M.; Yamada, S.; Lindblom, P.; Schallmeiner, E.; Stenzel, D.; Sauvaget, D.; Ledin, J.; Ringvall, M.; et al. Defective N-sulfation of heparin sulfate proteoglycans limits PDGF-BB binding and pericyte recruitment in vascular development. Gene Dev. 2007, 21, 316–331. [Google Scholar] [CrossRef] [Green Version]
- Sakata, H.; Stahl, S.J.; Taylor, W.G.; Rosenberg, J.M.; Sakaguchi, K.; Wingfield, P.T.; Rubin, J.S. Heparin-binding and oligomerization of hepatocyte growth factor/scatter factor isoforms. J. Biol. Chem. 1997, 272, 9457–9463. [Google Scholar] [CrossRef] [Green Version]
- Lyon, M.; Deakin, J.A.; Mizuno, K.; Nakamura, T.; Gallagher, J.T. Interaction of hepatocyte growth factor with heparan sulfate. Elucidation of the major heparan sulfate determinants. J. Biol. Chem. 1994, 269, 11216–11223. [Google Scholar]
- Ashikari, S.; Habuchi, H.; Kimata, K. Characterization of heparan sulfate oligosaccharides that bind to hepatocyte growth factor. J. Biol. Chem. 1995, 270, 29586–29593. [Google Scholar] [CrossRef] [Green Version]
- Ono, K.; Hattori, H.; Takeshita, S.; Kurita, A.; Ishihara, M. Structural features in heparin which interact with VEGF165 and modulate its biological activity. Glycobiology 1999, 9, 705–711. [Google Scholar] [CrossRef] [Green Version]
- Teran, M.; Nugent, M.A. Synergistic binding of vascular endothelial growth factor-A and its receptors to heparin selectively modulates complex affinity. J. Biol. Chem. 2015, 290, 16451–16462. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.R.; Wang, H.F.; Yu, D.F.; Chen, X.Y.; He, S.Y. Modulation of binding to vascular endothelial growth factor and receptor by heparin derived oligosaccharide. Carbohydr. Polym. 2017, 174, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Lyon, M.; Rushton, G.; Gallagher, J.T. The interaction of the transforming growth factor-betas with heparin/heparan sulfate is isoform-specific. J. Biol. Chem. 1997, 272, 18000–18006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rider, C.C.; Mulloy, B. Heparin, heparin sulphate and the TGF-β superfamily. Molecules 2017, 22, 713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCaffrey, T.A.; Falcone, D.J.; Du, B. Transforming growth factor-beta 1 is a heparin-binding protein: Identification of putative heparin-binding regions and isolation of heparins with varying affinity for TGF-beta 1. J. Cell. Physiol. 1992, 152, 430–440. [Google Scholar] [CrossRef]
- Muramatsu, T. Midkine, a heparin-binding cytokine with multiple roles in development, repair and diseases. Proc. Jpn. Acad. Ser. B 2010, 86, 410–425. [Google Scholar] [CrossRef] [Green Version]
- Zou, P.; Muramatsu, H.; Ichihara-Tanaka, K.; Habuchi, O.; Ohtake, S.; Ikematsu, S.; Sakuma, S.; Muramatsu, T. Glucosaminoglycan structures reqired for strong binding to midkine, a heparin-binding growth factor. Glycobiology 2003, 13, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Najjam, S.; Gibbs, R.V.; Gordon, M.Y.; Rider, C.C. Characterization of human recombinant interleukin 2 binding to heparin and heparin sulfate using an ELISA approach. Cytokine 1997, 9, 1013–1022. [Google Scholar] [CrossRef]
- Mummery, R.S.; Rider, C.C. Characterization of the heparin-binding properties of IL-6. J. Immunol. 2000, 165, 5671–5679. [Google Scholar] [CrossRef] [Green Version]
- Nordsieck, K.; Baumann, L.; Hintze, V.; Pisabarro, M.T.; Schnabelrauch, M.; Beck-Sickinger, A.G.; Samsonov, S.A. The effect of interleukin-8 truncations on its interactions with glycosaminoglycans. Biopolymers 2018, 109, e23103. [Google Scholar] [CrossRef]
- Salek-Ardakani, S.; Arrand, J.R.; Shaw, D.; Mackett, M. Heparin and heparin sulfate bind interleukin-10 and modulate its activity. Blood 2000, 96, 1879–1888. [Google Scholar] [CrossRef]
- Hasan, M.; Najjam, S.; Gordon, M.Y.; Gibbs, R.V.; Rider, C.C. IL-12 is a heparin-binding cytokine. J. Immunol. 1999, 162, 1064–1070. [Google Scholar] [PubMed]
- Jayanthi, S.; Koppolu, B.P.; Nguyen, K.G.; Smith, S.G.; Felber, B.K.; Kumar, T.K.S.; Zaharoff, D.A. Modulation of interleukin-12 activity in the presence of heparin. Sci. Rep. 2017, 7, 5360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maccarana, M.; Lindahl, U. Mode of interaction between platelet factor 4 and heparin. Glycobiology 1993, 3, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Stringer, S.E.; Gallagher, J.T. Specific binding of the chemokine platelet factor 4 to the heparan sulfate. J. Biol. Chem. 1997, 272, 20508–20514. [Google Scholar] [CrossRef] [Green Version]
- Sadir, D.; Forest, E.; Lortat-Jacob, H. The heparin sulfate binding sequence of interferon-γ increased the rate of the interferon-γ-interferon-γ receptor complex formation. J. Biol. Chem. 1998, 273, 10919–10925. [Google Scholar] [CrossRef] [Green Version]
- Sarrazin, S.; Bonnaffe, D.; Lubineau, A.; Lortat-Jacob, H. Heparan sulfate mimicry: A synthetic glycoconjugate that recognizes the heparin binding domain of interferon-gamma inhibits the cytokine activity. J. Biol. Chem. 2005, 280, 37556–37564. [Google Scholar] [CrossRef] [Green Version]
- Modrowski, D.; Lomri, A.; Marie, P.J. Glycosaminoglycans bind granulocyte-macrophage colony-stimulating factor and modulate its mitogenic activity and signaling in human osteoblastic cells. J. Cell. Physiol. 1998, 177, 187–195. [Google Scholar] [CrossRef]
- Sebollela, A.; Cagliari, T.C.; Limaverde, G.S.; Chapeaurouge, A.; Sorgine, M.H.; Ramos, C.H.; Ferreira, S.T. Heparin-binding sites in granulocyte-macrophage colony-stimulating factor. Localization and regulation by histidine ionization. J. Biol. Chem. 2005, 280, 31949–31956. [Google Scholar] [CrossRef] [Green Version]
- Higashiyama, S.; Lau, R.; Bener, G.E.; Abraham, J.A.; Klagsbrun, M. Structure of heparin-binding EGF-like growth factor. J. Biol. Chem. 1992, 267, 6205–6212. [Google Scholar]
- Douglus, M.S.; Ali, S.; Rix, D.A.; Zhang, J.-G.; Kirby, J.A. Endothelial production of MCP-1: Modulation by heparin and consequences for mononuclear cell activation. Immunology 1997, 92, 512–518. [Google Scholar] [CrossRef] [Green Version]
- Lau, E.K.; Paavola, C.D.; Johnson, Z.; Gaudry, J.-P.; Geretti, E.; Borlat, F.; Kung, A.J.; Proudfoot, A.E.; Handel, T.M. Identification of glycosaminoglycan binding site of the CC chemokine, MCP-1. J. Biol. Chem. 2004, 279, 22294–22305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishimoto, S.; Nakamura, S.; Hattori, H.; Nakamura, S.-I.; Oonuma, F.; Kanatani, Y.; Tanaka, Y.; Mori, Y.; Harada, Y.; Tagawa, M.; et al. Human stem cell factor (SCF) is a heparin-binding cytokine. J. Biochem. 2009, 145, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Sally, E.; Nelson, M.S.; Gupta, P. Identification of an MIP-1α-binding heparin sulfate oligosaccharide that support long-term in vitro maintenance of human LTC-ICs. Blood 2003, 101, 2243–2245. [Google Scholar]
- Koopmann, W.; Ediriwickrema, C.; Krangel, M.S. Structure and function of the glycosaminoglycan binding site of chemokine macrophage-inflammatory protein-1β. J. Immunol. 1999, 163, 2120–2127. [Google Scholar] [PubMed]
- Maccarana, M.; Casu, B.; Lindahl, U. Minimal sequence in heparin/heparan sulfate required for binding of basic fibroblast growth factor. J. Biol. Chem. 1993, 268, 23898–23905. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, J.E.; Fernig, D.G.; Ke, Y.; Wilkinson, M.C.; Gallagher, J.T. Identification of the basic fibroblast growth factor binding sequence in fibroblast heparan sulfate. J. Biol. Chem. 1992, 267, 10337–10341. [Google Scholar]
- Yu, H.; Munoz, E.M.; Edens, R.E.; Linhardt, R.J. Kinetic studies on thwe interactions of heparin and complement proteins using surface plasmon resonance. Biochim. Biophys. Acta 2005, 1726, 168–176. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, F.S.; Yang, S.K.; Lin, J.M.; Chen, Y.W.; Chen, C.S. Protein interacome analysis of iduronic acide-containing glycosaminoglycans reveals a novel flagellar invasion factor MbhA. J. Proteomics. 2019, 208, 103485. [Google Scholar] [CrossRef]
- Casu, B.; Petitou, M.; Provasoli, M.; Sinay, P. Conformational flexibility: A new concept for explaining binding and biological properties of iduronic acid-containing glycosaminoglycans. Trends Biochem. Sci. 1998, 13, 221–225. [Google Scholar] [CrossRef]
- Gunay, N.S.; Linhardt, R.J. Heparinoids: Structure, biological activities and therapeutic applications. Planta Med. 1999, 65, 301–306. [Google Scholar] [CrossRef] [Green Version]
- Casu, B.; Naggi, A.; Torri, G. Heparin-derived heparin sulfate mimics to modulate heparin sulfate-protein interaction in inflammation and cancer. Matrix Biol. 2010, 29, 442–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrini, L.; Burke, D.F.; von Delft, F.; Mulloy, B.; Blundell, T.L. Crystal structure of fibroblast growth factor receptor ectodomain bound to ligand and heparin. Nature 2000, 407, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahi, E.; Yamaguchi, Y. Proteoglycans as modulators of growth factor activities. Cell 1991, 64, 867–869. [Google Scholar] [CrossRef]
- Nader, H.B.; Dietrich, C.P.; Buonassisi, V.; Colburn, P. Heparin sequences in the heparin sulfate chains of an endothelial cell proteoglycan. Proc. Natl. Acad. Sci. USA 1987, 84, 3565–3569. [Google Scholar] [CrossRef] [Green Version]
- Park, P.W.; Reizes, O.; Bernfield, M. Cell surface heparin sulfate proteoglycans: Selective regulators of ligand-receptor encounters. J. Biol. Chem. 2000, 275, 29923–29926. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, J. Fell-Muir Lecture: Haparan sulphate and the art of cell regulator: A polymer chain conducts the protein orchestra. Int. J. Exp. Pathol. 2015, 96, 203–231. [Google Scholar] [CrossRef] [Green Version]
- Hirsh, J.; Warkentin, T.E.; Shaughnessy, S.G.; Anand, S.S.; Halperin, J.L.; Raschke, R. Heparin and low-molecular heparin, mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest 2001, 119, 64–94. [Google Scholar] [CrossRef] [Green Version]
- Fransson, L.A. Periodate oxidation of d-glucuronic acid residues in heparan sulfate and heparin. Carbohydr. Res. 1978, 62, 235–244. [Google Scholar] [CrossRef]
- Fransson, L.A.; Carlstedt, I. Alkaline and Smith degradation of oxidized dermatan sulfate-chondroitin sulfate copolymers. Carbohydr. Res. 1974, 36, 349–358. [Google Scholar] [CrossRef]
- Lin, C.C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef]
- Wang, S.C.; Chen, B.H.; Wang, L.F.; Chen, J.S. Characterization of chondroitin sulfate and its interpenetrating polymer network hydrogels for sustaining-drug release. Int. J. Pharmcol. 2007, 329, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.H.; Gao, J.; Chen, C.W.; Huard, J.; Wang, Y.D. Injectable fibroblast growth factor-2 coacervate for persistent angiogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 13444–13449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, M.; Ishihara, M.; Shimizu, M.; Obara, K.; Ishizuka, T.; Saito, Y.; Yura, H.; Morimoto, Y.; Takase, B.; Matsui, T.; et al. Vascularization in vivo caused by the controlled release of fibroblast growth factor-2 from an injectable chitosan/non-anticoagulant heparin hydrogel. Biomaterials 2004, 25, 699–706. [Google Scholar] [CrossRef]
- Fujita, M.; Ishihara, M.; Shimizu, M.; Obara, K.; Nakamura, S.; Kanatani, Y.; Morimoto, Y.; Takase, B.; Matui, T.; Kikuchi, M.; et al. Therapeutic angiogenesis induced by controlled release of fibroblast growth factor-2 from injectable chitosan/non-anticoagulant heparin hydrogel in rat hind limb ischemia model. Wound Repair Regen. 2007, 15, 58–65. [Google Scholar] [CrossRef]
- Nakamura, S.; Ishihara, M.; Obara, K.; Masuoka, K.; Ishizuka, T.; Kanatani, Y.; Takase, B.; Matsui, T.; Hattori, H.; Sato, T.; et al. Controlled release of fibroblast growth factor-2 from injectable 6-O-desulfated heparin hydrogel and subsequent effect on in vivo vascularization. J. Biomed. Mater. Res. A 2006, 78, 364–371. [Google Scholar] [CrossRef]
- Nakamura, S.; Nambu, M.; Ishizuka, T.; Hattori, H.; Kanatani, Y.; Kishimoto, S.; Amano, Y.; Aoki, H.; Kiyosawa, T.; Ishihara, M.; et al. Effect of fibroblast growth factor-2 from chitosan/fucoidan micro complex hydrogel on in vitro and in vivo neovascularization. J. Biomed. Mater. Res. A 2008, 85, 619–627. [Google Scholar] [CrossRef]
- Rele, S.M.; Cui, W.; Wang, L.; Hou, S.; Barr-Zarse, G.; Tatton, D.; Gnanou, Y.; Esko, J.D.; Chaikof, E.L. Dendrimer-like PEO glycopolymer exihibit anti-infmammatory properties. J. Am. Chem. Soc. 2005, 127, 10132–10133. [Google Scholar] [CrossRef] [Green Version]
- Paluck, S.J.; Nguyen, T.H.; Maynard, H.D. heparin-mimicking polymers: Synthesis and biological applications. Biomacromolecules 2016, 17, 3417–3440. [Google Scholar] [CrossRef]
- Ishihara, M.; Saito, Y.; Yura, H.; Ono, K.; Ishikawa, K.; Hattori, H.; Akaike, T.; Kurita, A. Heparin-carrying polystyrene to mediate cellular attachment and growth via interaction with growth factors. J. Biomed. Mater. Res. A 2000, 50, 144–152. [Google Scholar] [CrossRef]
- Ishihara, M.; Ono, K.; Ishikawa, K.; Hattori, H.; Saito, Y.; Yura, H.; Akaike, T.; Ozeki, Y.; Tanaka, S.; Mochizuki, H.; et al. Enhanced ability of heparin-carrying polystyrene (HCPS) to inhibit growth factor-induced endothelial cell growth. J. Biochem. 2000, 127, 797–803. [Google Scholar] [CrossRef]
- Ono, K.; Ishihara, M.; Ishikawa, K.; Ozeki, Y.; Deguchi, H.; Sato, M.; Hashimoto, H.; Saitoh, Y.; Yura, H.; Kurita, A.; et al. Periodate-treated, non-anticoagulant heparin carrying polystyrene (NAC-HCPS) affects angiogenesis and inhibits subcutaneous induced tumor growth and metastasis to the lung. Br. J. Cancer 2002, 86, 1803–1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, M.; Ishihara, M.; Ono, K.; Matsumura, K.; Saito, Y.; Yura, H.; Morimoto, Y.; Shimizu, M.; Takase, B.; Ozeki, S.; et al. Inhibition of neointimal proliferation in balloon-injured arteries using by non-anticoagulant heparin-carrying polystyrene (NAC-HCPS). J. Cardiovasc. Pharmacol. 2004, 43, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Sumitomo, H.; Ina, A. Synthesis and functions of polystylene derivatives having pendant oligosaccharides. Polym. J. 1985, 17, 567–575. [Google Scholar] [CrossRef]
- Hattori, H.; Nogami, Y.; Tanaka, T.; Amano, Y.; Fukuda, K.; Kishimoto, S.; Kanatani, Y.; Nakamura, S.; Takase, B.; Ishihara, M. Expansion and characterization of adipose tissue-derived stromal cells cultured with low serum medium. J. Biomed. Mater. Res. B 2008, 87, 229–236. [Google Scholar] [CrossRef]
- Ishihara, M.; Sato, M.; Hattori, H.; Saito, Y.; Yura, H.; Ono, K.; Masuoka, K.; Kikuchi, M.; Fujikawa, K.; Kurita, A. Heparin-carrying polystyrene (HCPS)-bound collegen substratum to immobilize heparin-binding growth factors and to enhance cellular growth. J. Biomed. Mater. Res. 2001, 56, 536–544. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Vanjari, Y.H.; Sancheti, K.H.; Patel, H.M.; Belgamwar, V.S.; Surana, S.J.; Pardeshi, C.V. Polyelectrolyte complexes: Mechanisms, critical experimental aspects, and applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1615–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Ishihara, M.; Kishimoto, S.; Nakamura, S.; Sato, Y.; Hattori, H. Polyelectrocyte complexes of natural polymers and their biomedical applications. Polymers 2019, 4, 672. [Google Scholar] [CrossRef] [Green Version]
- Khurshid, H.; Kim, S.H.; Bonder, M.J.; Colak, L.; Ali, B.; Shah, S.I.; Kiick, K.L.; Hadjipanayis, G.C. Development of heparin-coated magnetic nanoparticles for targeted drug delivery applications. J. Appl. Phys. 2009, 105, 07B308/1–07B308/3. [Google Scholar] [CrossRef]
- Huang, H.; Yang, X. Synthesis of polysaccharide-stabilized gold and silver nanoparticles: A green method. Carbohydr. Res. 2004, 339, 2627–2631. [Google Scholar] [CrossRef]
- Chauvierre, C.; Marden, M.C.; Vauthier, C.; Labarre, D.; Couvreur, P.; Leclerc, L. Heparin-coated poly (alkylcyanoacrylate) nanoparticles coupled to hemoglobin: A new oxygen carrier. Biomaterials 2004, 25, 3081–3086. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Lee, G.Y.; Kim, Y.S.; Yu, M.; Park, R.W.; Kim, I.S.; Kim, S.Y.; Byun, Y. Heparin-deoxycholic acid chemical conjugate as an anticancer drug carrier and its antitumor activity. J. Control. Release 2006, 114, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.; Albadawi, H.; Watkins, M.T.; Edelman, E.R.; Baker, A.B. Syndecan-4 proteoliposomes enhance fibroblast growth factor-2 (FGF-2)-induced proliferation, migration, and neovascularization of ischemic muscle. Proc. Natl. Acad. Sci. USA 2012, 109, 1679–1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagiwara, K.; Kishimoto, S.; Ishihara, M.; Koyama, Y.; Mazda, O.; Sato, T. In vivo gene transfer using pDNA/chitosan/chondroitin sulfate ternary complexes: Influence of chondroitin sulfate on the stability of freeze-dried complexes and transfer gene expression in vivo. J. Gene Med. 2013, 15, 83–92. [Google Scholar] [CrossRef]
- Houska, M.; Brynda, E.; Bahata, K. The effect of polyelectrolyte chain length on layer-by layer protein/polyelectrolyte assembly—An experimental study. J. Colloid Interface Sci. 2004, 273, 140–147. [Google Scholar] [CrossRef]
- Seyrek, E.; Dubin, P. Glycosaminoglycans as polyelectrolytes. Adv. Colloid Interface 2010, 158, 119–129. [Google Scholar] [CrossRef]
- Wolzt, M.; Wetermann, A.; Nieszpaur-Los, M.; Schneider, B.; Fassolt, A.; Lechner, K.; Kyrle, P.A. Studies on the neutralizing effects of protamine on unfractionated and low molecular weight heparin (Fragmin®) at the site of activation of the coagulation system in man. Thromb. Haemost. 1995, 73, 439–444. [Google Scholar] [CrossRef]
- Pan, M.; Lezo, J.S.; Medina, A.; Romero, M.; Hernandez, E.; Segura, J.; Melian, F.; Wanguemert, F.; Landin, M.; Benitez, F.; et al. In-laboratory removal of femoral sheath following protamine administration in patients having intracoronary stent implantation. Am. J. Cardiol. 1997, 80, 1336–1338. [Google Scholar] [CrossRef]
- Mori, Y.; Nakamura, S.; Kishimoto, S.; Kawakami, M.; Suzuki, S.; Matsui, T.; Ishihara, M. Preparation and characterization of low-molecular-weight heparin/protamine nanoparticles (LMW-H/P NPs) as FGF-2 carrier. Int. J. Nanomed. 2010, 5, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, S.; Kanatani, Y.; Kishimoto, S.; Nambu, M.; Ohno, C.; Hattori, H.; Fujita, M.; Hattori, H.; Tanaka, Y.; Kiyosawa, T.; et al. Controlled release of FGF-2 using fragmin/protamine microparticles and effect on neovascularization. J. Biomed. Mater. Res. A 2009, 91, 814–823. [Google Scholar] [CrossRef]
- Kishimoto, S.; Ishihara, M.; Nakamura, S.; Takikawa, M.; Fujita, M.; Sumi, Y.; Kiyosawa, T.; Sato, T.; Kanatani, Y. Fragmin/protamine microparticles to absorb and protect HGF and to function as local HGF carrier in vivo. Acta Biomaterilia 2013, 9, 4763–4770. [Google Scholar] [CrossRef] [PubMed]
- Nemeno, J.G.E.; Lee, S.; Yang, W.; Lee, K.M.; Lee, J.I.K. Applications and implications of heparin and protamine in tissue engineering and regenerative medicine. Biomed. Res. Int. 2014, 2014, 10. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, M.; Nakamura, S.-I.; Nakamura, S.; Nambu, M.; Ishihara, M.; Fujita, M.; Kishimoto, S.; Doumoto, T.; Yanagibayashi, S.; Azuma, R.; et al. Enhancement of vascularization and granulation tissue formation by growth factors in human platelet-rich plasma-containing fragmin/protamine microparticles. J. Biomed. Mater. Res. B 2011, 97, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Horio, T.; Fujita, M.; Tanaka, Y.; Ishihara, M.; Kishimoto, S.; Nakamura, S.; Hase, K.; Maehara, T. Efficacy of fragmin/protamine microparticles containing fibroblast growth factor-2 (F/P MPsFGF-2) to induce collateral vessels in a rabbit model of hindlimb ischemia. J. Vasc. Surg. 2011, 54, 791–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, S.; Ishihara, M.; Takikawa, M.; Kishimoto, S.; Isoda, S.; Fujita, M.; Sato, M.; Maehara, T. Attenuation of limb loss in an experimentally induced hindlimb ischemic model by fibroblast growth factor-2/fragmin/protamine microparticles as a delivery system. Tissue Eng. Part A 2012, 18, 2239–2247. [Google Scholar] [CrossRef] [Green Version]
- Fujita, M.; Horio, T.; Kishimoto, S.; Nakamura, S.; Takikawa, M.; Nakayama, T.; Yamamoto, Y.; Shimizu, M.; Hattori, H.; Tachibana, S.; et al. Effects of platelet-rich plasma-containing fragmin/protamine microparticles in enhancing endothelial and smooth muscle cell growth and inducing collateral vessels in a rabbit model of hindlimb ischemia. J. Biomed. Mater. Res. B. 2012, 101, 36–42. [Google Scholar] [CrossRef]
- Takabayashi, Y.; Mambu, M.; Ishihara, M.; Kuwabara, M.; Fukuda, K.; Nakamura, S.; Hattori, H.; Kiyosawa, T. Enhanced effect of fibroblast growth factor-2-containing dalteparin/protamine nanoparticles on hair growth. Clin. Cosmet. Investig. Dermatol. 2016, 9, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Takikawa, M.; Nakamura, S.-I.; Nakamura, S.; Nambu, M.; Ishihara, M.; Murakami, K.; Kishimoto, S.; Sasaki, K.; Yanagishita, S.; Azuma, R.; et al. Enhanced effect of platelet-rich plasma containing a new carrier on hair growth. Dermatol. Surg. 2011, 37, 1–9. [Google Scholar] [CrossRef]
- Takikawa, M.; Ishihara, M.; Takabayashi, Y.; Sumi, Y.; Takikawa, M.; Yoshida, R.; Nakamura, S.; Hattori, H.; Yanagibayashi, S.; Yamamoto, N.; et al. Enhanced healing of mitomycin C-treated healing-impaired wounds in rats with PRP-containing fragmin/protamine microparticles (PRP&F/P MPs). J. Plast. Surg. Hand Surg. 2015, 49, 268–274. [Google Scholar]
- Kinoda, J.; Ishihara, M.; Nakamura, S.; Fujita, M.; Fukuda, K.; Sato, Y.; Yokoe, H. Protective effect of FGF-2 and low-molecular-weight heparin/protamine nanoparticles on radiation-induced healing-impaired wound repair in rats. J. Radiat. Res. 2018, 59, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Takikawa, M.; Ishihara, M.; Kishimoto, S.; Nakamura, S.; Yanagibayashi, S.; Hattori, H.; Azuma, R.; Yamamoto, N.; Kiyosawa, T. PRP&F/P MPs improved survival of dorsal paired pedicle skin flaps in rats. J. Surg. Res. 2011, 170, 189–196. [Google Scholar]
- Takabayashi, Y.; Ishihara, M.; Sumi, Y.; Takikawa, M.; Nakamura, S.; Kiyosawa, T. Platelet-rich plasma-containing fragmin-protamine micro-nanoparticles promote epithelialization and angiogenesis in split-thickness skin graft donor sites. J. Surg. Res. 2015, 193, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, M.; Nakamura, S.; Ishihara, M.; Takabayashi, Y.; Fujita, M.; Hattori, H.; Kushibiki, T.; Ishihara, M. Improved angiogenesis and healing in crush syndrome by fibroblast growth factor-2-containing low-molecular-weight heparin (Fragmin)/protamine nanoparticles. J. Surg. Res. 2015, 196, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-I.K.; Edelman, E.R. Structural biomechanics modulate intramuscular distribution of locally delivery drug. J. Biomech. 2008, 41, 2884–2891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumano, I.; Kishimoto, S.; Nakamura, S.; Hattori, H.; Tanaka, Y.; Nakata, M.; Sato, T.; Fujita, M.; Maehara, T.; Ishihara, M. Fragmin/protamine microparticles (F/P MPs) as cell carriers enhance the formation and growth of tumors in vivo. Cell. Mol. Bioeng. 2011, 4, 476–483. [Google Scholar] [CrossRef]
- Volpe, J.P.; Milasm, L. Influence of tumor transplantation methods on tumor growth rate and metastatic potential of solitary tumors derived metastasis. Clin. Exp. Metasitasis 1990, 8, 381–389. [Google Scholar] [CrossRef]
- Nakamura, S.; Kishimoto, S.; Nakamura, S.I.; Nambu, M.; Fujita, M.; Tanaka, Y.; Mori, Y.; Tagawa, M.; Maehara, T.; Ishihara, M. Fragmin/protamine microparticles as cell carriers to enhance viability of adipose-derived stromal cells and their subsequent effect on in vivo neovascularization. J. Biomed. Mater. Res. A 2010, 92, 1614–1622. [Google Scholar] [CrossRef]
- Kishimoto, S.; Ishihara, M.; Mori, Y.; Takikawa, M.; Hattori, H.; Nakamura, S.; Sato, T. Effective expansion of human adipose-derived stromal cells and bone marrow-derived mesenchymal stem cells cultured on a fragmin/protamine nanoparticles-coated substratum with human platelet-rich plasma. J. Tissue Eng. Regen. Med. 2013, 7, 955–964. [Google Scholar] [CrossRef]
- Kishimoto, S.; Nakamura, S.; Nakamura, S.I.; Kanatani, Y.; Hattori, H.; Tanaka, Y.; Harada, M.; Tagawa, M.; Mori, Y.; Maehara, T.; et al. Fragmin/protamine microparticle-coated matrix immobilized cytokines to stimulate various cell proliferations with low serum media. Artif. Organs 2009, 33, 431–438. [Google Scholar] [CrossRef]
- Kishimoto, S.; Nakamura, S.; Nakamura, S.I.; Hattori, H.; Oomuma, F.; Kanatani, Y.; Tanaka, Y.; Harada, Y.; Tagawa, M.; Maehara, T.; et al. Cytokine-immobilized microparticle-coated plates for culturing hematopoietic progenitor cells. J. Control. Release 2009, 133, 185–190. [Google Scholar] [CrossRef]
- Kishimoto, S.; Ishihara, M.; Takikawa, M.; Takikawa, M.; Sumi, Y.; Nakamura, S.; Fujita, M.; Sato, T.; Kiyosawa, T. Three-dimensional culture using human plasma-medium gel with fragmin/protamine microparticles for proliferation of various human cells. Cytotechnology 2014, 66, 791–802. [Google Scholar] [CrossRef] [Green Version]
- Kishimoto, S.; Ishihara, M.; Mori, Y.; Takikawa, M.; Sumi, Y.; Nakamura, S.; Sato, T.; Kiyosawa, T. Three-dimensional expansion using plasma-medium gel with fragmin/protamine nanoparticles and FGF-2 to stimulate adipose-derived stromal cells and bone marrow-derived mesenchymal stem cells. BioRes. Open Access 2012, 1, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Rokstad, A.M.; Donati, I.; Borgogna, M.; Oberholzer, J.; Strand, B.L.; Espevikm, T.; Skjak-Braek, G. Cell-compatible covalently reinforced beads obtained from a chemoenzymatically engineered alginate. Biomaterials 2006, 27, 4726–4737. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Zhao, J.; Chen, Y.M.; Zhang, P.; Zhang, Q. Self-healing polysaccharide-based hydrogels as injectable carriers for neural stem cells. Sci. Rep. 2016, 6, 37841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumi, Y.; Ishihara, M.; Kishimoto, S.; Takikawa, M.; Doumoto, T.; Azuma, R.; Nakamura, S.; Hattori, H.; Fujita, M.; Kiyosawa, T. Transplantation of inbred adipose-derived stromal cells in rats with plasma gel containing fragmin/protamine microparticles and FGF-2. J. Biomed. Mater. Res. B 2013, 101, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Sumi, Y.; Ishihara, M.; Kishimoto, S.; Takikawa, M.; Hattori, H.; Takikawa, M.; Doumoto, T.; Azuma, R.; Nakamura, S.; Fujita, M.; et al. Effective wound healing in streptozotocin-induced diabetic rats by adipose-derived stromal cell-transplantation in plasma-gel containing fragmin/protamine microparticles. Ann. Plast. Surg. 2014, 72, 113–120. [Google Scholar] [CrossRef]
- Baker, A.B.; Gibson, W.J.; Kolachalama, V.B.; Golomb, M.; Indolfi, L.; Spruell, C.; Zcharia, E.; Vlodavsky, I.; Edelman, E.R. Heparanase regulates thrombosis in vascular injury and stent induced flow disturbance. J. Am. Coll. Cardiol. 2012, 59, 1551–1560. [Google Scholar] [CrossRef] [Green Version]
- Biran, R.; Pond, D. Heparin coatings for improving blood compatibility of medical devices. Adv. Drug Deliv. Rev. 2017, 112, 12–23. [Google Scholar] [CrossRef]
- Wendel, H.P.; Ziemer, G. Coating-techniques to improve the hemocompatibility of artificial devices used for extracorponeal circulation. Eur. J. Cardio Thorac. Surg. 1999, 16, 342–352. [Google Scholar] [CrossRef] [Green Version]
- Tanzi, M.C. Bioactive technologies for hemocompatibility. Expert Rev. Med. Devices 2005, 2, 473–492. [Google Scholar] [CrossRef]
- Murugesan, S.; Xie, J.; Linhardt, R.J. Immobilization of heparin: Approaches and applications. Curr. Top. Med. Chem. 2008, 8, 80–100. [Google Scholar] [PubMed]
- Hwang, C.W.; Wu, D.; Edelman, E.R. Physiological transport forces govern drug distribution for stent-based delivery. Circulation 2001, 104, 600–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obara, K.; Ishihara, M.; Ozeki, Y.; Ishizuka, T.; Hayashi, T.; Nakamura, S.; Saito, Y.; Yura, H.; Matsui, T.; Hattori, H.; et al. Controlled release of paclitaxel from photocrosslinked chitosan hydrogels and its subsequent effect on subcutaneous tumar growth in mice. J. Control. Release 2005, 110, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Gore, S.; Andersson, J.; Biran, R.; Underwood, C.; Riesenfeld, J. Heparin surfaces: Impact of immobilization chemistry on hemocompatibility and protein adsorption. J. Biomed. Mater. Res. B 2014, 102, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Butler, M.; Sikkander, S.A.; Toida, T.; Linhardt, R.J. Further evidence that periodate cleavage of heparin occurs primarily through the antithrombin binding site. Carbohydr. Res. 2002, 337, 2239–2243. [Google Scholar] [CrossRef]
| Full Name (Family) | Abbreviations | Functions | References |
|---|---|---|---|
| Fibroblast growth factor family | FGF-1 FGF-2 FGF-4 | Potential effects in the repair and regeneration of tissues and in development. | [20,70,71,72] [20,70,71,72] [20,73] |
| Platelet-derived growth factor | PDGF-A PDGF-BB | Blood vessel formation, mitogenesis, and proliferation of mesenchymal cells. | [74] [75] |
| Hepatocyte growth factor | HGF | Cell growth, cell motility, and morphogenesis by activating a tyrosine kinase. | [76,77,78] |
| Vascular endothelial growth factor | VEGF | Angiogenesis, bone formation, hematopoiesis, wound healing, and development. | [79,80,81] |
| Transforming growth factor-β family | TGF-β1 TG F-β2 | Cell growth, development, homeostasis, and regulation of the immune system. | [82,83,84] [82,83] |
| Midkines | MK | Development, reproduction, and repair, and in the pathogenesis of inflammatory diseases. | [85,86] |
| Interleukin family | IL-2, IL-6 IL-8, IL-10 IL-12 | Development and differentiation of T and B lymphocytes, and hematopoietic cells. | [87,88] [89,90] [91,92] |
| Platelet factor-4 | PF-4 | Chemoattractant for neutrophils and fibroblasts, a role in inflammation and repair. | [93,94] |
| Interferon-γ | IFN-γ | Antiviral, immunoregulatory, and anti-tumor properties. | [95,96] |
| Granulocyte/macrophage-colony stimulating factor | GM-CSF | Stimulation of stem cells to produce granulocytes and monocytes. | [97,98] |
| Heparin-binding epidermal growth factor | HB-EGF | Wound healing, cardiac hypertrophy, and heart development. | [99] |
| Monocyte chemotactic protein-1 | MCP-1 | Promotion of recruitment of monocytes and macrophages. | [100,101] |
| Stem cell factor | SCF | Hematopoiesis, supermagenesis, and melanogenesis. | [102] |
| Macrophage-inflammatory protein-1 | MIP-1α MIP-1β | Activation of granulocytes, which can lead to acute neutrophilic inflammation. | [103] [104] |
| Applications | Overview | References |
|---|---|---|
| Injection of NAC-heparin/CH-LA | Induction of angiogenesis and collateral circulation by subcutaneous injection of FGF-2 containing NAC-heparin/chitosan–lactose (CH-LA) | [123,124] |
| Inhibition of angiogenesis and tumor metastasis in vivo | NAC-HCPS inhibited angiogenesis and subcutaneous induced tumor growth and metastasis in vivo | [131] |
| Inhibition of neointimal proliferation of balloon-injured arteries | NAC-HCPS inhibited smooth muscle cell growth in vitro and neointimal proliferation of balloon-injured arteries in vivo | [132] |
| Substratum for cell cultures | NAC-HCPS is efficiently adsorbed onto plastic surfaces such as those of tissue culture plates, and heparin-binding cytokines are immobilized on the surface of NAC-HCPS-coated plates | [134,135] |
| Applications | Overview | References |
|---|---|---|
| Carrier for FGF-2, HGF, and cytokines from platelet-rich plasma | Adsorption, stabilization, controlled release, and activation of FGF-2, HGF, and cytokines from platelet-rich plasma (PRP). | [149,150] (FGF-2) [151] (HGF) [152] (Cytokines from PRP) |
| Neovascularization | Induction of collateral blood vessel formation in rabbit by FGF-2, HGF, and cytokines from PRP-containing LMWH/P N/MPs. | [150,154,155] (FGF-2) [151] (HGF) [153,156] (Cytokines from PRP) |
| Hair regrowth | Enhancement of human hair growth by FGF-2 and cytokines from PRP-containing LMWH/P N/MPs. | [157] (FGF-2) [158] (Cytokines from PRP) |
| Injection of cytokines from PRP into skin for healing-impaired wound | Enhancement of mitomycin C-treated healing-impaired wound by cytokines from PRP-containing LMWH/P N/MPs. | [159] |
| Injection of cytokines from PRP into skin for healing-impaired wound | Enhancement of radiation-induced healing-impaired wound repair by FGF-2-containing LMWH/P N/MPs. | [160] |
| Injection of cytokines from PRP into skin for skin flap necrosis | Prevention of skin flap necrosis by topical injection of cytokines from PRP-containing LMWH/P N/MPs. | [161] |
| Injection of cytokines from PRP into skin for split-thickness skin graft donor sites | Promotion of epithelialization and angiogenesis in split-thickness skin graft donor sites by pre-injection of cytokines from PRP-containing LMWH/P N/MPs. | [162] |
| Injection of FGF-2 into skin for wounds in crush syndrome | Promotion of survival and healing of wounds in crush syndrome model of rat by injection of FGF-2-containing LMWH/P N/MPs. | [163] |
| Applications | Overview | References |
|---|---|---|
| Formation of cell aggregates | Formation of cell aggregates by the interaction of cells with LMWH/P N/MPs and increase of cellular viability. | [165] (Tumor cells) [167] (ADSCs) |
| 2D expansion of cells | The ability of LMWH/P N/MPs to retain heparin-binding cytokines. Various cells two-dimensionally expand on those cytokine-coated plates. | [167,168] (ADSCs and BMSCs) [169] (Adhesive cells) [170] (Hematopoietic pro- genitor cells) |
| 3D expansion of cells | Various cells can also be grown efficiently in three-dimensional (3D) culture using low human plasma-DMEM gel containing LMWH/P N/MPs. | [171] (Adhesion cells) [172] (ADSCs and BMSCs) |
| Transplantation of ADSCs | Transplantation of 3D-cultured IR-ADSCs derived from inbred rats using injectable low IRP (3%)-DMEM gel with LMWH/P N/MPs. | [175] |
| Transplantation of 3D-cultured IR-ADSCs derived from inbred rats using injectable IR-ADSCs using IRP (6%)-DMEM gel with LMWH/P N/MPs/FGF-2. | [176] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishihara, M.; Nakamura, S.; Sato, Y.; Takayama, T.; Fukuda, K.; Fujita, M.; Murakami, K.; Yokoe, H. Heparinoid Complex-Based Heparin-Binding Cytokines and Cell Delivery Carriers. Molecules 2019, 24, 4630. https://doi.org/10.3390/molecules24244630
Ishihara M, Nakamura S, Sato Y, Takayama T, Fukuda K, Fujita M, Murakami K, Yokoe H. Heparinoid Complex-Based Heparin-Binding Cytokines and Cell Delivery Carriers. Molecules. 2019; 24(24):4630. https://doi.org/10.3390/molecules24244630
Chicago/Turabian StyleIshihara, Masayuki, Shingo Nakamura, Yoko Sato, Tomohiro Takayama, Koichi Fukuda, Masanori Fujita, Kaoru Murakami, and Hidetaka Yokoe. 2019. "Heparinoid Complex-Based Heparin-Binding Cytokines and Cell Delivery Carriers" Molecules 24, no. 24: 4630. https://doi.org/10.3390/molecules24244630






