Convergent Synthesis of Polysubstituted Furans via Catalytic Phosphine Mediated Multicomponent Reactions
Abstract
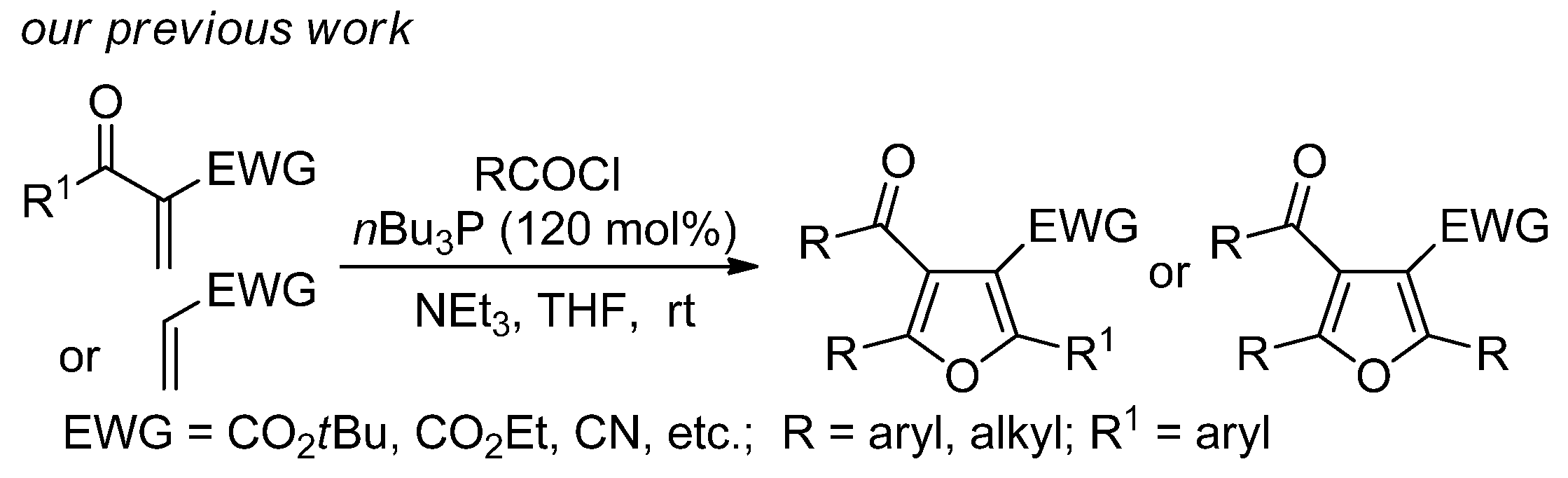
:1. Introduction
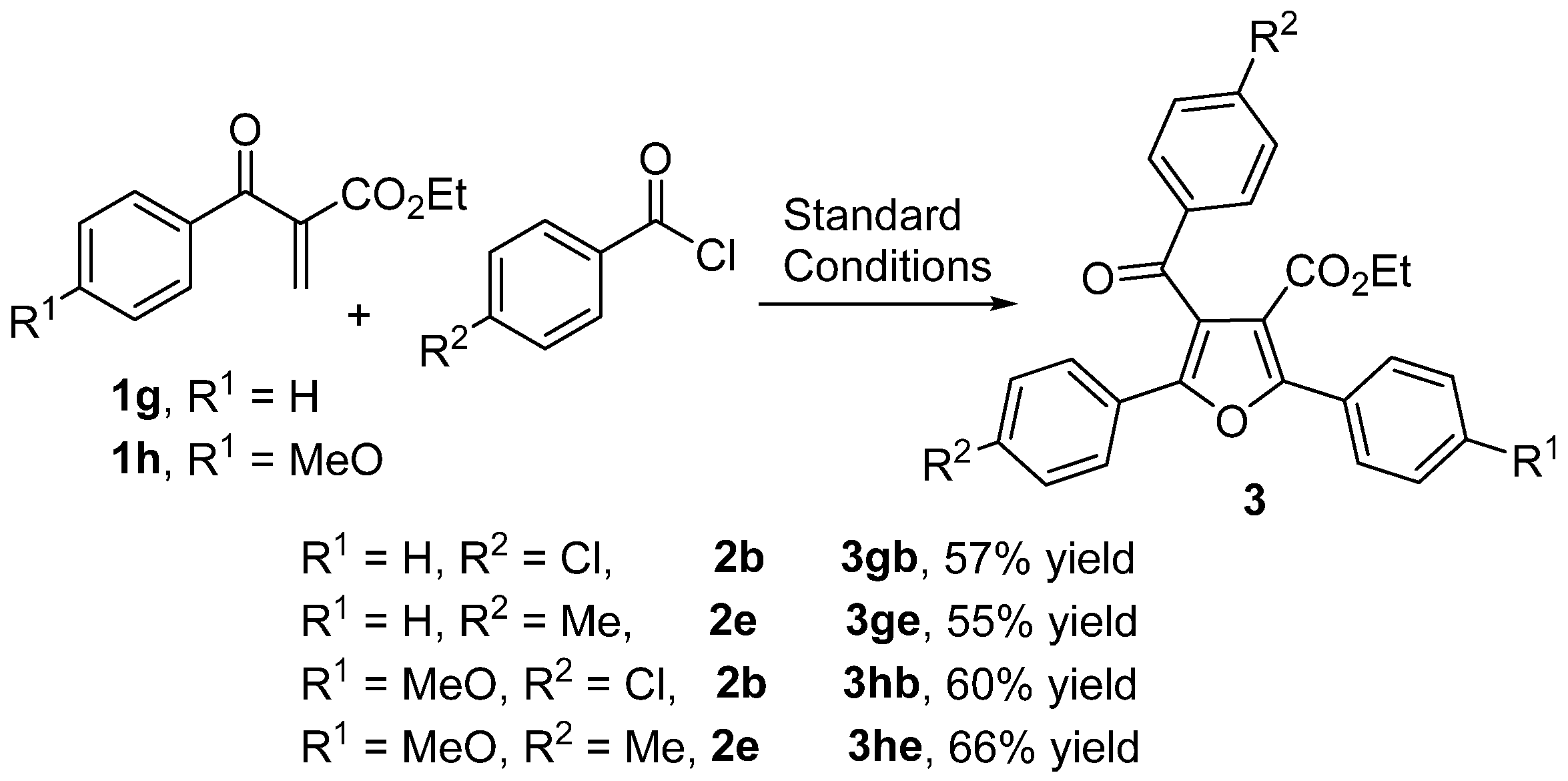
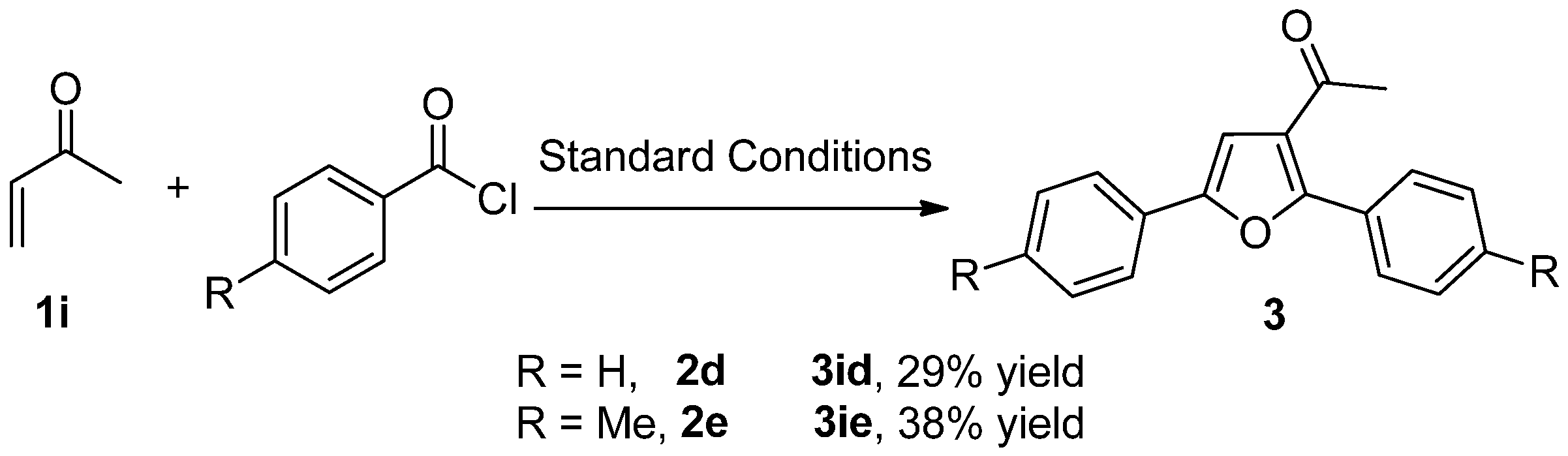
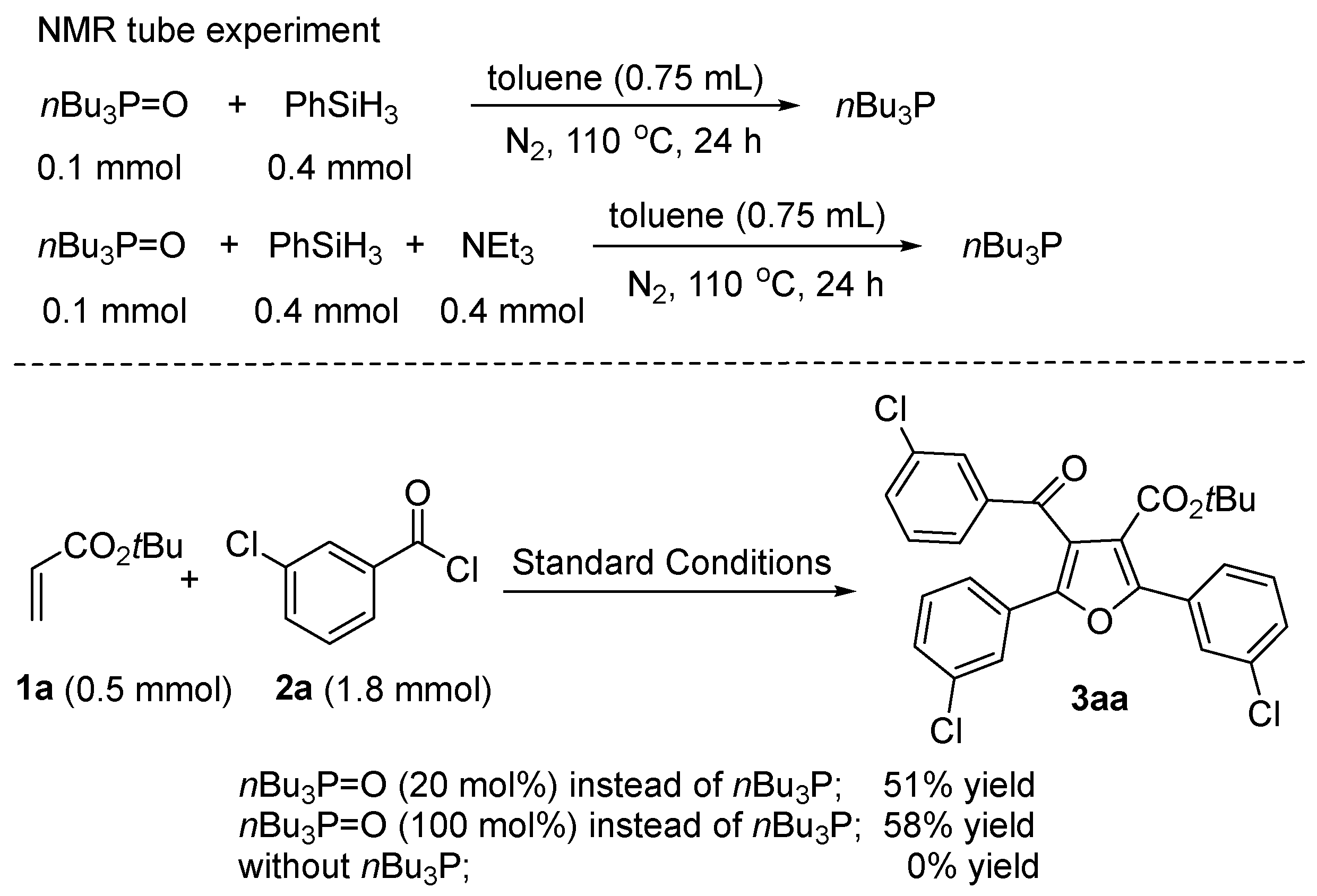
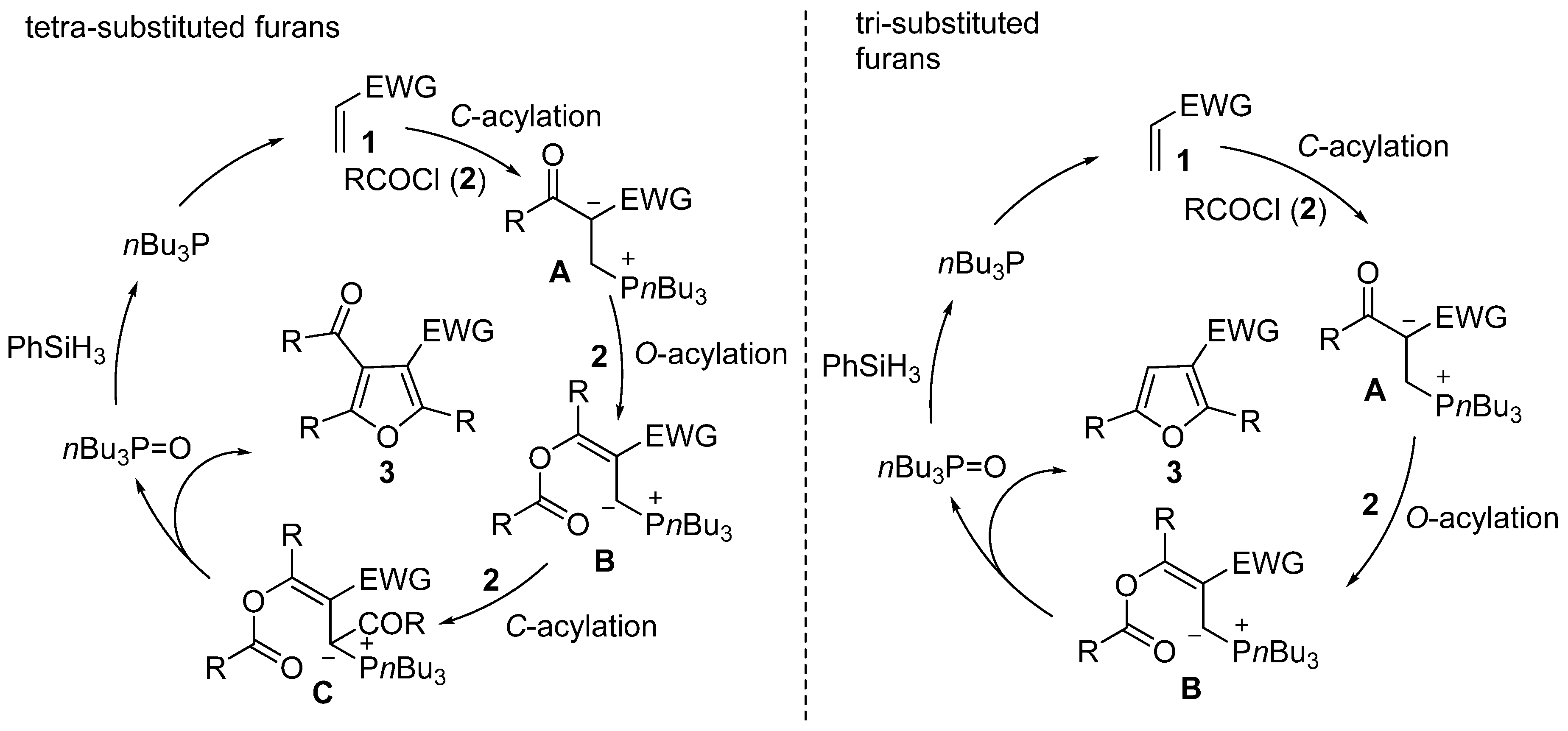
2. Results and Discussion
3. Experimental Section
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Byrne, P.A.; Gilheany, D.G. The modern interpretation of the Wittig reaction mechanism. Chem. Soc. Rev. 2013, 42, 6670–6696. [Google Scholar] [CrossRef] [Green Version]
- Chelucci, G. Synthesis and Metal-Catalyzed Reactions of gem-Dihalovinyl Systems. Chem. Rev. 2012, 112, 1344–1462. [Google Scholar] [CrossRef]
- Perin, G.; Lenardão, E.J.; Jacob, R.G.; Panatieri, R.B. Synthesis of Vinyl Selenides. Chem. Rev. 2009, 109, 1277–1301. [Google Scholar] [CrossRef]
- Flynn, A.B.; Ogilvie, W.W. Stereocontrolled Synthesis of Tetrasubstituted Olefins. Chem. Rev. 2007, 107, 4698–4745. [Google Scholar] [CrossRef]
- Batesky, D.C.; Goldfogel, M.J.; Weix, D.J. Removal of Triphenylphosphine Oxide by Precipitation with Zinc Chloride in Polar Solvents. J. Org. Chem. 2017, 82, 9931–9936. [Google Scholar] [CrossRef] [Green Version]
- Bergbreiter, D.E.; Yang, Y.-C.; Hobbs, C.E. Polyisobutylene-Supported Phosphines as Recyclable and Regenerable Catalysts and Reagents. J. Org. Chem. 2011, 76, 6912–6917. [Google Scholar] [CrossRef]
- Constable, D.J.C.; Dunn, P.J.; Hayler, J.D.; Humphrey, G.R.; Leazer, J.J.L.; Linderman, R.J.; Lorenz, K.; Manley, J.; Pearlman, B.A.; Wells, A.; et al. Key green chemistry research areas-a perspective from pharmaceutical manufacturers. Green Chem. 2007, 9, 411–420. [Google Scholar] [CrossRef]
- Dandapani, S.; Curran, D.P. Separation-Friendly Mitsunobu Reactions: A Microcosm of Recent Developments in Separation Strategies. Chem. Eur. J. 2004, 10, 3130–3138. [Google Scholar] [CrossRef]
- Dutartre, M.; Bayardon, J.; Juge, S. Applications and stereoselective syntheses of P-chirogenic phosphorus compounds. Chem. Soc. Rev. 2016, 45, 5771–5794. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, X.; Kwon, O. Phosphine catalysis of allenes with electrophiles. Chem. Soc. Rev. 2014, 43, 2927–2940. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Shi, M. Applications of Chiral Phosphine-Based Organocatalysts in Catalytic Asymmetric Reactions. Chem. Asian J. 2014, 9, 2720–2734. [Google Scholar] [CrossRef] [PubMed]
- Marinetti, A.; Voituriez, A. Enantioselective Phosphine Organocatalysis. Synlett 2010, 174–194. [Google Scholar] [CrossRef]
- Glueck, D.S. Catalytic asymmetric synthesis of chiral phosphanes. Chem. Eur. J. 2008, 14, 7108–7117. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-M.; Kwong, F.-Y.; Yu, W.-Y.; Chan, A.S.C. Recent advances in developing new axially chiral phosphine ligands for asymmetric catalysis. Coord. Chem. Rev. 2007, 251, 2119–2144. [Google Scholar] [CrossRef]
- Pietrusiewicz, K.M.; Zablocka, M. Preparation of Scalemic P-Chiral Phosphines and Their Derivatives. Chem. Rev. 1994, 94, 1375–1411. [Google Scholar] [CrossRef]
- Rein, T.; Pedersen, T.M. Palladium-catalyzed reaction of olefins with PhI(OAc)2-TBAB system: An efficient and highly selective bisfunctionalization strategy. Synthesis 2002, 2002, 579–594. [Google Scholar] [CrossRef]
- Rein, T.; Reiser, O. Recent advances in asymmetric Wittig-type reactions. Acta Chem. Scand. 1996, 50, 369–379. [Google Scholar] [CrossRef]
- Zhang, K.; Lu, L.-Q.; Yao, S.; Chen, J.-R.; Shi, D.-Q.; Xiao, W.-J. Enantioconvergent Copper Catalysis: In Situ Generation of the Chiral Phosphorus Ylide and Its Wittig Reactions. J. Am. Chem. Soc. 2017, 139, 12847–12854. [Google Scholar] [CrossRef]
- Wong, G.W.; Landis, C.R. Iterative Asymmetric Hydroformylation/Wittig Olefination Sequence. Angew. Chem. Int. Ed. 2013, 52, 1564–1567. [Google Scholar] [CrossRef]
- Enders, D.; Grossmann, A.; Gieraths, B.; Duezdemir, M.; Merkens, C. Organocatalytic One-Pot Asymmetric Synthesis of 4H,5H-Pyrano[2,3-c]pyrazoles. Org. Lett. 2012, 14, 4254–4257. [Google Scholar] [CrossRef]
- Lin, A.; Wang, J.; Mao, H.; Ge, H.; Tan, R.; Zhu, C.; Cheng, Y. Organocatalytic Asymmetric Michael-Type/Wittig Reaction of Phosphorus Ylides: Synthesis of Chiral α-Methylene-δ-Ketoesters. Org. Lett. 2011, 13, 4176–4179. [Google Scholar] [CrossRef] [PubMed]
- Gramigna, L.; Duce, S.; Filippini, G.; Fochi, M.; Franchini, M.C.; Bernardi, L. Organocatalytic asymmetric Wittig reactions: Generation of enantioenriched axially chiral olefins breaking a symmetry plane. Synlett 2011, 2745–2749. [Google Scholar] [CrossRef]
- Ye, L.-W.; Wang, S.-B.; Wang, Q.-G.; Sun, X.-L.; Tang, Y.; Zhou, Y.-G. Asymmetric tandem Michael addition-ylide olefination reaction for the synthesis of optically active cyclohexa-1,3-diene derivatives. Chem. Commun. 2009, 3092–3094. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-k.; Ma, C.; Jiang, K.; Liu, T.-Y.; Chen, Y.-C. Asymmetric Tandem Michael Addition-Wittig Reaction to Cyclohexenone Annulation. Org. Lett. 2009, 11, 2848–2851. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.-C.; Jan, R.-H.; Tsai, C.-W.; Nimje, R.Y.; Liao, J.-H.; Lee, G.-H. Organocatalytic Enantioselective Cascade Michael-Michael-Wittig Reactions of Phosphorus Ylides: One-Pot Synthesis of the all-cis Trisubstituted Cyclohexenecarboxylates via the [1 + 2 + 3] Annulation. Org. Lett. 2009, 11, 5246–5249. [Google Scholar] [CrossRef] [PubMed]
- Voituriez, A.; Saleh, N. From phosphine-promoted to phosphine-catalyzed reactions by in situ phosphine oxide reduction. Tetrahedron Lett. 2016, 57, 4443–4451. [Google Scholar] [CrossRef]
- Xu, S.; Tang, Y. Catalytic approaches to stoichiometric phosphine-mediated organic reactions. Lett. Org. Chem. 2014, 11, 524–533. [Google Scholar] [CrossRef]
- Van Kalkeren, H.A.; van Delft, F.L.; Rutjes, F.P.J.T. Organophosphorus Catalysis to Bypass Phosphine Oxide Waste. ChemSusChem 2013, 6, 1615–1624. [Google Scholar] [CrossRef]
- Zhao, Q.; Curran, D.P.; Malacria, M.; Fensterbank, L.; Goddard, J.-P.; Lacôte, E. N-Heterocyclic Carbene-Catalyzed Hydrosilylation of Styryl and Propargylic Alcohols with Dihydrosilanes. Chem. Eur. J. 2011, 17, 9911–9914. [Google Scholar] [CrossRef]
- Quin, L.D.; Caster, K.C.; Kisalus, J.C.; Mesch, K.A. Bridged ring systems containing phosphorus: Structural influences on the stereochemistry of silane reductions of P-oxides and on carbon-13 and phosphorus-31 NMR properties of phosphines. J. Am. Chem. Soc. 1984, 106, 7021–7032. [Google Scholar] [CrossRef]
- Busacca, C.A.; Raju, R.; Grinberg, N.; Haddad, N.; James-Jones, P.; Lee, H.; Lorenz, J.C.; Saha, A.; Senanayake, C.H. Reduction of Tertiary Phosphine Oxides with DIBAL-H. J. Org. Chem. 2008, 73, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Busacca, C.A.; Lorenz, J.C.; Grinberg, N.; Haddad, N.; Hrapchak, M.; Latli, B.; Lee, H.; Sabila, P.; Saha, A.; Sarvestani, M.; et al. A Superior Method for the Reduction of Secondary Phosphine Oxides. Org. Lett. 2005, 7, 4277–4280. [Google Scholar] [CrossRef] [PubMed]
- Imamoto, T.; Kikuchi, S.-i.; Miura, T.; Wada, Y. Stereospecific Reduction of Phosphine Oxides to Phosphines by the Use of a Methylation Reagent and Lithium Aluminum Hydride. Org. Lett. 2001, 3, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Henson, P.D.; Naumann, K.; Mislow, K. Stereomutation of phosphine oxide by lithium aluminum hydride. J. Amer. Chem. Soc. 1969, 91, 5645–5646. [Google Scholar] [CrossRef]
- Sowa, S.; Stankevic, M.; Flis, A.; Pietrusiewicz, K.M. Reduction of Tertiary Phosphine Oxides by BH3 Assisted by Neighboring Activating Groups. Synthesis 2018, 50, 2106–2118. [Google Scholar]
- Provis-Evans, C.B.; Emanuelsson, E.A.C.; Webster, R.L. Rapid Metal-Free Formation of Free Phosphines from Phosphine Oxides. Adv. Synth. Catal. 2018, 360, 3999–4004. [Google Scholar] [CrossRef]
- Elias, J.S.; Costentin, C.; Nocera, D.G. Direct Electrochemical P(V) to P(III) Reduction of Phosphine Oxide Facilitated by Triaryl Borates. J. Am. Chem. Soc. 2018, 140, 13711–13718. [Google Scholar] [CrossRef]
- Mehta, M.; Garcia de la Arada, I.; Perez, M.; Porwal, D.; Oestreich, M.; Stephan, D.W. Metal-Free Phosphine Oxide Reductions Catalyzed by B(C6F5)3 and Electrophilic Fluorophosphonium Cations. Organometallics 2016, 35, 1030–1035. [Google Scholar] [CrossRef]
- Stepen, A.J.; Bursch, M.; Grimme, S.; Stephan, D.W.; Paradies, J. Electrophilic Phosphonium Cation-Mediated Phosphane Oxide Reduction Using Oxalyl Chloride and Hydrogen. Angew. Chem. Int. Ed. 2018, 57, 15253–15256. [Google Scholar] [CrossRef]
- Li, P.; Wischert, R.; Metivier, P. Mild Reduction of Phosphine Oxides with Phosphites To Access Phosphines. Angew. Chem. Int. Ed. 2017, 56, 15989–15992. [Google Scholar] [CrossRef]
- Kuroboshi, M.; Kita, T.; Aono, A.; Katagiri, T.; Kikuchi, S.; Yamane, S.; Kawakubo, H.; Tanaka, H. Reduction of phosphine oxides to the corresponding phosphine derivatives in Mg/Me3SiCl/DMI system. Tetrahedron Lett. 2015, 56, 918–920. [Google Scholar] [CrossRef]
- Pehlivan, L.; Metay, E.; Delbrayelle, D.; Mignani, G.; Lemaire, M. Reduction of phosphine oxides to phosphines with the InBr3/TMDS system. Tetrahedron 2012, 68, 3151–3155. [Google Scholar] [CrossRef]
- Rajendran, K.V.; Gilheany, D.G. Simple unprecedented conversion of phosphine oxides and sulfides to phosphine boranes using sodium borohydride. Chem. Commun. 2012, 48, 817–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawakubo, H.; Kuroboshi, M.; Yano, T.; Kobayashi, K.; Kamenoue, S.; Akagi, T.; Tanaka, H. Electroreduction of triphenylphosphine oxide to triphenylphosphine in the presence of chlorotrimethylsilane. Synthesis 2011, 4091–4098. [Google Scholar]
- Yano, T.; Kuroboshi, M.; Tanaka, H. Electroreduction of triphenylphosphine dichloride and the efficient one-pot reductive conversion of phosphine oxide to triphenylphosphine. Tetrahedron Lett. 2010, 51, 698–701. [Google Scholar] [CrossRef]
- Longwitz, L.; Werner, T. Recent advances in catalytic Wittig-type reactions based on P(III)/P(V) redox cycling. Pure Appl. Chem. 2019, 91, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Podyacheva, E.; Kuchuk, E.; Chusov, D. Reduction of phosphine oxides to phosphines. Tetrahedron Lett. 2019, 60, 575–582. [Google Scholar] [CrossRef]
- Karanam, P.; Reddy, G.M.; Lin, W. Strategic Exploitation of the Wittig Reaction: Facile Synthesis of Heteroaromatics and Multifunctional Olefins. Synlett 2018, 29, 2608–2622. [Google Scholar]
- Kovacs, T.; Keglevich, G. The Reduction of Tertiary Phosphine Oxides by Silanes. Curr. Org. Chem. 2017, 21, 569–585. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, T.; Keglevich, G. The deoxygenation of phosphine oxides under green chemical conditions. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 359–366. [Google Scholar] [CrossRef]
- Lao, Z.; Toy, P.H. Catalytic Wittig and aza-Wittig reactions. Beilstein J. Org. Chem. 2016, 12, 2577–2587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herault, D.; Nguyen, D.H.; Nuel, D.; Buono, G. Reduction of secondary and tertiary phosphine oxides to phosphines. Chem. Soc. Rev. 2015, 44, 2508–2528. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.J.; Tellez, J.L.; Nixon, Z.S.; Kang, L.J.; Carter, A.L.; Kunkel, S.R.; Przeworski, K.C.; Chass, G.A. Recycling the Waste: The Development of a Catalytic Wittig Reaction. Angew. Chem. Int. Ed. 2009, 48, 6836–6839. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.J.; Lavigne, F.; Coyle, E.E.; Holohan, A.J.; Doonan, B.J. Breaking the Ring through a Room Temperature Catalytic Wittig Reaction. Chem. Eur. J. 2013, 19, 5854–5858. [Google Scholar]
- O’Brien, C.J.; Nixon, Z.S.; Holohan, A.J.; Kunkel, S.R.; Tellez, J.L.; Doonan, B.J.; Coyle, E.E.; Lavigne, F.; Kang, L.J.; Przeworski, K.C. Part I: The Development of the Catalytic Wittig Reaction. Chem. Eur. J. 2013, 19, 15281–15289. [Google Scholar] [CrossRef]
- Coyle, E.E.; Doonan, B.J.; Holohan, A.J.; Walsh, K.A.; Lavigne, F.; Krenske, E.H.; O’Brien, C.J. Catalytic Wittig Reactions of Semi- and Nonstabilized Ylides Enabled by Ylide Tuning. Angew. Chem. Int. Ed. 2014, 53, 12907–12911. [Google Scholar] [CrossRef]
- Li, Y.; Das, S.; Zhou, S.; Junge, K.; Beller, M. General and Selective Copper-Catalyzed Reduction of Tertiary and Secondary Phosphine Oxides: Convenient Synthesis of Phosphines. J. Am. Chem. Soc. 2012, 134, 9727–9732. [Google Scholar] [CrossRef]
- Li, Y.; Lu, L.-Q.; Das, S.; Pisiewicz, S.; Junge, K.; Beller, M. Highly Chemoselective Metal-Free Reduction of Phosphine Oxides to Phosphines. J. Am. Chem. Soc. 2012, 134, 18325–18329. [Google Scholar] [CrossRef]
- Cai, L.; Zhang, K.; Chen, S.; Lepage, R.J.; Houk, K.N.; Krenske, E.H.; Kwon, O. Catalytic Asymmetric Staudinger-aza-Wittig Reaction for the Synthesis of Heterocyclic Amines. J. Am. Chem. Soc. 2019, 141, 9537–9542. [Google Scholar] [CrossRef]
- Longwitz, L.; Spannenberg, A.; Werner, T. Phosphetane Oxides as Redox Cycling Catalysts in the Catalytic Wittig Reaction at Room Temperature. ACS Catal. 2019, 9, 9237–9244. [Google Scholar] [CrossRef]
- Lorton, C.; Castanheiro, T.; Voituriez, A. Catalytic and Asymmetric Process via PIII/PV=O Redox Cycling: Access to (Trifluoromethyl)cyclobutenes via a Michael Addition/Wittig Olefination Reaction. J. Am. Chem. Soc. 2019, 141, 10142–10147. [Google Scholar] [CrossRef]
- Fianchini, M.; Maseras, F. DFT characterization of the mechanism for Staudinger/aza-Wittig tandem organocatalysis. Tetrahedron 2019, 75, 1852–1859. [Google Scholar] [CrossRef]
- Han, X.; Saleh, N.; Retailleau, P.; Voituriez, A. Phosphine-Catalyzed Reaction between 2-Aminobenzaldehydes and Dialkyl Acetylenedicarboxylates: Synthesis of 1,2-Dihydroquinoline Derivatives and Toward the Development of an Olefination Reaction. Org. Lett. 2018, 20, 4584–4588. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, M.; Ding, M.-W. Catalytic Intramolecular Wittig Reaction Based on a Phosphine/Phosphine Oxide Catalytic Cycle for the Synthesis of Heterocycles. Eur. J. Org. Chem. 2017, 2568–2578. [Google Scholar] [CrossRef]
- Wang, L.; Xie, Y.-B.; Huang, N.-Y.; Yan, J.-Y.; Hu, W.-M.; Liu, M.-G.; Ding, M.-W. Catalytic aza-Wittig Reaction of Acid Anhydride for the Synthesis of 4H-Benzo[d][1,3]oxazin-4-ones and 4-Benzylidene-2-aryloxazol-5(4H)-ones. ACS Catal. 2016, 6, 4010–4016. [Google Scholar] [CrossRef]
- Saleh, N.; Voituriez, A. Synthesis of 9H-Pyrrolo[1,2-a]indole and 3H-Pyrrolizine Derivatives via a Phosphine-Catalyzed Umpolung Addition/Intramolecular Wittig Reaction. J. Org. Chem. 2016, 81, 4371–4377. [Google Scholar] [CrossRef]
- Schirmer, M.-L.; Adomeit, S.; Spannenberg, A.; Werner, T. Novel Base-Free Catalytic Wittig Reaction for the Synthesis of Highly Functionalized Alkenes. Chem. Eur. J. 2016, 22, 2458–2465. [Google Scholar] [CrossRef]
- Lee, C.-J.; Chang, T.-H.; Yu, J.-K.; Madhusudhan Reddy, G.; Hsiao, M.-Y.; Lin, W. Synthesis of Functionalized Furans via Chemoselective Reduction/Wittig Reaction Using Catalytic Triethylamine and Phosphine. Org. Lett. 2016, 18, 3758–3761. [Google Scholar] [CrossRef]
- Schirmer, M.-L.; Adomeit, S.; Werner, T. First Base-Free Catalytic Wittig Reaction. Org. Lett. 2015, 17, 3078–3081. [Google Scholar] [CrossRef]
- Tsai, Y.-L.; Lin, W. Synthesis of Multifunctional Alkenes from Substituted Acrylates and Aldehydes via Phosphine-Catalyzed Wittig Reaction. Asian J. Org. Chem. 2015, 4, 1040–1043. [Google Scholar] [CrossRef]
- Rommel, S.; Belger, C.; Begouin, J.-M.; Plietker, B. Dual [Fe+Phosphine] Catalysis: Application in Catalytic Wittig Olefination. Chemcatchem 2015, 7, 1292–1301. [Google Scholar] [CrossRef]
- Werner, T.; Hoffmann, M.; Deshmukh, S. First Enantioselective Catalytic Wittig Reaction. Eur. J. Org. Chem. 2014, 6630–6633. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Chen, M.; Ding, M.-W. Reversible P(III)/P(V) Redox: Catalytic Aza-Wittig Reaction for the Synthesis of 4(3H)-Quinazolinones and the Natural Product Vasicinone. Adv. Synth. Catal. 2014, 356, 1098–1104. [Google Scholar] [CrossRef]
- Werner, T.; Hoffmann, M.; Deshmukh, S. First Microwave-Assisted Catalytic Wittig Reaction. Eur. J. Org. Chem. 2014, 6873–6876. [Google Scholar] [CrossRef]
- Van Kalkeren, H.A.; te Grotenhuis, C.; Haasjes, F.S.; Hommersom, C.A.; Rutjes, F.P.J.T.; van Delft, F.L. Catalytic Staudinger/aza-Wittig sequence by in situ phosphane oxide reduction. Eur. J. Org. Chem. 2013, 7059–7066. [Google Scholar] [CrossRef]
- Beddoe, R.H.; Sneddon, H.F.; Denton, R.M. The catalytic Mitsunobu reaction: A critical analysis of the current state-of-the-art. Org. Biomol. Chem. 2018, 16, 7774–7781. [Google Scholar] [CrossRef]
- Hirose, D.; Gazvoda, M.; Košmrlj, J.; Taniguchi, T. The “Fully Catalytic System” in Mitsunobu Reaction Has Not Been Realized Yet. Org. Lett. 2016, 18, 4036–4039. [Google Scholar] [CrossRef]
- Buonomo, J.A.; Aldrich, C.C. Mitsunobu Reactions Catalytic in Phosphine and a Fully Catalytic System. Angew. Chem. Int. Ed. 2015, 54, 13041–13044. [Google Scholar] [CrossRef] [Green Version]
- Lenstra, D.C.; Wolf, J.J.; Mecinovic, J. Catalytic Staudinger Reduction at Room Temperature. J. Org. Chem. 2019, 84, 6536–6545. [Google Scholar] [CrossRef]
- Zhang, K.; Cai, L.; Yang, Z.; Houk, K.N.; Kwon, O. Bridged [2.2.1] bicyclic phosphine oxide facilitates catalytic γ-umpolung addition-Wittig olefination. Chem. Sci. 2018, 9, 1867–1872. [Google Scholar] [CrossRef] [Green Version]
- Lenstra, D.C.; Lenting, P.E.; Mecinovic, J. Sustainable organophosphorus-catalysed Staudinger reduction. Greem Chem. 2018, 20, 4418–4422. [Google Scholar] [CrossRef]
- Andrews, K.G.; Denton, R.M. A more critical role for silicon in the catalytic Staudinger amidation: Silanes as non-innocent reductants. Chem. Commun. 2017, 53, 7982–7985. [Google Scholar] [CrossRef] [PubMed]
- Saleh, N.; Blanchard, F.; Voituriez, A. Synthesis of Nitrogen-Containing Heterocycles and Cyclopentenone Derivatives via Phosphine-Catalyzed Michael Addition/Intramolecular Wittig Reaction. Adv. Synth. Catal. 2017, 359, 2304–2315. [Google Scholar] [CrossRef]
- Van Kalkeren, H.A.; Bruins, J.J.; Rutjes, F.P.J.T.; van Delft, F.L. Organophosphorus-catalyzed Staudinger reduction. Adv. Synth. Catal. 2012, 354, 1417–1421. [Google Scholar] [CrossRef]
- Longwitz, L.; Jopp, S.; Werner, T. Organocatalytic Chlorination of Alcohols by P(III)/P(V) Redox Cycling. J. Org. Chem. 2019, 84, 7863–7870. [Google Scholar] [CrossRef] [PubMed]
- Van Kalkeren, H.A.; Leenders, S.; Hommersom, C.R.A.; Rutjes, F.; van Delft, F.L. In situ phosphine oxide reduction: A catalytic Appel reaction. Chem. Eur. J. 2011, 17, 11290–11295. [Google Scholar] [CrossRef]
- Nykaza, T.V.; Ramirez, A.; Harrison, T.S.; Luzung, M.R.; Radosevich, A.T. Biphilic Organophosphorus-Catalyzed Intramolecular Csp2-H Amination: Evidence for a Nitrenoid in Catalytic Cadogan Cyclizations. J. Am. Chem. Soc. 2018, 140, 3103–3113. [Google Scholar] [CrossRef] [Green Version]
- White, P.B.; Rijpkema, S.J.; Bunschoten, R.P.; Mecinovic, J. Mechanistic Insight into the Catalytic Staudinger Ligation. Org. Lett. 2019, 21, 1011–1014. [Google Scholar]
- Nykaza, T.V.; Cooper, J.C.; Li, G.; Mahieu, N.; Ramirez, A.; Luzung, M.R.; Radosevich, A.T. Intermolecular Reductive C-N Cross Coupling of Nitroarenes and Boronic Acids by PIII/PV=O Catalysis. J. Am. Chem. Soc. 2018, 140, 15200–15205. [Google Scholar] [CrossRef] [Green Version]
- Hamstra, D.F.J.; Lenstra, D.C.; Koenders, T.J.; Rutjes, F.P.J.T.; Mecinovic, J. Poly(methylhydrosiloxane) as a green reducing agent in organophosphorus-catalyzed amide bond formation. Org. Biomol. Chem. 2017, 15, 6426–6432. [Google Scholar] [CrossRef] [Green Version]
- Fourmy, K.; Voituriez, A. Catalytic Cyclization Reactions of Huisgen Zwitterion with α-Ketoesters by in Situ Chemoselective Phosphine Oxide Reduction. Org. Lett. 2015, 17, 1537–1540. [Google Scholar] [CrossRef]
- Zhao, W.; Yan, P.K.; Radosevich, A.T. A Phosphetane Catalyzes Deoxygenative Condensation of α-Keto Esters and Carboxylic Acids via PIII/PV=O Redox Cycling. J. Am. Chem. Soc. 2015, 137, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Kosal, A.D.; Wilson, E.E.; Ashfeld, B.L. Phosphine-Based Redox Catalysis in the Direct Traceless Staudinger Ligation of Carboxylic Acids and Azides. Angew. Chem. Int. Ed. 2012, 51, 12036–12040. [Google Scholar] [CrossRef] [PubMed]
- Rotstein, B.H.; Zaretsky, S.; Rai, V.; Yudin, A.K. Small Heterocycles in Multicomponent Reactions. Chem. Rev. 2014, 114, 8323–8359. [Google Scholar] [CrossRef] [PubMed]
- Estevez, V.; Villacampa, M.; Menendez, J.C. Recent advances in the synthesis of pyrroles by multicomponent reactions. Chem. Soc. Rev. 2014, 43, 4633–4657. [Google Scholar] [CrossRef]
- Cioc, R.C.; Ruijter, E.; Orru, R.V.A. Multicomponent reactions: Advanced tools for sustainable organic synthesis. Green Chem. 2014, 16, 2958–2975. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, R.; He, Z.-R.; He, Z. Phosphane-Mediated Domino Synthesis of Tetrasubstituted Furans from Simple Terminal Activated Olefins. Eur. J. Org. Chem. 2012, 6033–6041. [Google Scholar] [CrossRef]
- Lawrence, N.J.; Crump, J.P.; McGown, A.T.; Hadfield, J.A. Reaction of Baylis–Hillman products with Swern and Dess–Martin oxidants. Tetrahedron Lett. 2001, 42, 3939–3941. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 3 are not available from the authors. |
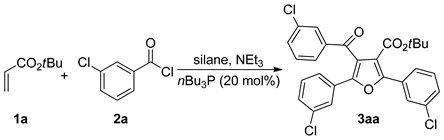
| Entry | Silane (mmol) | Solvent | Temp (°C) | 3 (%) b |
|---|---|---|---|---|
| 1 | Ph2SiH2 (0.6) | THF | rt | 11 |
| 2 | Ph2SiH2 (0.6) | THF | 60 | 12 |
| 3 | PhSiH3 (0.6) | Toluene | rt | trace |
| 4 | PhSiH3 (0.6) | Toluene | 110 | 74 |
| 5 | Ph2SiH2 (0.6) | Toluene | 110 | 42 |
| 6 | (MeO)3SiH (0.6) | Toluene | 110 | 33 |
| 7 | PMHS (0.034) | Toluene | 110 | 26 |
| 8 | PMHS (0.106) | Toluene | 110 | 30 |
| 9 | Ph3SiH (1.2) | Toluene | 110 | 18 |
| 10 | SiHCl3 (0.6) | Toluene | rt | trace |
| 11 | PhSiH3 (0.25) | Toluene | 110 | 65 |
| 12 | PhSiH3 (0.4) | Toluene | 110 | 84 |
| 13 | PhSiH3 (0.75) | Toluene | 110 | 49 |
| 14 | PhSiH3 (0.4) | Toluene | 80 | 56 |
| 15 | PhSiH3 (0.4) | Dioxane | 110 | 54 |
| 16 | PhSiH3 (0.4) | Xylene | 110 | 60 |
| 17 | PhSiH3 (0.4) | DMF | 110 | 11 |
| 18 | PhSiH3 (0.4) | CH3CN | 80 | trace |
| 19 c | PhSiH3 (0.4) | Toluene | 110 | 48 |

| Entry | EWG in 1 | R in 2 or 2′ | 3 (%) b |
|---|---|---|---|
| 1 | CO2tBu (1a) | 3-ClC6H4 (2a) | 3aa, 84 |
| 2 | CO2tBu (1a) | 4-ClC6H4 (2b) | 3ab, 75 |
| 3 | CO2tBu (1a) | 2-ClC6H4 (2c) | 3ac, 33 |
| 4 | CO2tBu (1a) | Ph(2d) | 3ad, 70 |
| 5 | CO2tBu (1a) | 4-MeC6H4 (2e) | 3ae, 90 |
| 6 | CO2tBu (1a) | 4-NO2C6H4 (2f) | 3af, 37 |
| 7 | CO2tBu (1a) | 2-thienyl (2g) | 3ag, 46 |
| 8 | CO2Me (1b) | 4-ClC6H4 (2b) | 3bb, 81 |
| 9 | CO2Me (1b) | 4-MeC6H4 (2e) | 3be, 84 |
| 10 | CO2Et (1c) | 4-ClC6H4 (2b) | 3cb, 71 |
| 11 | CO2Et (1c) | 4-MeC6H4 (2e) | 3ce, 84 |
| 12 | CO2nBu (1d) | 4-ClC6H4 (2b) | 3db, 85 |
| 13 | CO2nBu (1d) | 4-MeC6H4 (2e) | 3de, 83 |
| 14 | CO2Bn (1e) | Ph (2d) | 3ed, 87 |
| 15 | CN (1f) | 4-ClC6H4 (2b) | 3fb, 38 |
| 16 | CN (1f) | Ph (2d) | 3fd, 57 |
| 17 | CN (1f) | 4-MeC6H4 (2e) | 3fe, 44 |
| 18 | CO2tBu (1a) | Me (2′a) | 3aa’, 27 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Chen, R.; Han, J.; He, Z. Convergent Synthesis of Polysubstituted Furans via Catalytic Phosphine Mediated Multicomponent Reactions. Molecules 2019, 24, 4595. https://doi.org/10.3390/molecules24244595
Fan X, Chen R, Han J, He Z. Convergent Synthesis of Polysubstituted Furans via Catalytic Phosphine Mediated Multicomponent Reactions. Molecules. 2019; 24(24):4595. https://doi.org/10.3390/molecules24244595
Chicago/Turabian StyleFan, Xia, Rongshun Chen, Jie Han, and Zhengjie He. 2019. "Convergent Synthesis of Polysubstituted Furans via Catalytic Phosphine Mediated Multicomponent Reactions" Molecules 24, no. 24: 4595. https://doi.org/10.3390/molecules24244595
APA StyleFan, X., Chen, R., Han, J., & He, Z. (2019). Convergent Synthesis of Polysubstituted Furans via Catalytic Phosphine Mediated Multicomponent Reactions. Molecules, 24(24), 4595. https://doi.org/10.3390/molecules24244595






