Preparation and Characterisation of Polyphenol-HP-β-Cyclodextrin Inclusion Complex that Protects Lamb Tripe Protein against Oxidation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Encapsulation Efficiency and Loading Capacity
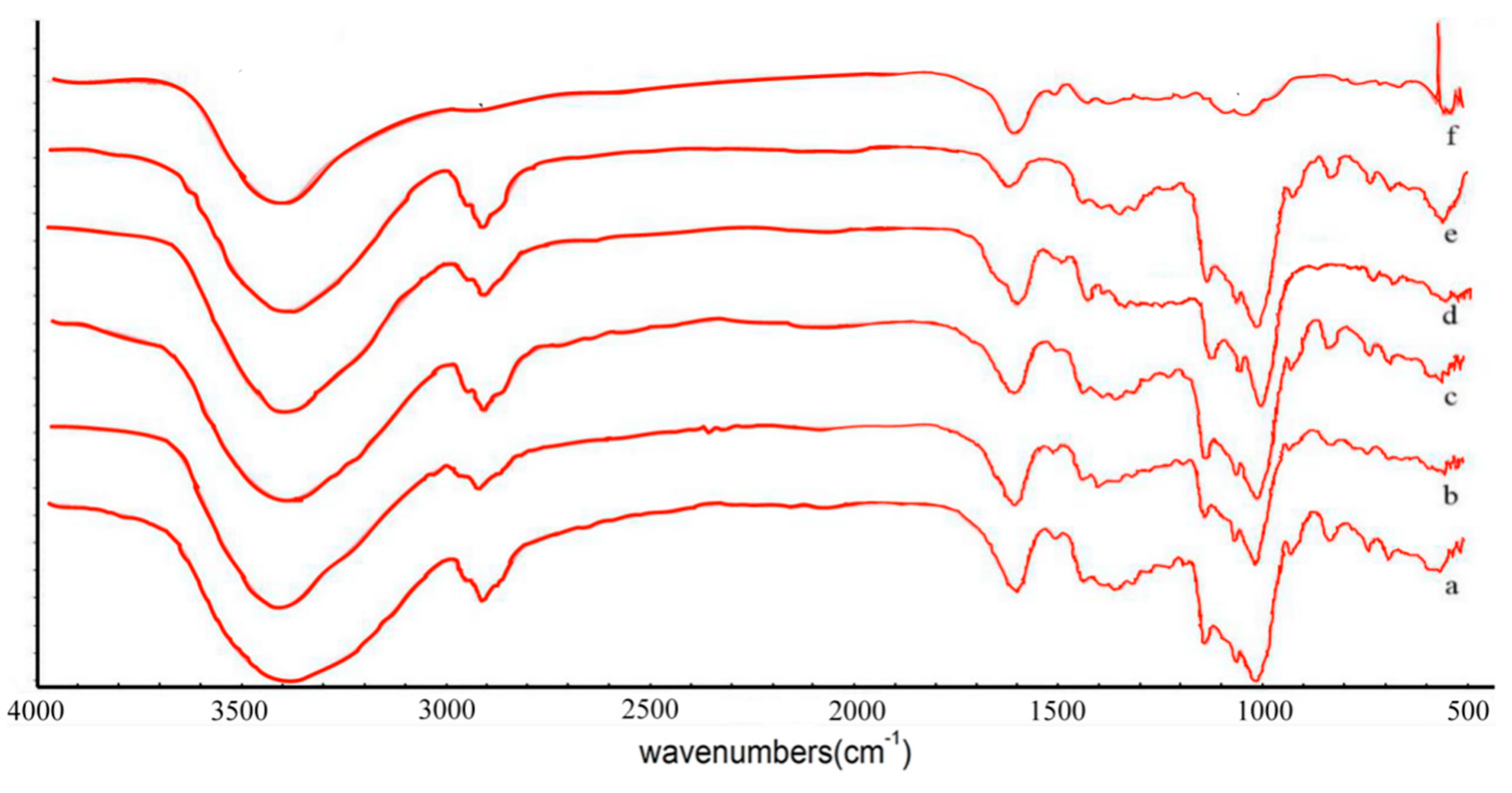
2.2. FTIR Spectra
2.3. XRD Analysis
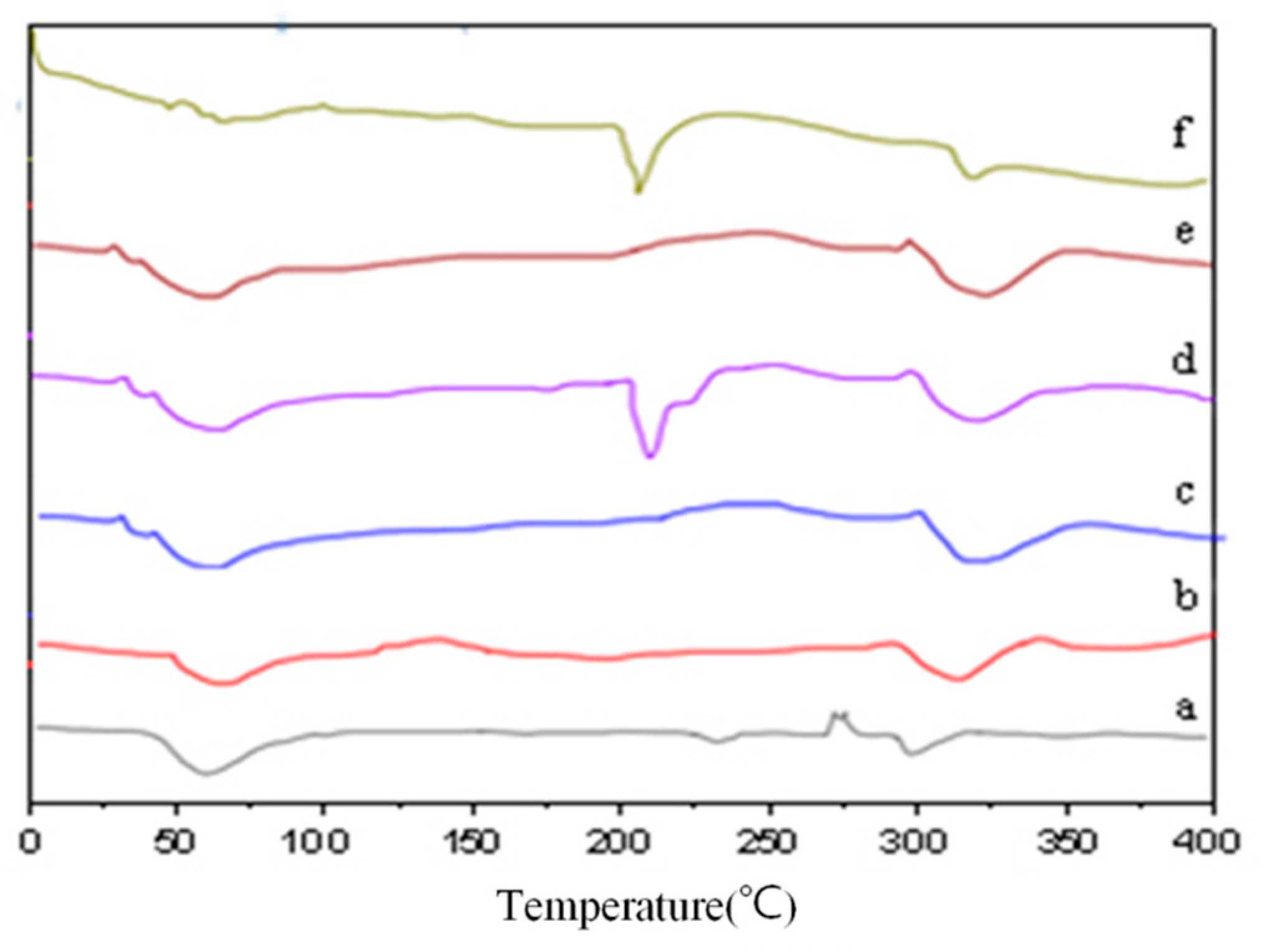
2.4. DSC Analysis
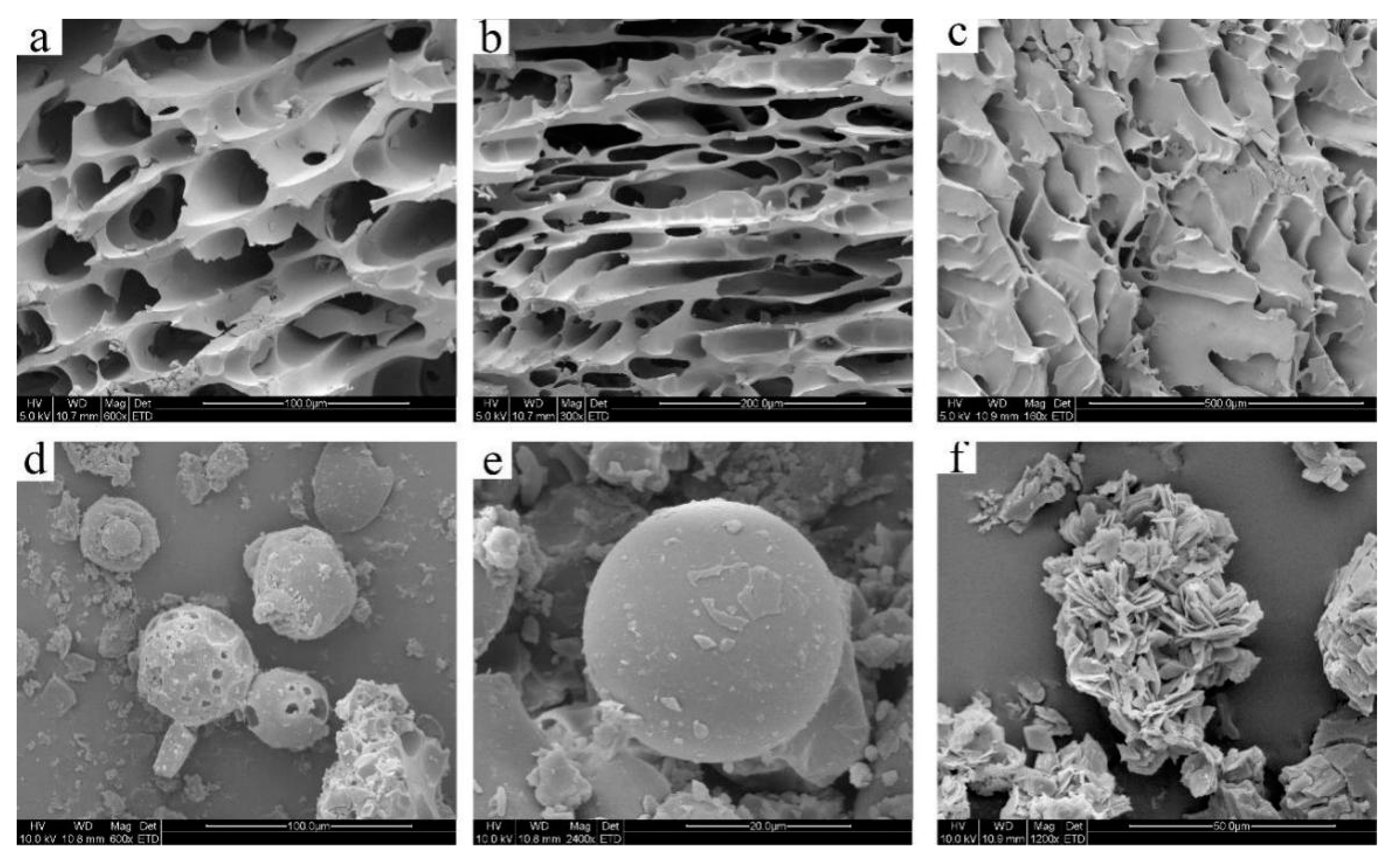
2.5. SEM Analysis
2.6. DPPH Free Radical Scavenging Activity
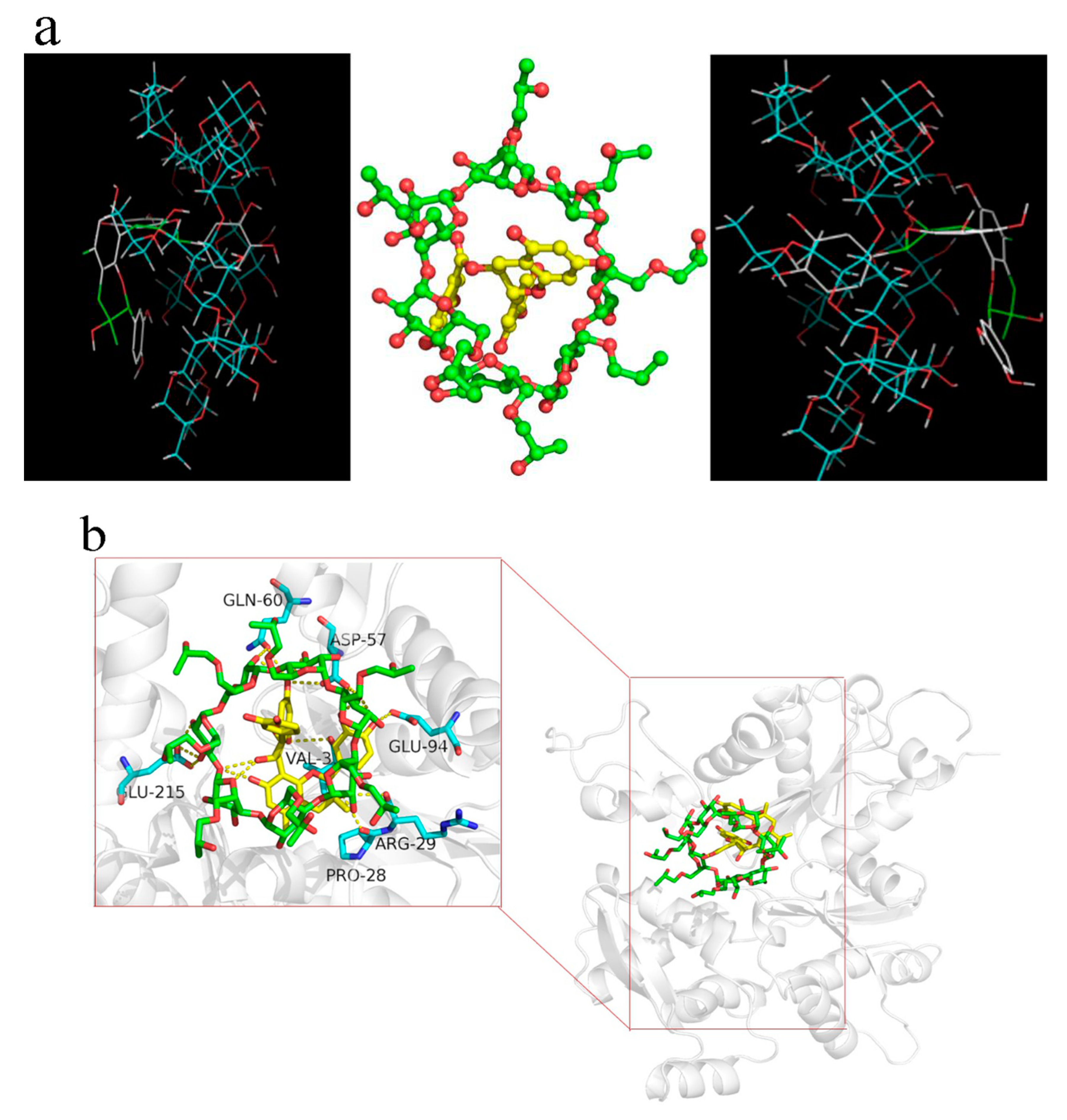
2.7. Molecular Docking
2.8. Effect of HP-β-CD/GSE Inclusion Complex on the Oxidation of Myofibrillar Protein
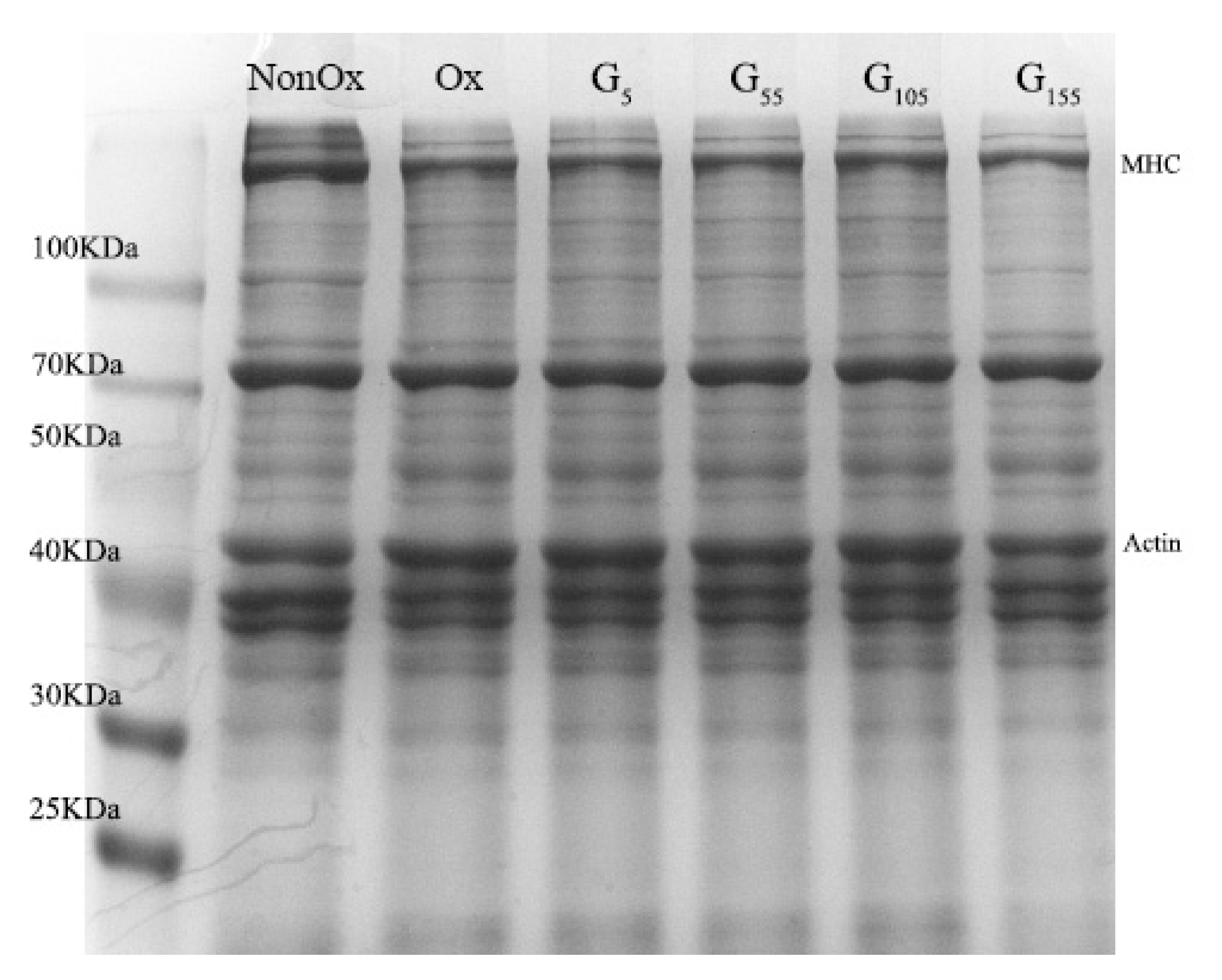
2.9. SDS-PAGE Analysis
3. Material and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Inclusion Complexes (IC)
3.2.2. Determination of Encapsulation Efficiency and Loading Capacity
3.2.3. Fourier-Transform Infrared Spectroscopy (FTIR)
3.2.4. X-Ray Diffraction (XRD)
3.2.5. Differential Scanning Calorimetry (DSC)
3.2.6. Scanning Electron Microscopy (SEM)
3.2.7. 2,2-diphenyl-1-picrylhydrazyl (DPPH) Free Radical Scavenging Activity
3.2.8. Molecular Docking
3.2.9. Extraction of Myofibrillar Protein (MP) and MALDI-TOF-MS Analysis
3.2.10. Molecular Modelling Between Inclusion Complexes and MP
3.2.11. Determination of Carbonyl Content
3.2.12. Determination of Total Sulfhydryl Content
3.2.13. Determination of Surface Hydrophobicity
4. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GSE | Grape Seed Extract |
| FTIR | Fourier-transform infrared spectroscopy |
| XRD | X-ray diffraction |
| DSC | Differential scanning calorimetry |
| HP-β-CD | 2-hydroxypropyl-β-cyclodextrin |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| GSP | Grape seed proanthocyanidin |
| DNPH | 2,4-dinitrophenylhydrazine |
| BPB | Bromophenol blue |
| MP | Myofibrillar protein |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| TBHQ | Tertiary butylhydroquinone |
| -S-S- | Disulfide bonds |
| MHC | Myosin heavy chain |
References
- Liu, B.; Aisa, H.A.; Yili, A. Isolation and identification of two potential antioxidant peptides from sheep abomasum protein hydrolysates. Eur. Food. Res. Technol. 2018, 244, 1615–1625. [Google Scholar] [CrossRef]
- Zhang, W.G.; Xiao, S.; Ahn, D.U. Protein oxidation: Basic principles and implications for meat quality. Crit. Rev. Food Sci. Nutr. 2013, 53, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Sørensen, K.M.; Engelsen, S.B.; Sun, D.-W.; Pu, H. Lipid oxidation degree of pork meat during frozen storage investigated by near-infrared hyperspectral imaging: Effect of ice crystal growth and distribution. J. Food. Eng. 2019, 263, 311–319. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant polyphenols as antioxidant and antibacterial agents for shelf-life extension of meat and meat products: Classification, structures, sources, and action mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef] [Green Version]
- Krishnaswamy, K.; Orsat, V.; Thangavel, K. Synthesis and characterization of nano-encapsulated catechin by molecular inclusion with beta-cyclodextrin. J. Food. Eng. 2012, 111, 255–264. [Google Scholar] [CrossRef]
- Nardoia, M.; Ruiz-Capillas, C.; Casamassima, D.; Herrero, A.M.; Pintado, T.; Jimenez-Colmenero, F.; Chamorro, S.; Brenes, A. Effect of polyphenols dietary grape by-products on chicken patties. Eur. Food. Res. Technol. 2017, 244, 367–377. [Google Scholar] [CrossRef]
- Rubilar, J.F.; Rms, C.; Silva, H.D.; Vicente, A.A.; Khmelinskii, I.; Vieira, M.C. Physico-mechanical properties of chitosan films with carvacrol and grape seed extract. J. Food. Eng. 2013, 115, 466–474. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.Y.; Zhang, L.M.; Li, W.; Zhang, S.T.; Luo, L.X.; Wang, J. In vitro evaluation of the anti-digestion and antioxidant effects of grape seed procyanidins according to their degrees of polymerization. J. Funct. Foods 2018, 49, 85–95. [Google Scholar] [CrossRef]
- Valls, J.; Agnolet, S.; Haas, F.; Struffi, I.; Ciesa, F.; Robatscher, P. Valorization of lagrein grape pomace as a source of phenolic compounds: Analysis of the contents of anthocyanins, flavanols and antioxidant activity. Eur. Food. Res. Technol. 2017, 243, 2211–2224. [Google Scholar] [CrossRef]
- Poverenov, E.; Granit, R.; Gabai, S. Encapsulation and controlled release of antifungal propionic acid utilizing biodegradable active films based on natural polymers. Eur. Food. Res. Technol. 2013, 237, 19–26. [Google Scholar] [CrossRef]
- Bagchi, D.; Swaroop, A.; Preuss, H.G.; Bagchi, M. Free radical scavenging, antioxidant and cancer chemoprevention by grape seed proanthocyanidin: An overview. Mutat. Res.-Fund. Mol. Mech. Mutagenesis 2014, 768, 69–73. [Google Scholar]
- Nowshehri, J.A.; Bhat, Z.A.; Shah, M.Y. Blessings in disguise: Bio-functional benefits of grape seed extracts. Food. Res. Int. 2015, 77, 333–348. [Google Scholar] [CrossRef]
- Perumalla, A.V.S.; Hettiarachchy, N.S. Green tea and grape seed extracts-potential applications in food safety and quality. Food Res. Int. 2011, 44, 0–839. [Google Scholar] [CrossRef]
- Ren, H.; Lyu, Y.; Li, X.Y.; Zhang, S.X.; Ye, Y.S.; Li, D.F. Preparation and characterization of dialdehyde β-cyclodextrin with broad-spectrum antibacterial activity. Food. Res. Int. 2018, 111, 237–243. [Google Scholar] [CrossRef]
- Garrido, E.M.P.J.; Cerqueira, A.S.; Chavarria, D.; Silva, T.; Borges, F. Microencapsulation of caffeic acid phenethyl ester and caffeic acid phenethyl amide by inclusion in hydroxypropyl-β-cyclodextrin. Food Chem. 2018, 254, 260–265. [Google Scholar] [CrossRef]
- Ramírez-Ambrosi, M.; Caldera, F.; Trotta, F.; Berrueta, L.A.; Gallo, B. Encapsulation of apple polyphenols in β-cd nanosponges. J. Incl. Phenom. Macrocycl. Chem. 2014, 80, 85–92. [Google Scholar] [CrossRef]
- Ferrati, S.; Nicolov, E.; Bansal, S.; Hosali, S.; Landis, M.; Grattoni, A. Docetaxel/2-hydroxypropyl β-cyclodextrin inclusion complex increases docetaxel solubility and release from a nanochannel drug delivery system. Curr. Drug Targets 2015, 16, 1645–1649. [Google Scholar] [CrossRef]
- Stancanelli, R.; Ficarra, R.; Cannavà, C.; Guardo, M.; Calabrò, M.L.; Ficarra, P.; Ottanà, R.; Maccari, R.; Crupi, V.; Majolino, D.; et al. U-vis and FTIR-ATR characterization of 9-fluorenon-2-carboxyester/(2-hydroxypropyl)-β-cyclodextrin inclusion complex. J. Pharm. Biomed. Anal. 2008, 47, 704–709. [Google Scholar] [CrossRef]
- Ol’khovich, M.V.; Sharapova, A.V.; Blokhina, S.V.; Skachilova, S.Y.; Kesarev, O.G.; Perlovic, G.L. Physicochemical characteristics of the inclusion complexes of biologically active compounds with 2-hydroxypropyl-β-cyclodextrin. Thermochim. Acta 2016, 639, 1–9. [Google Scholar] [CrossRef]
- Liao, Y.H.; Zhang, X.F.; Li, C.L.; Huang, Y.J.; Lei, M.; Yan, M.N. Inclusion complexes of hp-β-cyclodextrin with agomelatine: Preparation, characterization, mechanism study and in vivo evaluation. Carbohydr. Polym. 2016, 147, 415–425. [Google Scholar] [CrossRef]
- Ho, S.; Thoo, Y.Y.; Young, D.J.; Siow, L.F. Cyclodextrin encapsulated catechin: Effect of pH relative humidity and various food models on antioxidant stability. LWT-Food Sci. Technol. 2017, 85, 232–239. [Google Scholar] [CrossRef]
- You, G.J.; Sun, L.L.; Cao, X.X.; Li, H.H.; Wang, M.; Liu, Y.N.; Ren, X.L. Comprehensive evaluation of solubilization of flavonoids by various cyclodextrins using high performance liquid chromatography and chemometry. LWT-Food Sci. Technol. 2018, 94, 172–177. [Google Scholar] [CrossRef]
- Roy, P.; Dinda, A.K.; Chaudhury, S.; Dasgupta, S. Beta-cyclodextrin encapsulated polyphenols as effective antioxidants. Biopolymers 2017, 109, e23084. [Google Scholar] [CrossRef] [PubMed]
- Budryn, G.; Zaczyńska, D.; Rachwa-Rosiak, D.; Oracz, J. Changes in properties of food proteins after interaction with free and β-cyclodextrin encapsulated hydroxycinnamic acids. Eur. Food. Res. Technol. 2015, 240, 1157–1166. [Google Scholar] [CrossRef] [Green Version]
- Suvarna, V.; Thorat, S.; Nayak, U.; Sherje, A.; Murahari, M. Host-guest interaction study of Efavirenz with hydroxypropyl-β-cyclodextrin and l-arginine by computational simulation studies: Preparation and characterization of supramolecular complexes. J. Mol. Liq. 2018, 259, 55–64. [Google Scholar] [CrossRef]
- Shi, J.-H.; Chen, K.; Xu, Y. Characterization of the inclusion interaction between prednisolone and di-O-methyl-β-cyclodextrin: Spectroscopic methods and molecular modeling. J. Mol. Liq. 2014, 194, 172–178. [Google Scholar] [CrossRef]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in the solid state: A review. J. Pharmaceut. Biomed. 2015, 113, 226–238. [Google Scholar] [CrossRef]
- Zeng, Z.; Fang, Y.; Ji, H. Side chain influencing the interaction between β-cyclodextrin and vanillin. Flavour. Frag. J. 2012, 27, 378–385. [Google Scholar] [CrossRef]
- Qian, L.; Pu, H.Y.; Tang, P.X.; Tang, B.; Sun, Q.M.; Li, H. Propyl gallate/cyclodextrin supramolecular complexes with enhanced solubility and radical scavenging capacity. Food Chem. 2017, 245, 1062–1069. [Google Scholar]
- Pu, H.; Sun, Q.; Tang, P.; Zhao, L.; Li, Q.; Liu, Y. Characterization and antioxidant activity of the complexes of tertiary butylhydroquinone with β-cyclodextrin and its derivatives. Food Chem. 2018, 260, 183–192. [Google Scholar] [CrossRef]
- Amiri, S.; Nalbandi, B. Improve solubility and bioavailability of silver sulfadiazine via formation of inclusion complex by cyclodextrin. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1764–1774. [Google Scholar] [CrossRef]
- Patel, K.K.; Gade, S.; Anjum, M.M.; Singh, S.K.; Maiti, P.; Agrawal, A.K.; Singh, S. Effect of penetration enhancers and amorphization on transdermal permeation flux of raloxifene-encapsulated solid lipid nanoparticles: An ex vivo study on human skin. Appl. Nanosci. 2019, 1–12. [Google Scholar] [CrossRef]
- Meng, X.; Pan, Q.; Liu, Y. Preparation and properties of phytosterols with hydroxypropyl β-cyclodextrin inclusion complexes. Eur. Food. Res. Technol. 2012, 235, 1039–1047. [Google Scholar] [CrossRef]
- Ho, S.; Thoo, Y.Y.; Young, D.J.; Siow, L.F. Inclusion complexation of catechin by β-cyclodextrins: Characterization and storage stability. LWT-Food Sci. Technol. 2017, 86, 555–565. [Google Scholar] [CrossRef]
- Rajamohan, R.; Kothainayaki, S.; Swaminatan, M. Spectrofluorimetricstudy on inclusion complexation of 2-amino-6-fluorobenzothiazole with β-cyclodextrin. Collect. Czechoslov. Chem. Commun. 2008, 73, 147–160. [Google Scholar] [CrossRef]
- Venuti, V.; Crupi, V.; Fazio, B.; Majolino, D.; Acri, G.; Testagrossa, B.; Stancanelli, R.; De Gaetano, F.; Gagliardi, A.; Paolino, D.; et al. Physicochemical Characterization and Antioxidant Activity Evaluation of Idebenone/Hydroxypropyl-β-Cyclodextrin Inclusion Complex†. Biomolecules 2019, 9, 531. [Google Scholar] [CrossRef] [Green Version]
- Veras, K.S.; Silveira Fachel, F.N.; Delagustin, M.G.; Teixeira, H.F.; Barcellos, T.; Henriques, A.T.; Bassni, V.L.; Koester, L.S. Complexation of rosmarinic acid with hydroxypropyl-β-cyclodextrin and methyl-β-cyclodextrin: Formation of 2:1 complexes with improved antioxidant activity. J. Mol. Struct. 2019, 1195, 582–590. [Google Scholar] [CrossRef]
- Adjimani, J.P.; Asare, P. Antioxidant and free radical scavenging activity of iron chelators. Toxicol. Rep. 2015, 2, 721–728. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.M.; Chen, L.; Lv, Y.; Wang, S.X.; Suo, Z.Y.; Cheng, X.G. Inhibition of interaction between epigallocatechin-3-gallate and myofibrillar protein by cyclodextrin derivatives improves gel quality under oxidative stress. Food. Res. Int. 2018, 108, 8–17. [Google Scholar] [CrossRef]
- Zhang, B.; Fang, C.D.; Hao, G.J. Effect of kappa-carrageenan oligosaccharides on myofibrillar protein oxidation in peeled shrimp (litopenaeus vannamei) during long-term frozen storage. Food Chem. 2018, 245, 254–261. [Google Scholar] [CrossRef]
- Mohan, P.R.K.; Sreelakshmi, G.; Muraleedharan, C.V.; Joseph, R. Water soluble complexes of curcumin with cyclodextrins: characterization by ft-raman spectroscopy. Vib. Spectrosc. 2012, 62, 77–84. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Belgamwar, V.S. Inclusion complex of chrysin with sulfobutyl ether-β-cyclodextrin (captisol®): preparation, characterization, molecular modelling and in vitro anticancer activity. J. Mol. Struct. 2017, 1128, 563–571. [Google Scholar] [CrossRef]
- Abarca, R.L.; Rodriguez, F.J.; Guarda, A.; Galotto, M.J.; Bruna, J.E. Characterization of beta-cyclodextrin inclusion complexes containing an essential oil component. Food Chem. 2016, 196, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.S.; Sun, Z.P.; Zhang, L.Z.; Qin, Y.R.; Zhang, K. Inclusion complex of grape seeds extracts with sulfobutyl ether β-cyclodextrin: Preparation, characterization, stability and evaluation of α-glucosidase and α-amylase inhibitory effects in vitro. LWT-Food Sci. Technol. 2019, 101, 819–826. [Google Scholar] [CrossRef]
- Xu, J.N.; Zhang, Y.M.; Li, X.Y.; Zheng, Y. Inclusion complex of nateglinide with sulfobutyl ether β-cyclodextrin: preparation, characterization and water solubility. J. Mol. Struct. 2017, 1141, 328–334. [Google Scholar] [CrossRef]
- Olga, G.; Styliani, C.; Ioannis, R.G. Coencapsulation of ferulic and gallic acid in hp-β-cyclodextrin. Food Chem. 2015, 185, 33–40. [Google Scholar] [CrossRef]
- Suliman, F.O.; Elbashir, A.A. Enantiodifferentiation of chiral baclofen by β-cyclodextrin using capillary electrophoresis: A molecular modeling approach. J. Mol. Struct. 2012, 1019, 43–49. [Google Scholar] [CrossRef]
- Li, J.Q.; Geng, S.; Liu, B.G.; Wang, H.B.; Liang, G.Z. Self-assembled mechanism of hydrophobic amino acids and β-cyclodextrin based on experimental and computational methods. Food. Res. Int. 2018, 112, 136–142. [Google Scholar] [CrossRef]
- Cao, Y.G.; Xiong, Y.L. Chlorogenic acid-mediated gel formation of oxidatively stressed myofibrillar protein. Food Chem. 2015, 180, 235–243. [Google Scholar] [CrossRef]
- Rakotondramavo, A.; Rabesona, H.; Brou, C.; Lamballerie, M.D.; Pottier, L. Ham processing: effects of tumbling, cooking and high pressure on proteins. Eur Food Res Technol. 2018, 245, 273–284. [Google Scholar] [CrossRef]
Sample Availability: Samples of the lamb tripe and grape seed extract/2-hydroxypropyl-β-cyclodextrin are available from the authors. |


| HP-β-CD/GSE Ratio | Encapsulation Efficiency | Loading Capacity |
|---|---|---|
| 1:0.5 | 63.2 ± 3.31% | 5.41 ± 0.24% |
| 1:1 | 26.5 ± 2.54% | 2.59 ± 0.13% |
| 1:2 | 40.7 ± 1.33% | 4.60 ± 0.37% |
| Sample | Carbonyl Content (nmol/mg Protein) | Sulphydryl Content (nmol/mg Protein) | BPB Binding (μg) |
|---|---|---|---|
| NonOx | 1.19 ± 0.12 a | 67.54 ± 0.78 a | 2.93 ± 1.15 e |
| Ox | 2.23 ± 0.22 c | 58.33 ± 1.16 e | 7.16 ± 1.94 a |
| Ox + HP-β-CD/GSE (5 μmol/g) | 1.86 ± 0.56 bc | 60.02 ± 1.97 d | 6.04 ± 0.37 b |
| Ox + HP-β-CD/GSE (55 μmol/g) | 1.31 ± 0.17 ab | 61.43 ± 2.06 c | 5.89 ± 2.34 c |
| Ox + HP-β-CD/GSE (105 μmol/g) | 1.26 ± 0.09 a | 62.21 ± 1.93 b | 4.96 ± 1.71 d |
| Ox + HP-β-CD/GSE (155 μmol/g) | 1.43 ± 0.38 ab | 61.37 ± 1.85 c | 6.07 ± 0.83 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Ran, L.; Liu, F.; Hou, R.; Zhao, W.; Li, Y.; Wang, C.; Dong, J. Preparation and Characterisation of Polyphenol-HP-β-Cyclodextrin Inclusion Complex that Protects Lamb Tripe Protein against Oxidation. Molecules 2019, 24, 4487. https://doi.org/10.3390/molecules24244487
Li W, Ran L, Liu F, Hou R, Zhao W, Li Y, Wang C, Dong J. Preparation and Characterisation of Polyphenol-HP-β-Cyclodextrin Inclusion Complex that Protects Lamb Tripe Protein against Oxidation. Molecules. 2019; 24(24):4487. https://doi.org/10.3390/molecules24244487
Chicago/Turabian StyleLi, Wenhui, Lidan Ran, Fei Liu, Ran Hou, Wei Zhao, Yingbiao Li, Chunyan Wang, and Juan Dong. 2019. "Preparation and Characterisation of Polyphenol-HP-β-Cyclodextrin Inclusion Complex that Protects Lamb Tripe Protein against Oxidation" Molecules 24, no. 24: 4487. https://doi.org/10.3390/molecules24244487






