Purine Catabolism Shows a Dampened Circadian Rhythmicity in a High-fat Diet-Induced Mouse Model of Obesity
Abstract
1. Introduction
2. Results
2.1. Feeding a High Fat Diet Induced Obesity in Mice
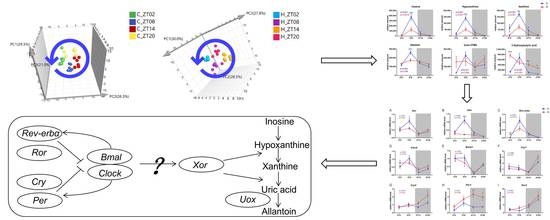
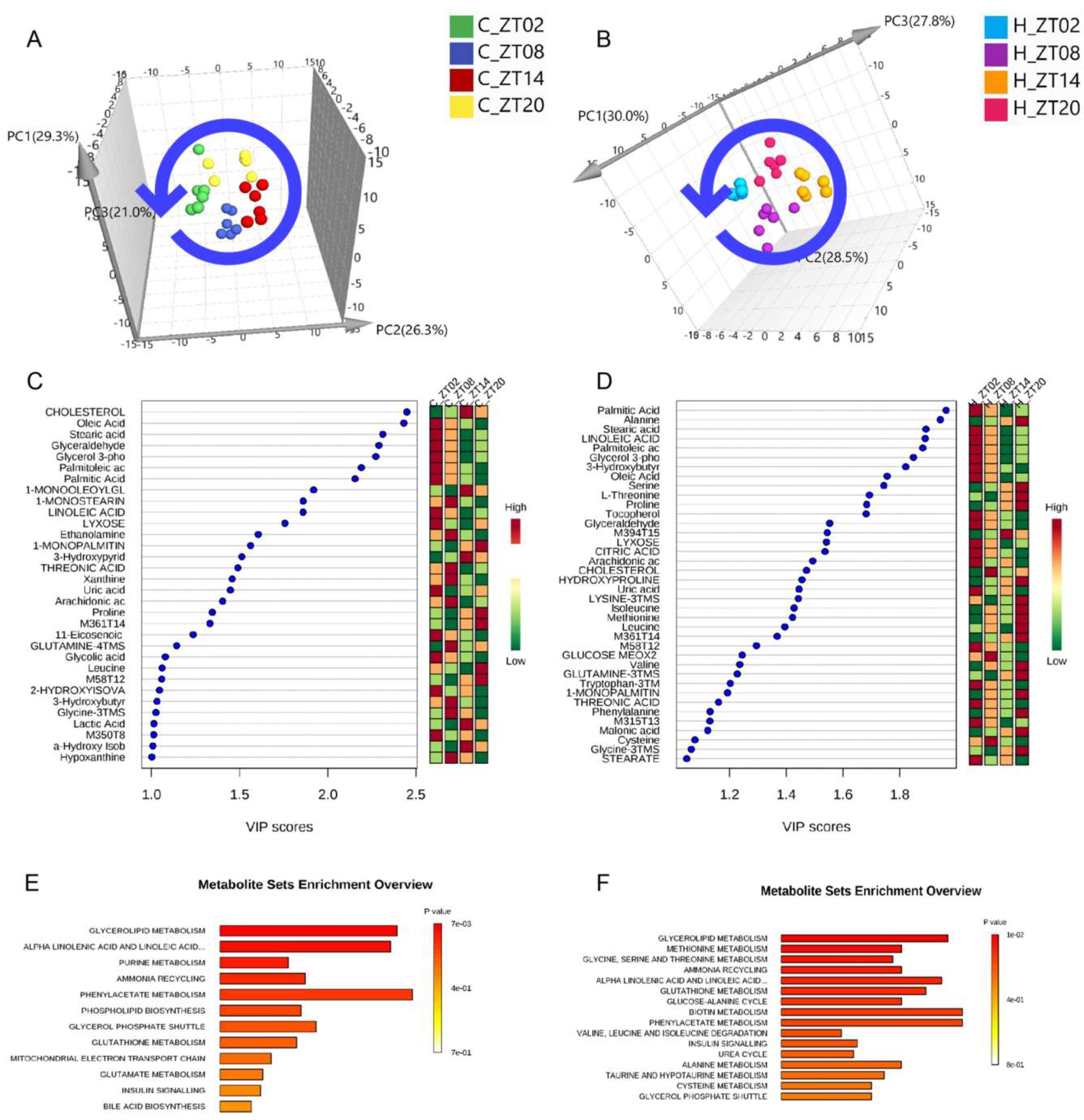
2.2. Obesity Induced Significant Changes in the Circadian Serum Metabolome
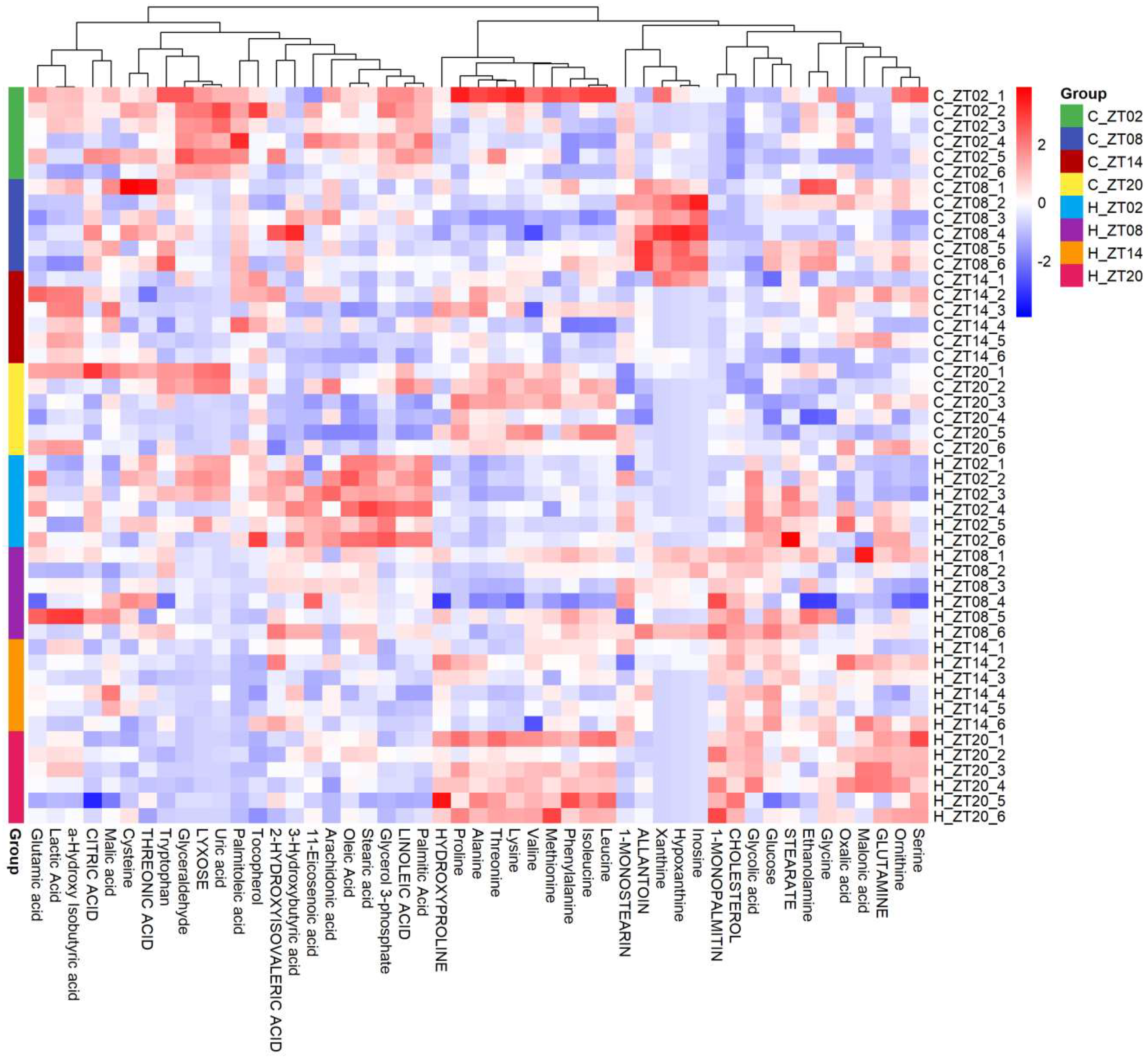
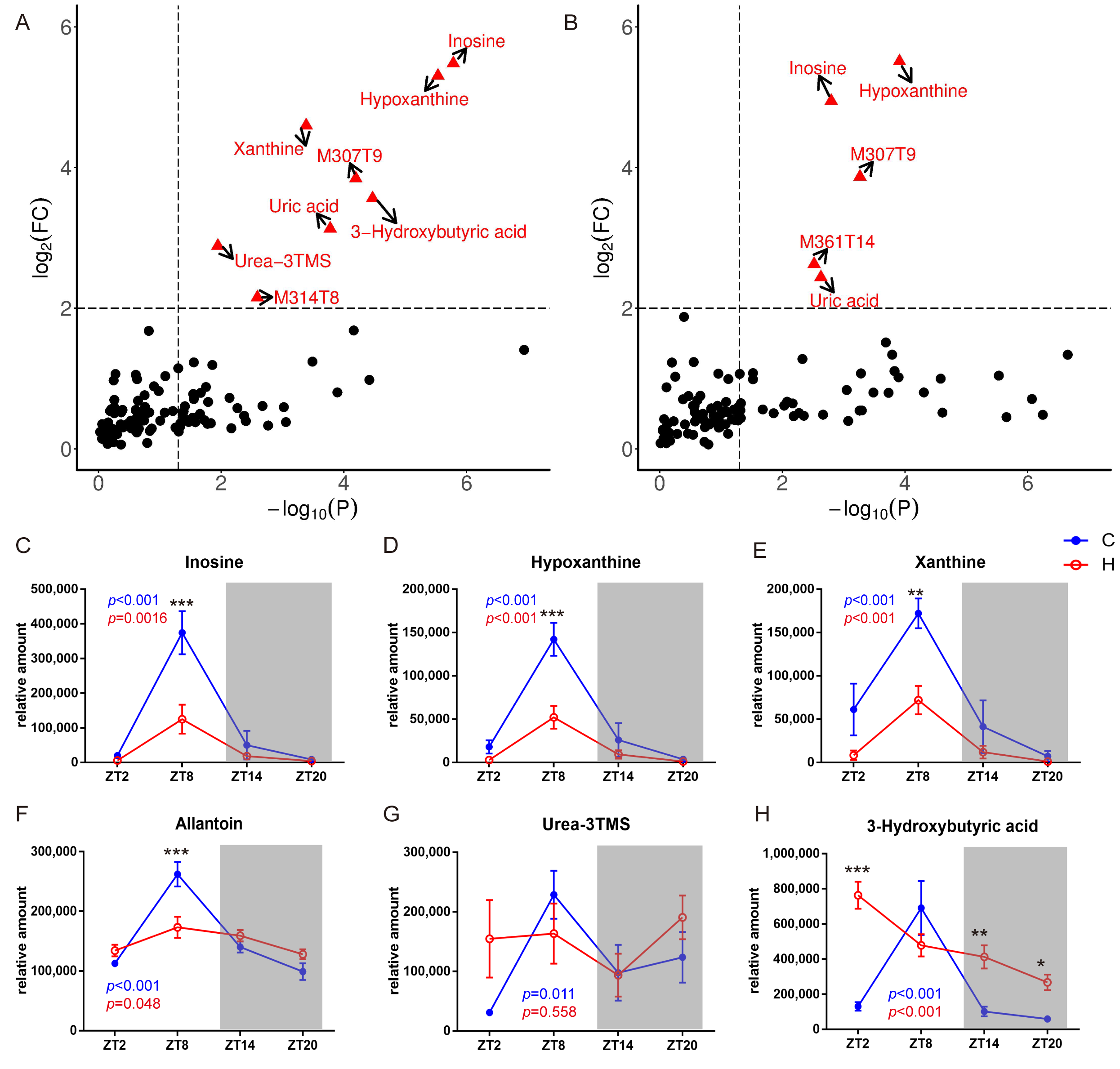
2.3. Metabolic Biomarkers Affected by Circadian Rhythms in Normal Mice and High-Fat Diet-Induced Obese Mice
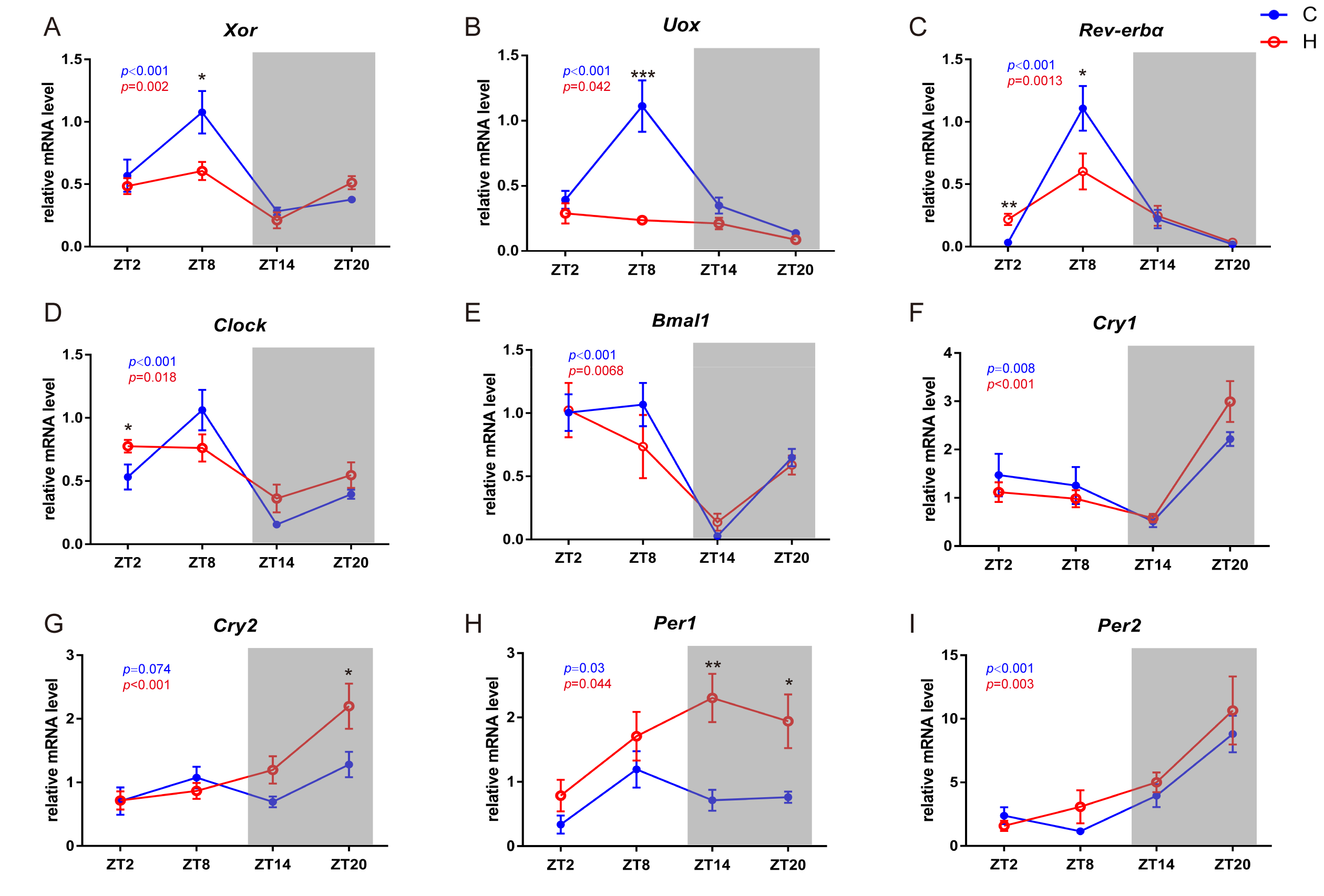
2.4. Remodelling Metabolic Circadian Rhythms by High-Fat Diet-Induced Obesity
2.5. Circadian-Related Expression of Xanthine Oxidase and Uricase
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animal Studies
4.3. Measurement of Serum Glucose and Cholesterol
4.4. Sample Preparation and GC/MS Analysis
4.5. Compound Identification and Statistical Analysis
4.6. qRT-PCR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bernard, S.; Gonze, D.; Čajavec, B.; Herzel, H.; Kramer, A. Synchronization-Induced Rhythmicity of Circadian Oscillators in the Suprachiasmatic Nucleus. PLoS Comput. Biol. 2007, 3, e68. [Google Scholar] [CrossRef] [PubMed]
- Tischkau, S.A.; Mitchell, J.W.; Tyan, S.H.; Buchanan, G.F.; Gillette, M.U. CREB-dependent activation of Per1 is required for light-induced signaling in the Suprachiasmatic Nucleus Circadian clock. J. Biol. Chem. 2002, 278, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, C.; Van, D.S.L.; Edlund, K.; Lohr, M.; Hellwig, B.; Marchan, R.; Schmidt, M.; Rahnenführer, J.; Oster, H.; Hengstler, J.G. Loss of circadian clock gene expression is associated with tumor progression in breast cancer. Cell Cycle 2014, 13, 3282–3291. [Google Scholar] [CrossRef] [PubMed]
- Cermakian, N.; Lange, T.; Golombek, D.; Sarkar, D.; Nakao, A.; Shibata, S.; Mazzoccoli, G. Crosstalk between the circadian clock circuitry and the immune system. Chronobiol. Int. 2013, 30, 870–888. [Google Scholar] [CrossRef] [PubMed]
- Naylor, E.; Bergmann, B.M.; Krauski, K.; Zee, P.C.; Takahashi, J.S.; Vitaterna, M.H.; Turek, F.W. The circadian clock mutation alters sleep homeostasis in the mouse. J. Neurosci. 2000, 20, 8138–8143. [Google Scholar] [CrossRef] [PubMed]
- Sadacca, L.A.; Lamia, K.A.; Delemos, A.S.; Blum, B.; Weitz, C.J. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia 2011, 54, 120–124. [Google Scholar] [CrossRef]
- Masri, S.; Sassone-Corsi, P. The emerging link between cancer, metabolism, and circadian rhythms. Nat. Med. 2018, 24, 1795. [Google Scholar] [CrossRef]
- Poggiogalle, E.; Jamshed, H.; Peterson, C.M. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism 2018, 84, 11–27. [Google Scholar] [CrossRef]
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Losee-Olson, S.; Easton, A.; Jensen, D.R. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005, 308, 1043–1045. [Google Scholar] [CrossRef]
- He, B.; Nohara, K.; Park, N.; Park, Y.S.; Guillory, B.; Zhao, Z.; Garcia, J.M.; Koike, N.; Lee, C.C.; Takahashi, J.S. The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab. 2016, 23, 610–621. [Google Scholar] [CrossRef]
- Sitaula, S.; Billon, C.; Kamenecka, T.M.; Solt, L.A.; Burris, T.P. Suppression of atherosclerosis by synthetic REV-ERB agonist. Biochem. Biophys. Res. Commun. 2015, 460, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Solt, L.A.; Wang, Y.; Banerjee, S.; Hughes, T.; Kojetin, D.J.; Lundasen, T.; Shin, Y.; Liu, J.; Cameron, M.D.; Noel, R. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 2012, 485, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Stephan, F.K.; Davidson, A.J. Glucose, but not fat, phase shifts the feeding-entrained circadian clock. Physiol. Behav. 1998, 65, 277–288. [Google Scholar] [PubMed]
- Stokkan, K.A.; Yamazaki, S.; Tei, H.; Sakaki, Y.; Menaker, M. Entrainment of the circadian clock in the liver by feeding. Science 2001, 291, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Kruszon-Moran, D.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Trends in obesity among adults in the United States, 2005 to 2014. Jama 2016, 315, 2284–2291. [Google Scholar]
- Froy, O. Metabolism and circadian rhythms—implications for obesity. Endocr. Rev. 2010, 31, 1–24. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Lindon, J.C.; Holmes, E. Metabonomics: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 1999, 29, 1181–1189. [Google Scholar]
- Dallmann, R.; Viola, A.U.; Tarokh, L.; Cajochen, C.; Brown, S.A. The human circadian metabolome. Off. Sci. Tech. Inf. Tech. Rep. 2000, 33, 787–791. [Google Scholar]
- Abbondante, S.; Eckelmahan, K.L.; Ceglia, N.J.; Baldi, P.; Sassonecorsi, P. Comparative Circadian Metabolomics reveal Differential Effects of Nutritional Challenge in the Serum and Liver. J. Biol. Chem. 2015, 291, 2812–2828. [Google Scholar] [CrossRef]
- Asher, G.; Schibler, U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab. 2011, 13, 125–137. [Google Scholar]
- Dyar, K.A.; Lutter, D.; Artati, A.; Ceglia, N.J.; Liu, Y.; Armenta, D.; Jastroch, M.; Schneider, S.; de Mateo, S.; Cervantes, M.; et al. Atlas of Circadian Metabolism Reveals System-wide Coordination and Communication between Clocks. Cell 2018, 174, 1571–1585. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Ushijima, K.; Shimba, S.; Fujimura, A. Daily fasting blood glucose rhythm in male mice: A role of the circadian clock in the liver. Endocrinology 2016, 157, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Hardin, P.E.; Hall, J.C.; Rosbash, M. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature 1990, 343, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Young, M.W.; Kay, S.A. Time zones: A comparative genetics of circadian clocks. Nat. Rev. Genet. 2001, 2, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Aa, J.; Feng, S.; Wang, G.; Huang, Q.; Zha, W.; Yan, B.; Zheng, T.; Liu, L.; Cao, B.; Shi, J. Gas chromatography time-of-flight mass spectrometry based metabolomic approach to evaluating toxicity of triptolide. Metabolomics 2011, 7, 217–225. [Google Scholar] [CrossRef]
- Altmaier, E.; Fobo, G.; Heier, M.; Thorand, B.; Meisinger, C.; Römisch-Margl, W.; Waldenberger, M.; Gieger, C.; Illig, T.; Adamski, J. Metabolomics approach reveals effects of antihypertensives and lipid-lowering drugs on the human metabolism. Eur. J. Epidemiol. 2014, 29, 325–336. [Google Scholar] [CrossRef]
- Jänis, M.T.; Laaksonen, R.; Oresic, M. Metabolomic strategies to identify tissue-specific effects of cardiovascular drugs. Expert Opin. Drug Metab. Toxicol. 2008, 4, 665–680. [Google Scholar] [CrossRef]
- Liu, L.; Cao, B.; Aa, J.; Zheng, T.; Jian, S.; Li, M.; Wang, X.; Zhao, C.; Xiao, W.; Yu, X. Prediction of the pharmacokinetic parameters of triptolide in rats based on endogenous molecules in pre-dose baseline serum. PLoS ONE 2012, 7, e43389. [Google Scholar] [CrossRef]
- Milburn, M.V.; Lawton, K.A. Application of metabolomics to diagnosis of insulin resistance. Annu. Rev. Med. 2013, 64, 291–305. [Google Scholar] [CrossRef]
- Tiziani, S.; Lopes, V.; Günther, U.L. Early Stage Diagnosis of Oral Cancer Using 1 H NMR–Based Metabolomics 1 2. Neoplasia 2009, 11, 269–276. [Google Scholar] [CrossRef]
- Minami, Y.; Kasukawa, T.; Kakazu, Y.; Iigo, M.; Sugimoto, M.; Ikeda, S.; Ueda, H.R. Measurement of internal body time by blood metabolomics. Proc. Natl. Acad. Sci. USA 2009, 106, 9890–9895. [Google Scholar] [CrossRef] [PubMed]
- Adamovich, Y.; Rousso-Noori, L.; Zwighaft, Z.; Neufeld-Cohen, A.; Golik, M.; Kraut-Cohen, J.; Wang, M.; Han, X.; Asher, G. Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides. Cell Metab. 2014, 19, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Oishi, K.; Yamamoto, S.; Itoh, N.; Miyazaki, K.; Nemoto, T.; Nakakita, Y.; Kaneda, H. Disruption of behavioral circadian rhythms induced by psychophysiological stress affects plasma free amino acid profiles without affecting peripheral clock gene expression in mice. Biochem. Biophys. Res. Commun. 2014, 450, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Adeva, M.M.; Calviño, J.; Souto, G.; Donapetry, C. Insulin resistance and the metabolism of branched-chain amino acids in humans. Amino Acids 2012, 43, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Ramsey, K.M.; Marcheva, B.; Bass, J. Circadian rhythms, sleep, and metabolism. J. Clin. Investig. 2011, 121, 2133. [Google Scholar] [CrossRef]
- Pochini, L.; Scalise, M.; Galluccio, M.; Indiveri, C. Membrane transporters for the special amino acid glutamine: Structure/function relationships and relevance to human health. Front. Chem. 2014, 2, 61. [Google Scholar] [CrossRef]
- Amores-Sánchez, M.A.I.; Medina, M.Á. Glutamine, as a precursor of glutathione, and oxidative stress. Mol. Genet. Metab. 1999, 67, 100–105. [Google Scholar] [CrossRef]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef]
- Seegmiller, J.E.; Laster, L.; Howell, R.R. Biochemistry of uric acid and its relation to gout. N. Engl. J. Med. 1963, 268, 764–773. [Google Scholar] [CrossRef]
- Fang, J.; Alderman, M.H. Serum Uric Acid and Cardiovascular MortalityThe NHANES I Epidemiologic Follow-up Study, 1971–1992. Jama 2000, 283, 2404–2410. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Li, C.; Cook, S.; Choi, H.K. Serum Concentrations of Uric Acid and the Metabolic Syndrome Among US Children and Adolescents. Circulation 2007, 115, 2526–2532. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Hu, H.; Zharikov, S.; Tuttle, K.R.; Short, R.A.; Glushakova, O.; Ouyang, X.; Feig, D.I.; Block, E.R.; Herrera-Acosta, J. A causal role for uric acid in fructose-induced metabolic syndrome. Am. J. Physiol. Ren. Physiol. 2006, 290, F625–F631. [Google Scholar] [CrossRef] [PubMed]
- Onat, A.; Uyarel, H.; Hergenç, G.; Karabulut, A.; Albayrak, S.; Sarı, I.; Yazıcı, M.; Keleş, I. Serum Uric Acid Is a Determinant of Metabolic Syndrome in a Population-Based Study. Am. J. Hypertens. 2006, 19, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Kanabrocki, E.L.; Third, J.L.; Ryan, M.D.; Nemchausky, B.A.; Shirazi, P.; Scheving, L.E.; Mccormick, J.B.; Hermida, R.C.; Bremner, W.F.; Hoppensteadt, D.A. Circadian relationship of serum uric acid and nitric oxide. Jama 2000, 283, 2240–2241. [Google Scholar] [CrossRef]
- De Sa, V.C.; Herna, R.; Sua, J.; Vidrio, S.; Di, M. Day-night variations of adenosine and its metabolizing enzymes in the brain cortex of the rat—possible physiological significance for the energetic homeostasis and the sleep-wake cycle. Brain Res. 1993, 612, 115–121. [Google Scholar]
- Deng, T.S.; Roenneberg, T. The flavo-enzyme xanthine oxidase is under circadian control in the marine alga Gonyaulax. Sci. Nat. 2002, 89, 171–175. [Google Scholar] [CrossRef]
- Panda, S. The arrival of circadian medicine. Nat. Rev. Endocrinol. 2019, 15, 67. [Google Scholar] [CrossRef]
- Jiye, A.; Trygg, J.; Gullberg, J.; Johansson, A.I.; Jonsson, P.; Antti, H.; Moritz, T. Extraction and GC/MS analysis of the human blood plasma metabolome. Anal. Chem. 2005, 77, 8086–8094. [Google Scholar]
- Aa, J.Y.; Wang, G.J.; Hao, H.P.; Huang, Q.; Lu, Y.H.; Yan, B.; Zha, W.B.; Liu, L.S.; Kang, A. Differential regulations of blood pressure and perturbed metabolism by total ginsenosides and conventional antihypertensive agents in spontaneously hypertensive rats. Acta Pharmacol. Sin. 2010, 31, 930–937. [Google Scholar] [CrossRef]
- Xia, J.; Sinelnikov, I.V.; Han, B.; Wishart, D.S. MetaboAnalyst 3.0—Making metabolomics more meaningful. Nucleic Acids Res. 2015, 43, W251–W257. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: The samples and datasets used and analyzed during the current study are available from the corresponding author on reasonable request. |


| AIN-93M | High-Fat Diet | |
|---|---|---|
| kcal/% | kcal/% | |
| Protein | 15 | 16 |
| Carbohydrate | 76 | 25 |
| Fat | 9 | 59 |
| Total | 100 | 100 |
| Kcal/gm | ||
| kcal | kcal | |
| Casein | 560 | 793 |
| Corn starch | 1983 | 0 |
| Maltodextrin | 500 | 880 |
| Sucrose | 400 | 363 |
| Soybean oil | 360 | 306 |
| Lard | 0 | 2757 |
| Cellulose | 0 | 0 |
| Mineral mix | 0 | 0 |
| Vitamin mix | 40 | 57 |
| L-cystine | 7 | 0 |
| Choline bitartrate | 0 | 0 |
| t-Butylhydroquinone | 0 | 0 |
| Cholesterol | 0 | 0 |
| Total | 3850 | 5156 |
| Primer | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|
| GAPDH | TGACGTGCCGCCTGGAGAAA | AGTGTAGCCCAAGATGCCCTTCAG |
| Xor | AAAGGACCAGACGATTGCTCC | TCACACGTTCCCCTTCAAAAC |
| Uox | GGCCCTATGACAAAGGTGAA | GCAGCAAAACCTCTTCCTTG |
| Bmal1 | GGACTTCGCCTCTACCTGTTCA | AACCATGTGCGAGTGCAGGCGC |
| Clock | CACTCTCACAGCCCCACTGTAC | CCCCACAAGCTACAGGAGCAGT |
| Rev-erbα | CTACTGGCTCCCTCACCCAGGA | GACACTCGGCTGCTGTCTTCCA |
| Cry1 | AGCGCAGGTGTCGGTTATGAGC | ATAGACGCAGCGGATGGTGTCG |
| Cry2 | AAGAAGCCCGCGGTGGCTGTGA | CCGTTCCAAGTGCTTGTCCAGG |
| Per1 | AACGGGATGTGTTTCGGGGTGC | AGGACCTCCTCTGATTCGGCAG |
| Per2 | TGATCGAGACGCCTGTGCTCGT | CTCCACGGGTTGATGAAGCTGG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, R.; Huang, J.; Yang, N.; He, J.; Yu, X.; Feng, S.; Xie, Y.; Wang, G.; Ye, H.; Aa, J. Purine Catabolism Shows a Dampened Circadian Rhythmicity in a High-fat Diet-Induced Mouse Model of Obesity. Molecules 2019, 24, 4524. https://doi.org/10.3390/molecules24244524
Sun R, Huang J, Yang N, He J, Yu X, Feng S, Xie Y, Wang G, Ye H, Aa J. Purine Catabolism Shows a Dampened Circadian Rhythmicity in a High-fat Diet-Induced Mouse Model of Obesity. Molecules. 2019; 24(24):4524. https://doi.org/10.3390/molecules24244524
Chicago/Turabian StyleSun, Runbin, Jingqiu Huang, Na Yang, Jun He, Xiaoyi Yu, Siqi Feng, Yuan Xie, Guangji Wang, Hui Ye, and Jiye Aa. 2019. "Purine Catabolism Shows a Dampened Circadian Rhythmicity in a High-fat Diet-Induced Mouse Model of Obesity" Molecules 24, no. 24: 4524. https://doi.org/10.3390/molecules24244524
APA StyleSun, R., Huang, J., Yang, N., He, J., Yu, X., Feng, S., Xie, Y., Wang, G., Ye, H., & Aa, J. (2019). Purine Catabolism Shows a Dampened Circadian Rhythmicity in a High-fat Diet-Induced Mouse Model of Obesity. Molecules, 24(24), 4524. https://doi.org/10.3390/molecules24244524