Antioxidant Activity Improvement of Apples Juice Supplemented with Chitosan-Galactose Maillard Reaction Products
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Galactose Concentration on the Extent of the Maillard Reaction
2.2. Color Measurement of CG-MRPs
2.3. FT-IR Analysis of CG-MRPs
2.4. Antioxidant Activities of CG-MRPs
2.4.1. DPPH Radical-Scavenging Activity
2.4.2. Chelating Effects on Ferrous Ions
2.4.3. Reducing Power Assay
3. Materials and Methods
3.1. Preparation of the Chitosan–Galactose Maillard Reaction
3.2. Extent of the Maillard Reaction
3.3. FT-IR Analysis
3.4. Antioxidant Activities
3.4.1. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical-Scavenging Activity
3.4.2. Chelating Ability on Ferrous Ions
3.4.3. Reducing Power
3.5. Antioxidant Activity of Apple Juice Containing MRPs
3.6. Statistical Analysis
4. Conclusions
Highlights
- The chitosan–galactose Maillard reaction products (CG-MRPs) were prepared.
- The extent of CG-MRPs increase along with increases of galactose concentration.
- All CG-MRPs showed better antioxidant activities than chitosan.
- CG-MRPs supplemented in apple juice improved significantly the antioxidant activity.
- The CG-MRPs might be useful for application as a food preservative.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kurita, K. Chemistry and application of chitin and chitosan. Polym. Degrad. Stab. 1998, 59, 117–120. [Google Scholar] [CrossRef]
- Hafsa, J.; Smach, M.A.; Charfeddine, B.; Limem, K.; Majdoub, H.; Rouatbi, S. Antioxidant and antimicrobial proprieties of chitin and chitosan extracted from Parapenaeus Longirostris shrimp shell waste. Ann. Pharm. Françaises 2016, 74, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, S. Competitive Biological Activities of Chitosan and Its Derivatives: Antimicrobial, Antioxidant, Anticancer, and Anti-Inflammatory Activities. Int. J. Polym. Sci. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Hafsa, J.; ali Smach, M.; Ben Khedher, M.R.; Charfeddine, B.; Limem, K.; Majdoub, H.; Rouatbi, S. Physical, antioxidant and antimicrobial properties of chitosan films containing Eucalyptus globulus essential oil. LWT Food Sci. Technol. 2016, 68, 356–364. [Google Scholar] [CrossRef]
- Zhu, K.-X.; Li, J.; Li, M.; Guo, X.-N.; Peng, W.; Zhou, H.-M. Functional properties of chitosan–xylose Maillard reaction products and their application to semi-dried noodle. Carbohydr. Polym. 2013, 92, 1972–1977. [Google Scholar] [CrossRef]
- Muzzarelli, R.; Muzzarelli, C. Chitosan as a Dietary Supplement and a Food Technology Agent. In Functional Food Carbohydrates; Biliaderis, C., Izydorczyk, M., Eds.; CRC Press: Orlando, FL, USA, 2006; Volume 10, pp. 215–248. [Google Scholar]
- Chung, Y.-C.; Yeh, J.-Y.; Tsai, C.-F. Antibacterial Characteristics and Activity of Water-Soluble Chitosan Derivatives Prepared by the Maillard Reaction. Molecules 2011, 16, 8504–8514. [Google Scholar] [CrossRef]
- Shelma, R.; Sharma, C.P. Acyl modified chitosan derivatives for oral delivery of insulin and curcumin. J. Mater. Sci. Mater. Med. 2010, 21, 2133–2140. [Google Scholar] [CrossRef]
- Kurita, Y.; Isogai, A. Reductive N-alkylation of chitosan with acetone and levulinic acid in aqueous media. Int. J. Biol. Macromol. 2010, 47, 184–189. [Google Scholar] [CrossRef]
- Gullón, B.; Montenegro, M.I.; Ruiz-Matute, A.I.; Cardelle-Cobas, A.; Corzo, N.; Pintado, M.E. Synthesis, optimization and structural characterization of a chitosan–glucose derivative obtained by the Maillard reaction. Carbohydr. Polym. 2016, 137, 382–389. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, J.; Zhao, Y. High intensity ultrasound assisted heating to improve solubility, antioxidant and antibacterial properties of chitosan-fructose Maillard reaction products. LWT Food Sci. Technol. 2015, 60, 253–262. [Google Scholar] [CrossRef]
- Hodge, J.E. Dehydrated Foods, Chemistry of Browning Reactions in Model Systems. J. Agric. Food Chem. 1953, 1, 928–943. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R. Antioxidant activity of water-soluble Maillard reaction products. Food Chem. 2005, 93, 273–278. [Google Scholar] [CrossRef]
- Amarowicz, R. Antioxidant activity of Maillard reaction products. Eur. J. Lipid Sci. Technol. 2009, 111, 109–111. [Google Scholar] [CrossRef]
- Bakry, A.M.; Ma, C.; Xiong, S.; Yin, T.; Zhang, B.; Huang, Q. Chitosan-glucose Maillard reaction products and their preservative effects on fresh grass carp (Ctenopharyngodon idellus) fillets during cold storage: Maillard reaction products and their preservative effects on fresh grass carp. J. Sci. Food Agric. 2019, 99, 2158–2164. [Google Scholar] [CrossRef] [PubMed]
- Chien, P.-J.; Sheu, F.; Huang, W.-T.; Su, M.-S. Effect of molecular weight of chitosans on their antioxidative activities in apple juice. Food Chem. 2007, 102, 1192–1198. [Google Scholar] [CrossRef]
- Roller, S.; Covill, N. The antifungal properties of chitosan in laboratory media and apple juice. Int. J. Food Microbiol. 1999, 47, 67–77. [Google Scholar] [CrossRef]
- Lee, B.; Seo, J.D.; Rhee, J.-K.; Kim, C.Y. Heated apple juice supplemented with onion has greatly improved nutritional quality and browning index. Food Chem. 2016, 201, 315–319. [Google Scholar] [CrossRef]
- Ajandouz, E.H.; Tchiakpe, L.S.; Ore, F.D.; Benajiba, A.; Puigserver, A. Effects of pH on Caramelization and Maillard Reaction Kinetics in Fructose-Lysine Model Systems. J. Food Sci. 2001, 66, 926–931. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Chander, R.; Sharma, A. Chitosan glucose complex—A novel food preservative. Food Chem. 2008, 106, 521–528. [Google Scholar] [CrossRef]
- Kosaraju, S.L.; Weerakkody, R.; Augustin, M.A. Chitosan−Glucose Conjugates: Influence of Extent of Maillard Reaction on Antioxidant Properties. J. Agric. Food Chem. 2010, 58, 12449–12455. [Google Scholar] [CrossRef]
- Matiacevich, S.B.; Santagapita, P.R.; Buera, M.P. Fluorescence from the Maillard Reaction and its Potential Applications in Food Science. Crit. Rev. Food Sci. Nutr. 2005, 45, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-C.; Kuo, C.-L.; Chen, C.-C. Preparation and important functional properties of water-soluble chitosan produced through Maillard reaction. Bioresour. Technol. 2005, 96, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Kogan, G.; Skorik, Y.; Itanova, I.; Krikova, L.; Urakova, Z.; Gomes, C.; Yatluk, Y.; Krajovi, J. Antioxidant and antimutagenic activity of -(2-carboxyethyl)chitosan. Toxicol. Appl. Pharmacol. 2004, 201, 303–310. [Google Scholar] [CrossRef]
- Paulino, A.T.; Simionato, J.I.; Garcia, J.C.; Nozaki, J. Characterization of chitosan and chitin produced from silkworm crysalides. Carbohydr. Polym. 2006, 64, 98–103. [Google Scholar] [CrossRef]
- Huang, X.; Tu, Z.; Xiao, H.; Wang, H.; Zhang, L.; Hu, Y.; Zhang, Q.; Niu, P. Characteristics and antioxidant activities of ovalbumin glycated with different saccharides under heat moisture treatment. Food Res. Int. 2012, 48, 866–872. [Google Scholar] [CrossRef]
- Guo, M.; Ma, Y.; Wang, C.; Liu, H.; Li, Q.; Fei, M. Synthesis, anti-oxidant activity, and biodegradability of a novel recombinant polysaccharide derived from chitosan and lactose. Carbohydr. Polym. 2015, 118, 218–223. [Google Scholar] [CrossRef]
- Yamauchi, R.; Tatsumi, Y.; Asano, M.; Kato, K.; Ueno, Y. Effect of Metal Salts and Fructose on the Autoxidation of Methyl Linoleate in Emulsions. Agric. Biol. Chem. 1988, 52, 849–850. [Google Scholar]
- Gülçin, İ. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef]
- Ying, G.; Xiong, W.; Wang, H.; Sun, Y.; Liu, H. Preparation, water solubility and antioxidant activity of branched-chain chitosan derivatives. Carbohydr. Polym. 2011, 83, 1787–1796. [Google Scholar] [CrossRef]
- Silván, J.M.; van de Lagemaat, J.; Olano, A.; del Castillo, M.D. Analysis and biological properties of amino acid derivates formed by Maillard reaction in foods. J. Pharm. Biomed. Anal. 2006, 41, 1543–1551. [Google Scholar] [CrossRef]
- Morales, F.J.; Jiménez-Pérez, S. Free radical scavenging capacity of Maillard reaction products as related to colour and fluorescence. Food Chem. 2001, 72, 119–125. [Google Scholar] [CrossRef]
- Song, Y.; Babiker, E.E.; Usui, M.; Saito, A.; Kato, A. Emulsifying properties and bactericidal action of chitosan–lysozyme conjugates. Food Res. Int. 2002, 35, 459–466. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, C.; Wang, J.; Wang, M.; Wu, Y.; Ruan, Y. Determination of degree of substitution for N-acylated chitosan using IR spectra. Sci. China Ser. B Chem. 2001, 44, 216–224. [Google Scholar] [CrossRef]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, L.M. Action of Phenolic Derivatives (Acetaminophen, Salicylate, and 5-Aminosalicylate) as Inhibitors of Membrane Lipid Peroxidation and as Peroxyl Radical Scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
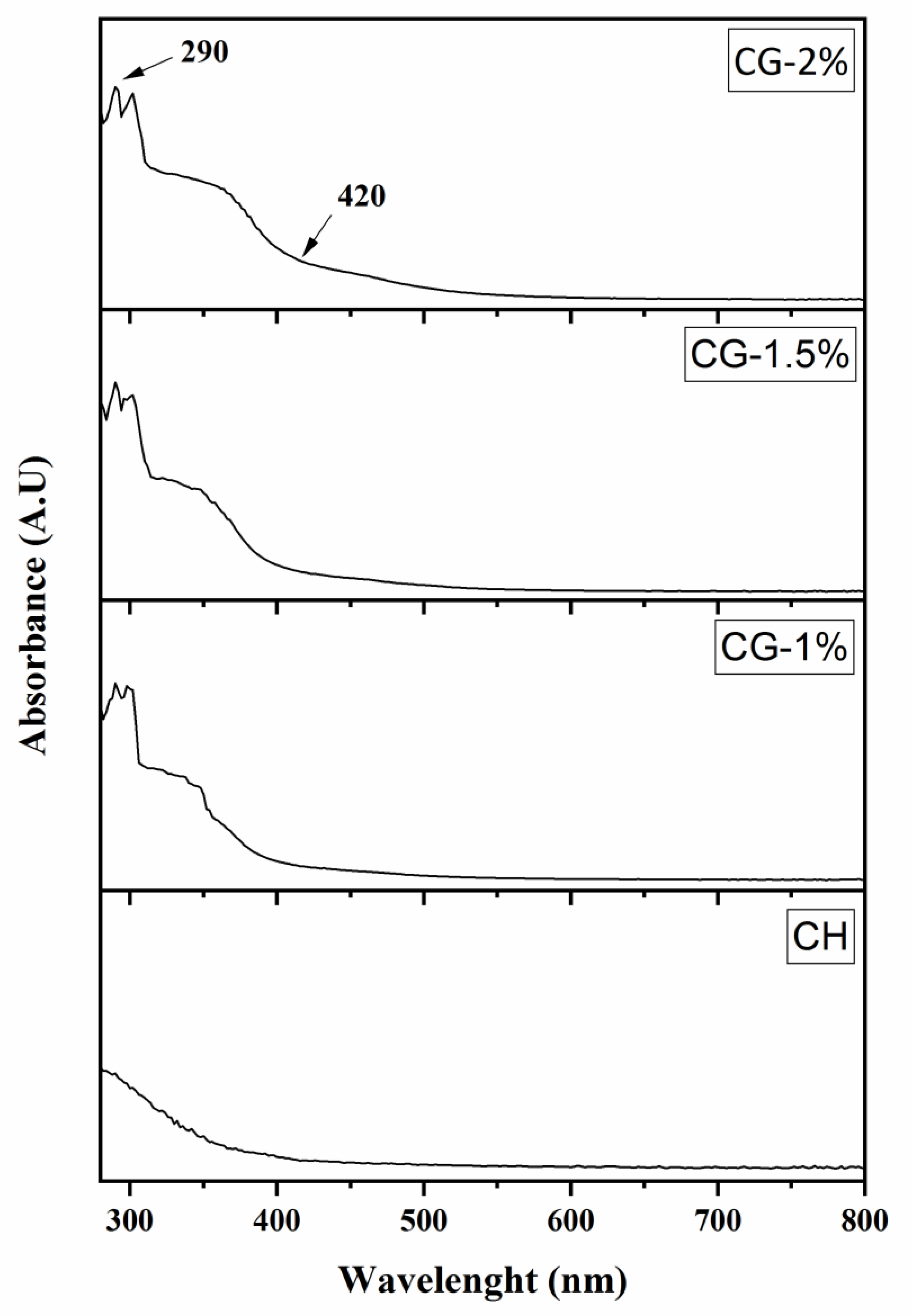

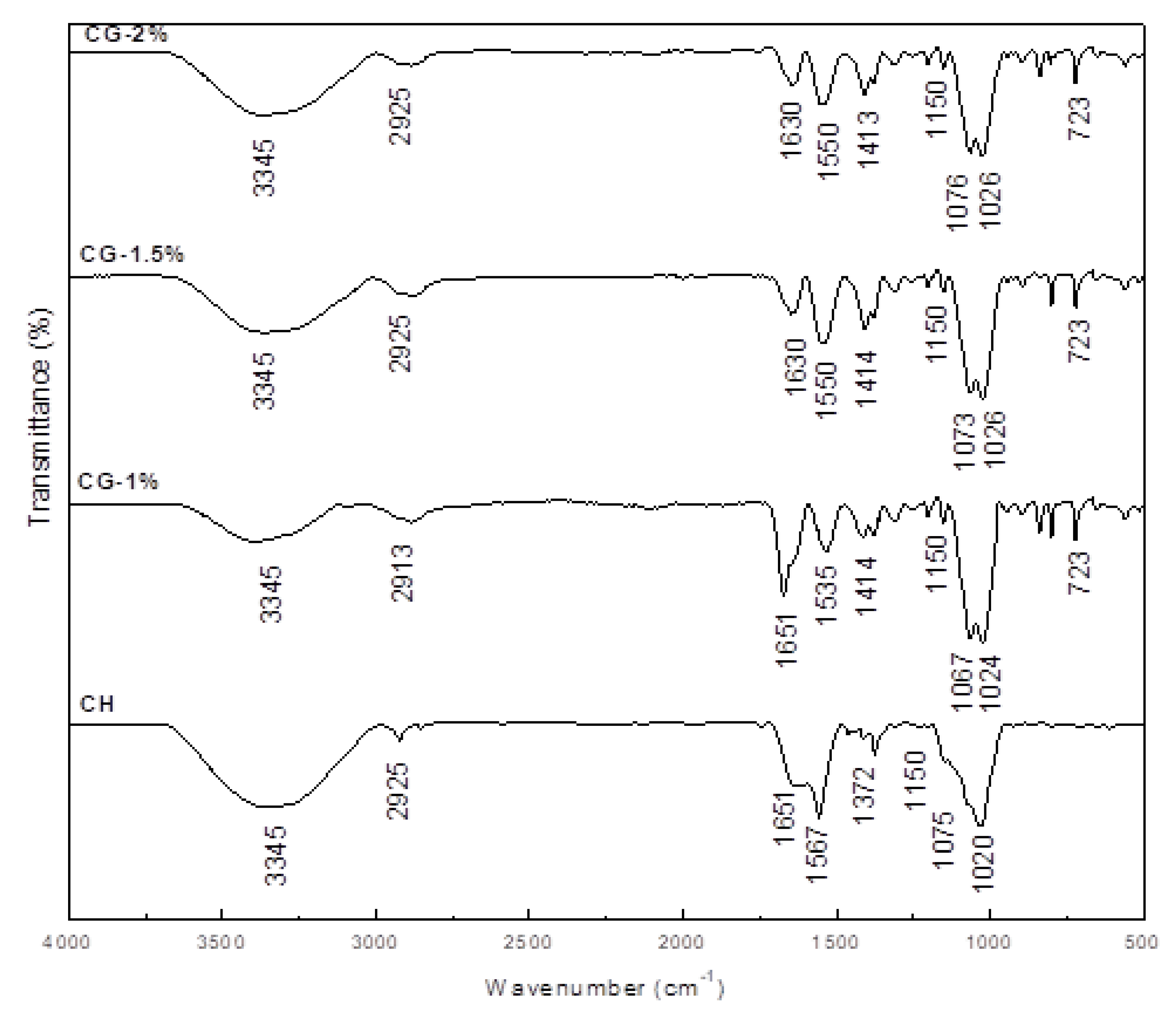
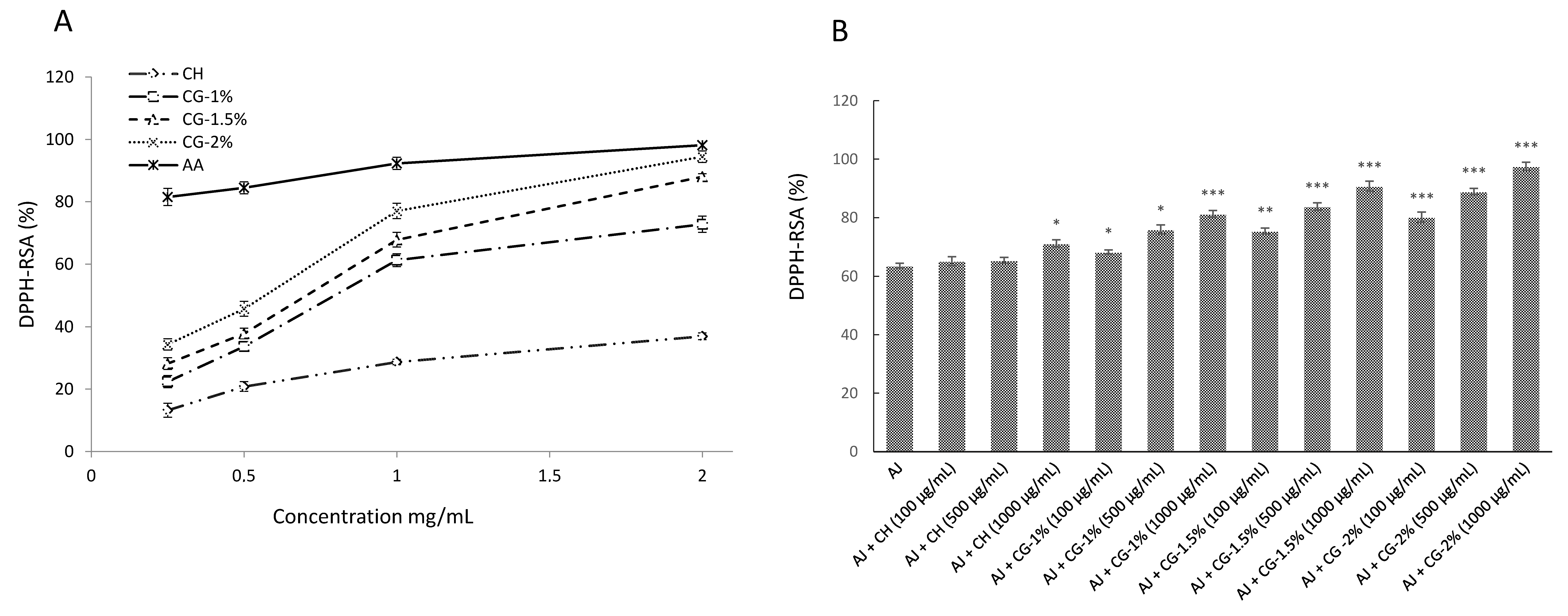
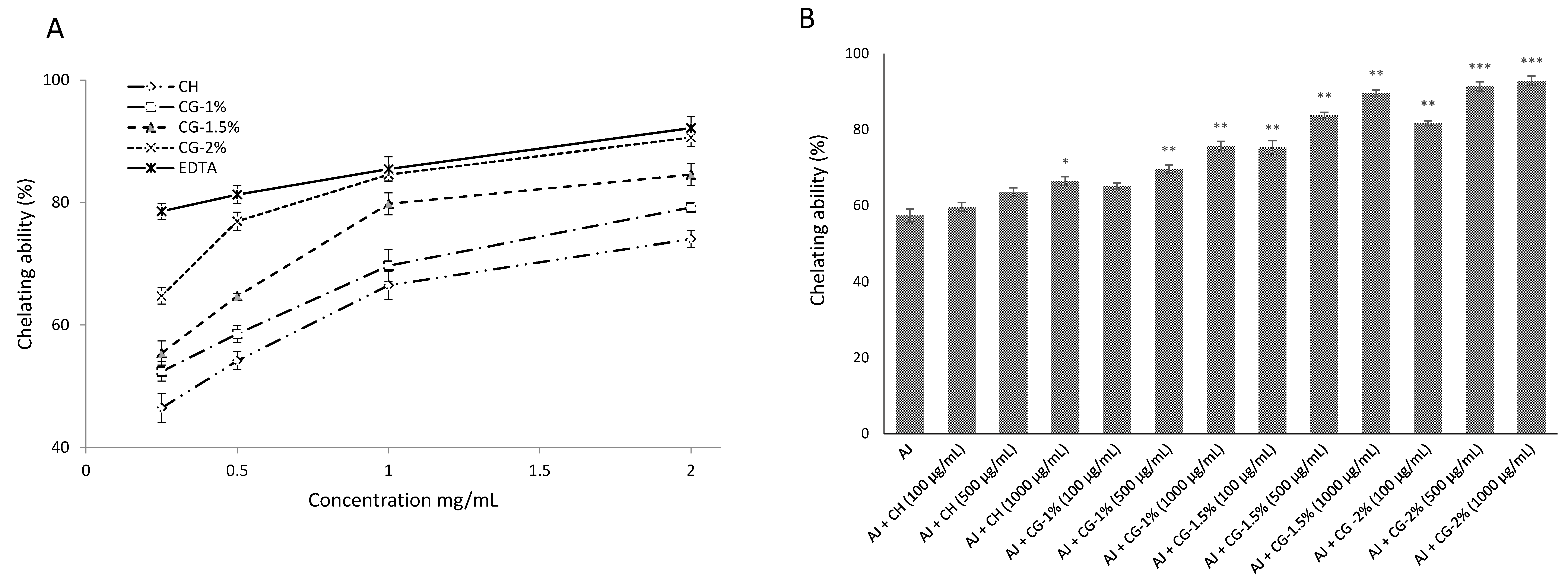

| Treatment Group a | DDA (%) | L* | a* | b* | ΔE |
|---|---|---|---|---|---|
| CH | 79.3 | 89.81 ± 0.17 a | −0.24 ± 0.05 a | 0.10 ± 0.04 a | - |
| CG-1% | 65.6 | 85.36 ± 0.14 b | −0.35 ± 0.11 b | 0.75 ± 0.05 b | 5.46 ± 0.21 a |
| CG-1.5% | 56.7 | 85.04 ± 0.07 c | −0.36 ± 0.10 b | 0.92 ± 0.04 c | 6.09 ± 0.10 b |
| CG-2% | 48.0 | 83.95 ± 0.07 d | −0.38 ± 0.01 c | 1.11 ± 0.06 d | 7.59 ± 0.07 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafsa, J.; Smach, M.a.; Sobeh, M.; Majdoub, H.; Yasri, A. Antioxidant Activity Improvement of Apples Juice Supplemented with Chitosan-Galactose Maillard Reaction Products. Molecules 2019, 24, 4557. https://doi.org/10.3390/molecules24244557
Hafsa J, Smach Ma, Sobeh M, Majdoub H, Yasri A. Antioxidant Activity Improvement of Apples Juice Supplemented with Chitosan-Galactose Maillard Reaction Products. Molecules. 2019; 24(24):4557. https://doi.org/10.3390/molecules24244557
Chicago/Turabian StyleHafsa, Jawhar, Mohamed ali Smach, Mansour Sobeh, Hatem Majdoub, and Aziz Yasri. 2019. "Antioxidant Activity Improvement of Apples Juice Supplemented with Chitosan-Galactose Maillard Reaction Products" Molecules 24, no. 24: 4557. https://doi.org/10.3390/molecules24244557
APA StyleHafsa, J., Smach, M. a., Sobeh, M., Majdoub, H., & Yasri, A. (2019). Antioxidant Activity Improvement of Apples Juice Supplemented with Chitosan-Galactose Maillard Reaction Products. Molecules, 24(24), 4557. https://doi.org/10.3390/molecules24244557






