Antimicrobial Secondary Metabolites from the Seawater-Derived Fungus Aspergillus sydowii SW9
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation
3.4. Extraction and Isolation
3.5. Antimicrobial Assays
3.6. Computational Section
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Smith, G.W.; Ives, L.D.; Nagelkerken, I.A.; Richie, K.B. Caribbean sea-fan mortalities. Nature 1996, 383, 487. [Google Scholar] [CrossRef]
- Kim, K.; Harvell, C.D. The rise and fall of a six-year coral-fungal epizootic. Am. Nat. 2004, 164, S52–S63. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Crombie, A.; Lacey, E.; Richardson, A.J.; Vuong, D.; Piggott, A.M.; Hallegraeff, G. Aspergillus Sydowii marine fungal bloom in Australian coastal waters, its metabolites and potential impact on Symbiodinium dinoflagellates. Mar. Drugs 2016, 14, 59. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Raja, H.A.; Darveaux, B.A.; Chen, W.-L.; Swanson, S.M.; Pearce, C.J.; Oberlies, N.H. New diketopiperazine dimer from a filamentous fungal isolate of Aspergillus sydowii. Magn. Reson. Chem. 2015, 53, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Wiese, J.; Aldemir, H.; Schmaljohann, R.; Gulder, T.A.M.; Imhoff, J.F. Asperentin B, a new inhibitor of the protein tyrosine phosphatase 1B. Mar. Drugs 2017, 15, 191. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-N.; Mou, Y.-H.; Dong, Y.; Wu, Y.; Liu, B.-Y.; Bai, J.; Yan, D.-J.; Zhang, L.; Feng, D.-Q.; Pei, Y.-H.; et al. Diphenyl ethers from a marine-derived Aspergillus sydowii. Mar. Drugs 2018, 16, 451. [Google Scholar] [CrossRef] [PubMed]
- Song, X.-Q.; Zhang, X.; Han, Q.-J.; Li, X.-B.; Li, G.; Li, R.-J.; Jiao, Y.; Zhou, J.-C.; Lou, H.-X. Xanthone derivatives from Aspergillus sydowii, an endophytic fungus from the liverwort Scapania ciliata S. Lac and their immunosuppressive activities. Phytochemistry Lett. 2013, 6, 318–321. [Google Scholar] [CrossRef]
- Liu, X.; Song, F.; Ma, L.; Chen, C.; Xiao, X.; Ren, B.; Liu, X.; Dai, H.; Piggott, A.M.; Av-Gay, Y.; et al. Sydowiols A–C: Mycobacterium tuberculosis protein tyrosine phosphatase inhibitors from an East China Sea marine-derived fungus, Aspergillus sydowii. Tetrahedron Lett. 2013, 54, 6081–6083. [Google Scholar] [CrossRef]
- Yang, S.-Q.; Li, X.-M.; Li, X.; Li, H.-L.; Meng, L.-H.; Wang, B.-G. New citrinin analogues produced by coculture of the marine algal-derived endophytic fungal strains Aspergillus sydowii EN-534 and Penicillium citrinum EN-535. Phytochem. Lett. 2018, 25, 191–195. [Google Scholar] [CrossRef]
- Hamasaki, T.; Nagayama, K.; Hatsuda, Y. Two new metabolites, sydonic acid and hydroxysydonic acid, from Aspergillus sydowi. Agric. Biol. Chem. 1978, 42, 37–40. [Google Scholar] [CrossRef]
- Hamasaki, T.; Sato, Y.; Hatsuda, Y. Isolation of new metabolites from Aspergillus sydowi and structure of sydowic acid. Agric. Biol. Chem. 1975, 39, 2337–2340. [Google Scholar] [CrossRef]
- Trisuwan, K.; Rukachaisirikul, V.; Kaewpet, M.; Phongpaichit, S.; Hutadilok-Towatana, N.; Preedanon, S.; Sakayaroj, J. Sesquiterpene and xanthone derivatives from the sea fan-derived fungus Aspergillus sydowii PSU-F154. J. Nat. Prod. 2011, 74, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhao, S.; Yin, L.; Chen, Z.; Shen, H.; Zhou, L. A new sydonic acid derivative from a marine derived-fungus Aspergillus sydowii. Chem. Nat. Compd. 2017, 53, 1056–1058. [Google Scholar] [CrossRef]
- Liu, N.; Peng, S.; Yang, J.; Cong, Z.; Lin, X.; Liao, S.; Yang, B.; Zhou, X.; Zhou, X.; Liu, Y.; et al. Structurally diverse sesquiterpenoids and polyketides from a sponge associated fungus Aspergillus sydowii SCSIO41301. Fitoterapia 2019, 135, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, H.; Su, M.; Hwang, G.J.; Hong, J.; Jun, J.H. New metabolites from the sponge-derived fungus Aspergillus sydowii J05B-7F-4. Nat. Prod. Res. 2017, 31, 1682–1686. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Li, Y.; Niu, S.; Zhang, H.; Liu, X.; Che, Y. N-Hydroxypyridones, Phenylhydrazones, and a Quinazolinone from Isaria farinose. J. Nat. Prod. 2011, 74, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Alberg, D.G.; Lauhon, C.T.; Nyfeler, R.; Faessler, A.; Bartlett, P.A. Inhibition of EPSP synthase by analogues of the tetrahedral intermediate and of EPSP. J. Am. Chem. Soc. 1992, 114, 3535–3546. [Google Scholar] [CrossRef]
- Li, C.-S.; An, C.-Y.; Li, X.-M.; Gao, S.-S.; Cui, C.-M.; Sun, H.-F.; Wang, B.-G. Triazole and dihydroimidazole alkaloids from the marine sediment-derived fungus Penicillium paneum SD-44. J. Nat. Prod. 2011, 74, 1331–1334. [Google Scholar] [CrossRef] [PubMed]
- An, C.-Y.; Li, X.-M.; Li, C.-S.; Gao, S.-S.; Shang, Z.; Wang, B.-G. Triazoles and other N-containing metabolites from the marine-derived endophytic fungus Penicillium chrysogenum EN-118. Helv. Chim. Acta 2013, 96, 682–687. [Google Scholar] [CrossRef]
- Li, X.-D.; Li, X.-M.; Yin, X.-L.; Li, X.; Wang, B.-G. Antimicrobial sesquiterpenoid derivatives and monoterpenoids from the deep-sea sediment-derived fungus Aspergillus versicolor SD-330. Mar. Drugs 2019, 17, 563. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
Sample Availability: Samples of the compounds 1–5 are available from the authors. |

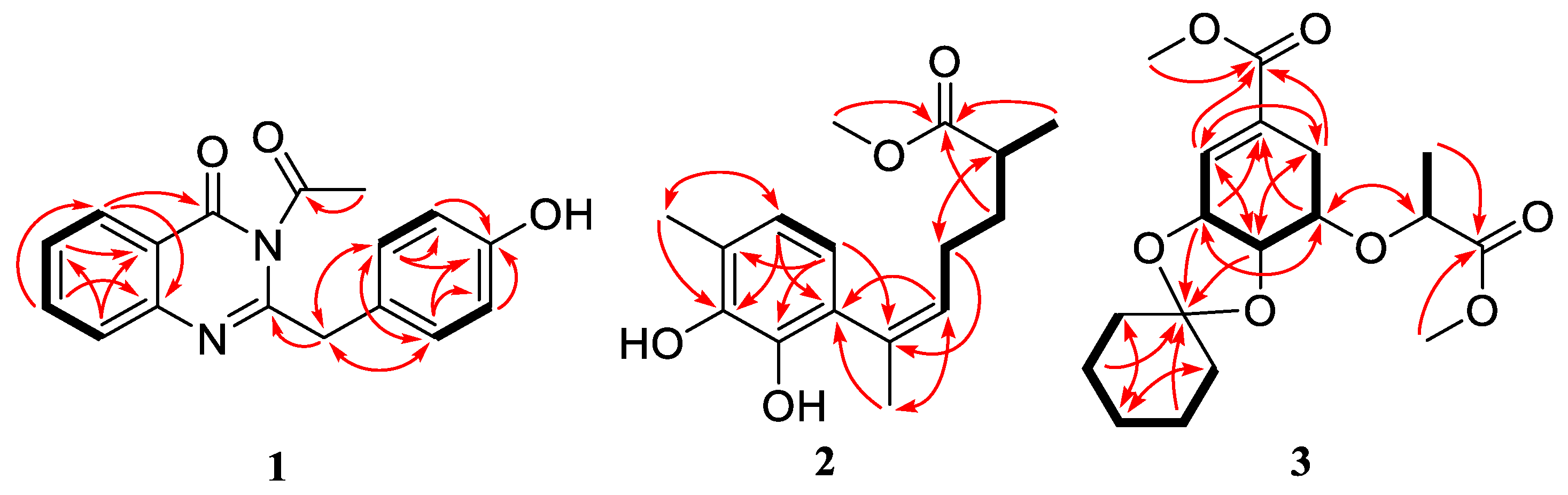
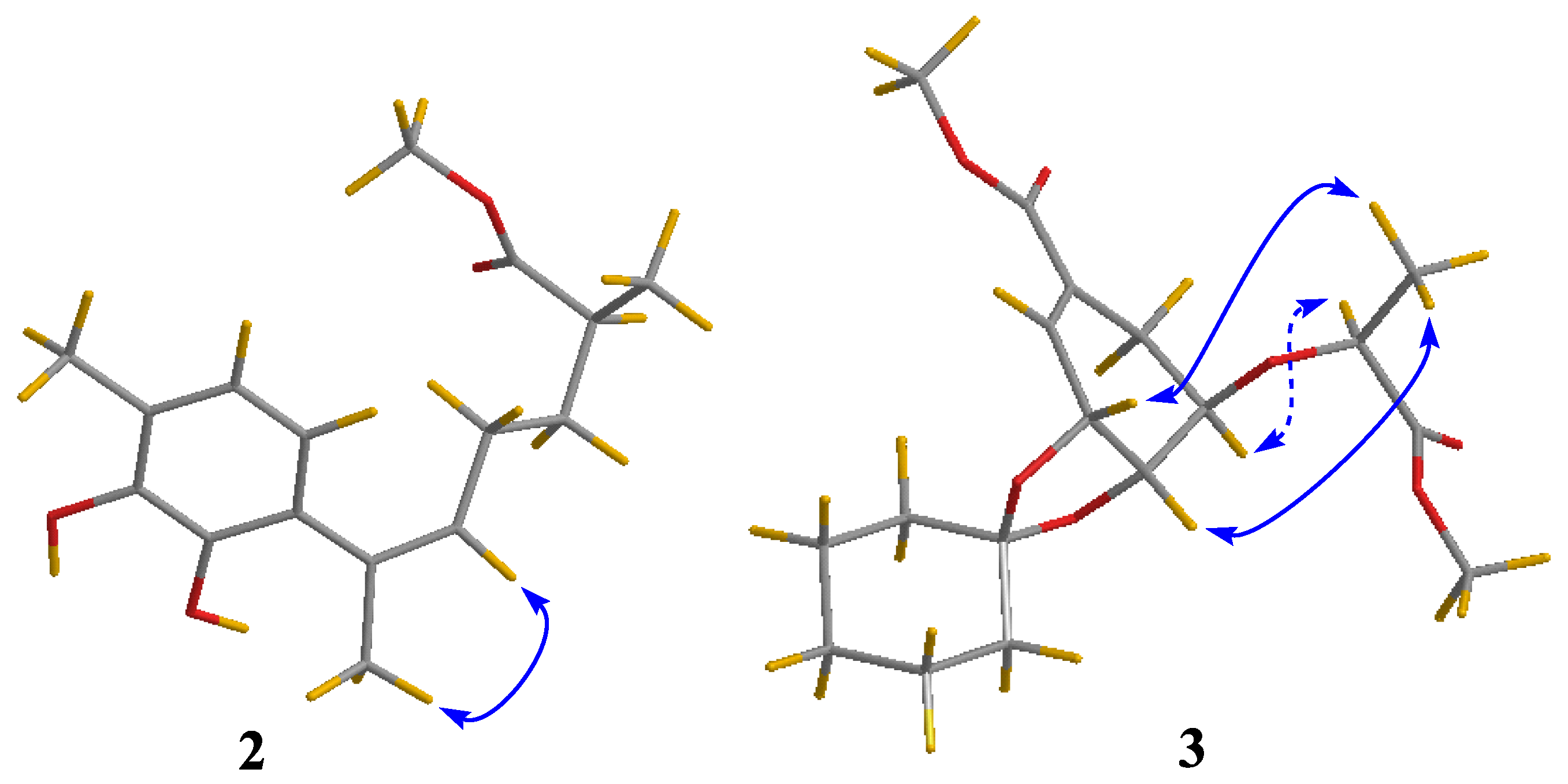
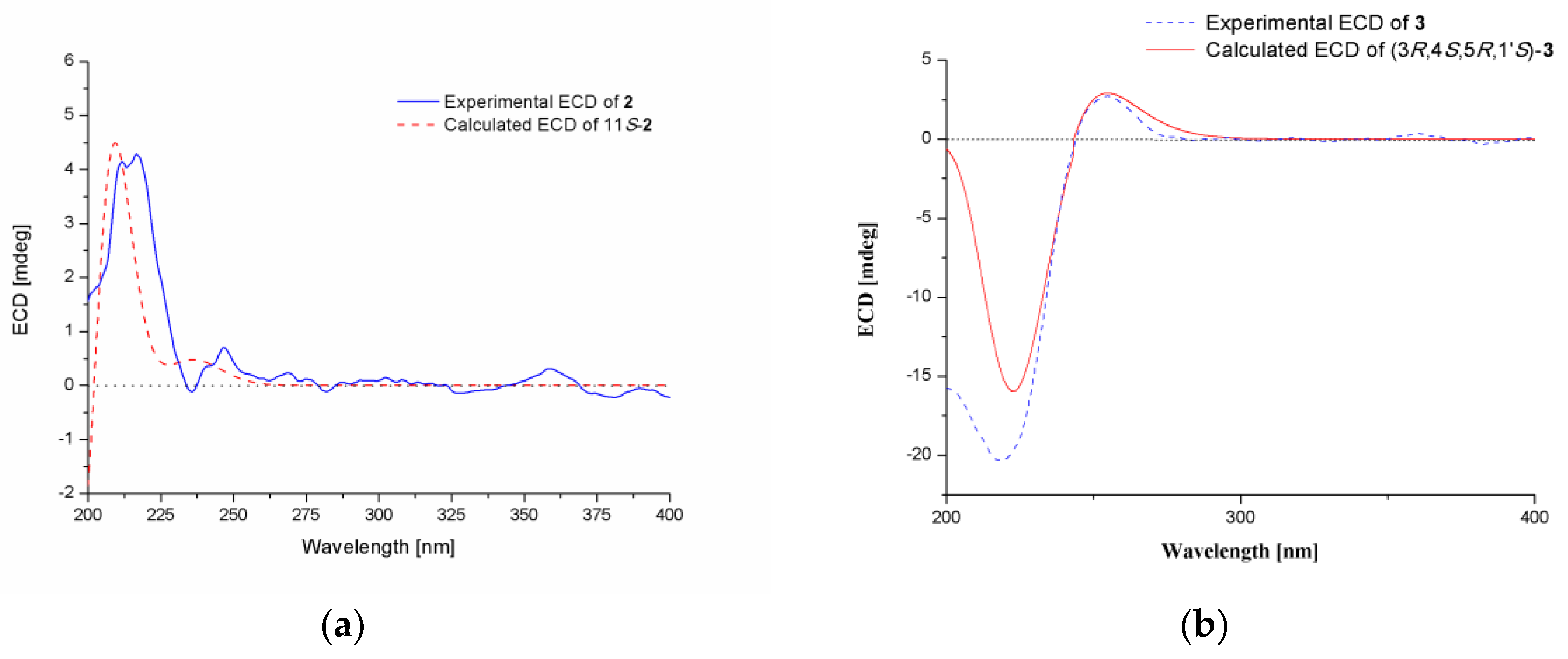
| 1 | 2 | ||||
|---|---|---|---|---|---|
| No. | δH (Mult, J in Hz) a | δC, Type b | No. | δH (Mult, J in Hz) a | δC, Type b |
| 1 | - | - | 1 | - | 126.6, qC |
| 2 | - | 157.7, qC | 2 | - | 119.1, CH |
| 3 | - | - | 3 | 6.28, d (7.6) | 120.5, CH |
| 4 | - | 162.9, qC | 4 | 6.49, d (7.6) | 123.2, qC |
| 4a | - | 121.1, qC | 5 | - | 143.2, qC |
| 5 | 8.04, d (8.0) | 126.1, CH | 6 | - | 142.1, qC |
| 6 | 7.42, t (7.5) | 126.3, CH | 7 | - | 134.2, qC |
| 7 | 7.73, t (7.5) | 134.5, CH | 8 | 5.34, t (6.6) | 126.7, CH |
| 8 | 7.57, d (8.0) | 127.1, CH | 9 | 1.74, m | 26.3, CH2 |
| 8a | - | 149.5, qC | 10a | 1.54, m | 33.1, CH2 |
| 9 | 3.79, s | 40.7, CH2 | 10b | 1.33, m | - |
| 10 | - | 127.0, qC | 11 | 2.33, m | 38.0, CH |
| 11 | 7.14, d (7.8) | 130.2, CH | 12 | - | 176.1, qC |
| 12 | 6.70, d (7.8) | 115.7, CH | 13 | 0.94, d (6.6) | 16.1, CH3 |
| 13 | - | 157.0, qC | 14 | 1.88, s | 24.6, CH3 |
| 14 | 6.70, d (7.8) | 115.7, CH | 15 | 2.10, s | 16.5, CH3 |
| 15 | 7.14, d (7.8) | 130.2, CH | 1′ | 3.50, s | 51.1, CH3 |
| 1′ | - | 175.8, qC | |||
| 2′ | 1.67, s | 25.3, CH3 | |||
| No. | δH (Mult, J in Hz) a | δC, Type b | No. | δH (Mult, J in Hz) a | δC, Type b |
|---|---|---|---|---|---|
| 1 | - | 129.4, qC | 1′′ | - | 109.5, qC |
| 2 | 6.71, s | 135.0, CH | 2′′ | 1.46, m | 37.7, CH2 |
| 3 | 4.71, s | 71.7, CH | 3′′ | 1.51, m | 24.0, CH2 |
| 4 | 4.16, t (6.2) | 75.1, CH | 4′′ | 1.31, m | 25.0, CH2 |
| 5 | 3.72, m | 75.5, CH | 5′′ | 1.48, m | 23.8, CH2 |
| 6a | 2.26, dd (6.5, 17.4) | 26.8, CH2 | 6′′ | 1.52, m | 35.4, CH2 |
| 6b | 2.46, dd (6.5, 17.4) | ||||
| 7 | - | 166.6, qC | 1′′′ | 3.63, s | 52.0, CH3 |
| 1′ | 4.23, m | 73.8, CH | 1′′′′ | 3.68, s | 52.5, CH3 |
| 2′ | - | 173.6, qC | |||
| 3′ | 1.22, d (6.8) | 19.5, CH3 |
| Strains | 1 | 2 | 3 | 4 | 5 | Positive Control |
|---|---|---|---|---|---|---|
| Escherichia colib | 16 | 2.0 | – | 8.0 | – | 2.0 |
| Staphylococcus aureusb | 8.0 | 16 | – | 8.0 | 32 | 1.0 |
| Staphylococcus. epidermidisb | 4.0 | 16 | 32 | 16 | – | 2.0 |
| Streptococcus pneumoniaeb | 16 | 4.0 | 32 | 8.0 | 32 | 1.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.-J.; Zhang, J.-L.; Li, C.; Mu, X.-G.; Liu, X.-L.; Wang, L.; Zhao, Y.-C.; Zhang, P.; Li, X.-D.; Zhang, X.-X. Antimicrobial Secondary Metabolites from the Seawater-Derived Fungus Aspergillus sydowii SW9. Molecules 2019, 24, 4596. https://doi.org/10.3390/molecules24244596
Liu Y-J, Zhang J-L, Li C, Mu X-G, Liu X-L, Wang L, Zhao Y-C, Zhang P, Li X-D, Zhang X-X. Antimicrobial Secondary Metabolites from the Seawater-Derived Fungus Aspergillus sydowii SW9. Molecules. 2019; 24(24):4596. https://doi.org/10.3390/molecules24244596
Chicago/Turabian StyleLiu, Yu-Jing, Jian-Long Zhang, Chen Li, Xue-Gen Mu, Xiao-Li Liu, Lei Wang, Yan-Cui Zhao, Peng Zhang, Xiao-Dong Li, and Xing-Xiao Zhang. 2019. "Antimicrobial Secondary Metabolites from the Seawater-Derived Fungus Aspergillus sydowii SW9" Molecules 24, no. 24: 4596. https://doi.org/10.3390/molecules24244596
APA StyleLiu, Y.-J., Zhang, J.-L., Li, C., Mu, X.-G., Liu, X.-L., Wang, L., Zhao, Y.-C., Zhang, P., Li, X.-D., & Zhang, X.-X. (2019). Antimicrobial Secondary Metabolites from the Seawater-Derived Fungus Aspergillus sydowii SW9. Molecules, 24(24), 4596. https://doi.org/10.3390/molecules24244596








