Stereoselective Bioreduction of α-diazo-β-keto Esters
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Synthesis of α-Diazo-β-keto Esters 2a–i and Racemic α-Diazo-β-hydroxy Esters 3a–i
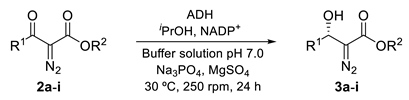
2.2. Bioreduction of α-Diazo-β-keto Esters 3a–i using Alcohol Dehydrogenases
2.2.1. Bioreduction of Ethyl 4-Azido-2-diazo-3-oxobutanoate (2a)
2.2.2. Bioreduction of α-Diazo-β-keto Esters 2b–i
2.2.3. Absolute Configuration Assignment for the Optically Active Hydroxy Esters 3a–i Obtained through the Bioreduction Process
3. Materials and Methods
3.1. General Methods
3.2. Chemical Synthesis of Ethyl 4-Azido-2-diazo-3-oxobutanoate (2a)
3.3. Chemical Synthesis of α-Diazo-β-keto Esters 2b–i
3.4. Chemical Reduction of α-Diazo-β-keto Esters 2a–i using Sodium Borohydride
3.5. Bioreduction of α- Diazo-β-keto Esters 2a–i using Sy-ADH, TES-ADH, and ADH-T
3.6. Bioreduction of α- Diazo-β-keto Esters 2a–i using LB-ADH
3.7. Bioreduction of α- Diazo-β-keto Esters 2a–i using ADH-A
3.8. Bioreduction of α- Diazo-β-keto Esters 2a–i using Ras-ADH
3.9. Bioreduction of α- Diazo-β-keto Esters 2a–i using evo-1.1.200
3.10. Bioreduction of α- Diazo-β-keto Esters 2a–i using Commercially Available ADHs from Codexis Inc.
3.11. Semi-preparative Bioreduction of α- Diazo-β-keto esters 2a–c,e,f,h,i using Commercially Available ADHs from Codexis Inc.
- (S)-3a: [α = +5.4 (c 0.1, CHCl3, 97% ee) after bioreduction with KRED-P2-D12 (83% conversion, 74% isolated yield).
- (R)-3b: [α = +7.5 (c 0.1, CHCl3, 96% ee) after bioreduction with KRED-P2-D12 (99% conversion, 86% isolated yield).
- (R)-3c: [α = +7.1 (c 0.1, CHCl3, 98% ee) after bioreduction with KRED-P2-D12 (99% conversion, 96% isolated yield).
- (R)-3e: [α = +9.4 (c 0.1, CHCl3, 99% ee) after bioreduction with KRED-P1-C01 (82% conversion, 73% isolated yield).
- (S)-3f: [α = +19.3 (c 0.42, CHCl3, 98% ee) after bioreduction with KRED-P1-B02 (91% conversion, 83% isolated yield).
- (R)-3h: [α = +8.5 (c 0.1, CHCl3, 96% ee) after bioreduction with KRED-P2-D11 (97% conversion, 89% isolated yield).
- (R)-3i: [α = +7.8 (c 0.1, CHCl3, 96% ee) after bioreduction with KRED-P2-D12 (97% conversion, 91% isolated yield).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maas, G. New Syntheses of Diazo Compounds. Angew. Chem. Int. Ed. 2009, 48, 8186–8195. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.; Miel, H.; Ring, A.; Slattery, C.N.; Maguire, A.R.; McKervey, M.A. Modern Organic Synthesis with α-Diazocarbonyl Compounds. Chem. Rev. 2015, 115, 9981–10080. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-P.; Li, Z.-Q.; Zhu, S.-F. Recent advances in transition-metal-catalyzed asymmetric reactions of diazo compounds with electron-rich (hetero) arenes. Tetrahedron Lett. 2018, 59, 2307–2318. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, C.; Ding, Q.; Peng, Y. Diazo Compounds: Versatile Synthons for the Synthesis of Nitrogen Heterocycles via Transition Metal-Catalyzed Cascade C–H Activation/Carbene Insertion/Annulation Reactions. Adv. Synth. Catal. 2019, 361, 919–944. [Google Scholar] [CrossRef]
- Empel, C.; Koenlgs, R.M. Sustainable Carbene Transfer Reactions with Iron and Light. Synlett 2019, 30, 1929–1934. [Google Scholar] [CrossRef]
- Dutta, N.B.; Sharma, S.; Chetry, R.L.; Baishya, G. A Green Protocol for the Synthesis of α-Diazo-β-hydroxy esters and One-Pot Conversion to β-Keto-Esters and Imidazo[1,2-a]pyridine-3-carboxylates. ChemistrySelect 2019, 4, 5817–5822. [Google Scholar] [CrossRef]
- Chen, D.F.; Wu, P.-Y.; Gong, L.-Z. Rhodium/Chiral Urea Relay Catalysis Enables an Enantioselective Semipinacol Rearrangement/Michael Addition Cascade. Org. Lett. 2013, 15, 3958–3961. [Google Scholar] [CrossRef]
- Bayir, A.; Draghici, C.; Brewer, M. Preparation of Tethered Aldehyde Ynoates and Ynones by Ring Fragmentation of Cyclic γ-Oxy-β-hydroxy-α-diazo Carbonyls. J. Org. Chem. 2010, 75, 296–302. [Google Scholar]
- Zhang, Y.; Wang, J. Recent development of reactions with α-diazocarbonyl compounds as nucleophiles. Chem. Commun. 2009, 36, 5350–5361. [Google Scholar] [CrossRef]
- Xiao, F.; Liu, Y.; Wang, J. DBU-catalyzed condensation of acyldiazomethanes to aldehydes in water and a new approach to ethyl β-hydroxy α-arylacrylates. Tetrahedron Lett. 2007, 48, 1147–1149. [Google Scholar] [CrossRef]
- Zhdanova, O.V.; Korneev, S.M.; Nikolaev, V.A. Chemistry of Diazocarbonyl Compounds: XVIII. Synthesis and Spectral Parameters of 1,3-Dialkyl-3-hydroxy-2-diazoketones. Russ. J. Org. Chem. 2004, 40, 316–328. [Google Scholar] [CrossRef]
- Moody, C.J.; Morfitt, C.N. Zinc Derivative of Ethyl Diazoacetate in the Synthesis of α-Diazo-β-hydroxy Esters. Synthesis 1998, 1039–1042. [Google Scholar] [CrossRef]
- Jiang, N.; Wang, J. DBU-promoted condensation of acyldiazomethanes to aldehydes and imines under catalytic conditions. Tetrahedron Lett. 2002, 43, 1285–1287. [Google Scholar] [CrossRef]
- Cuevas-Yañez, E.; Muchowski, J.M.; Cruz-Almanza, R. Metalation of α-diazocarbonyl compounds using Grignard reagents. A convenient synthesis of α-diazo-β-ketoesters and mixed esters of α-diazomalonate. Tetrahedron Lett. 2004, 45, 2417–2419. [Google Scholar] [CrossRef]
- Arai, S.; Hasegawa, K.; Nishida, A. One-pot synthesis of α-diazo-β-hydroxyesters under phase-transfer catalysis and application to the catalytic asymmetric aldol reaction. Tetrahedron Lett. 2004, 45, 1023–1026. [Google Scholar] [CrossRef]
- Li, P.; Majireck, M.M.; Korboukh, I.; Weinreb, S.M. A mild, efficient method for the oxidation of α-diazo-β-hydroxy esters to α-diazo-β-ketoesters. Tetrahedron Lett. 2008, 49, 3162–3164. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hasegawa, K.; Arai, S.; Nishida, A. Synthesis of α-diazo-β-hydroxyesters through a one-pot protocol by phase-transfer catalysis: Application to enantioselective aldol-type reaction and diastereoselective synthesis of α-amino-β-hydroxyester derivatives. Tetrahedron 2006, 62, 1390–1401. [Google Scholar] [CrossRef]
- Yao, W.; Wang, J. Direct catalytic asymmetric aldol-type reaction of aldehydes with ethyl diazoacetate. Org. Lett. 2003, 5, 1527–1530. [Google Scholar] [CrossRef]
- Trost, B.M.; Malhotra, S.; Fried, B.A. Magnesium-catalyzed asymmetric direct aldol addition of ethyl diazoacetate to aromatic, aliphatic, and α,β-unsaturated aldehydes. J. Am. Chem. Soc. 2009, 131, 1674–1675. [Google Scholar] [CrossRef]
- Trost, B.M.; Malhotra, S.; Koschker, P.; Ellerbrock, P. The development of the enantioselective addition of ethyl diazoacetate to aldehydes: Asymmetric synthesis of 1,2-diols. J. Am. Chem. Soc. 2012, 134, 2075–2084. [Google Scholar] [CrossRef]
- Pagar, V.V.; Jadhav, A.M.; Liu, R.-S. Gold-Catalyzed Formal [3 + 3] and [4 + 2] Cycloaddition Reactions of Nitrosobenzenes with Alkenylgold Carbenoids. J. Am. Chem. Soc. 2011, 133, 20728–20731. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.M.L.; Huby, N.J.S. Enantioselective synthesis of tropanes by reaction of rhodium-stabilized vinylcarbenoids with pyrroles. Tetrahedron Lett. 1992, 33, 6935–6938. [Google Scholar] [CrossRef]
- Applegate, G.A.; Berkowitz, D.B. Exploiting Enzymatic Dynamic Reductive Kinetic Resolution (DYRKR) in Stereocontrolled Synthesis. Adv. Synth. Catal. 2015, 357, 1619–1632. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, F.; Arends, I.W.C.E.; Holtmann, D. Enzymatic reductions for the chemist. Green Chem. 2011, 13, 2285–2313. [Google Scholar] [CrossRef]
- Kallergi, M.; Kalaitzakis, D.; Smonou, I. Enzymatic Total Synthesis of Banana Volatile (S)-2-Pentyl (R)-3-Hydroxyhexanoate. Eur. J. Org. Chem. 2011, 3946–3950. [Google Scholar] [CrossRef]
- Cuetos, A.; Rioz-Martínez, A.; Bisogno, F.R.; Grischek, B.; Lavandera, I.; de Gonzalo, G.; Kroutil, W.; Gotor, V. Access to Enantiopure α-Alkyl-β-hydroxy Esters through Dynamic Kinetic Resolutions Employing Purified/Overexpressed Alcohol Dehydrogenases. Adv. Synth. Catal. 2012, 354, 1743–1749. [Google Scholar] [CrossRef]
- Zadlo, A.; Schrittwieser, J.H.; Koszelewski, D.; Kroutil, W.; Ostaszewski, R. Enantioselective Reduction of Ethyl 3-Oxo-5-phenylpentanoate with Whole-Cell Biocatalysts. Eur. J. Org. Chem. 2016, 1007–1011. [Google Scholar] [CrossRef]
- Barik, R.; Halder, J.; Nanda, S. Biocatalytic dynamic kinetic reductive resolution with ketoreductase from Klebsiella pneumoniae: The asymmetric synthesis of functionalized tetrahydropyrans. Org. Biomol. Chem. 2019, 17, 8571–8588. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Brady, D. The Limits to Biocatalysis: Pushing the Envelope. Chem. Commun. 2018, 54, 6088–6104. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Pereira, P.C. Biocatalysis engineering: The big picture. Chem. Soc. Rev. 2017, 46, 2678–2691. [Google Scholar] [CrossRef]
- Ferreira-Leitão, V.S.; Cammarota, M.C.; Aguieiras, E.C.G.; Sá, L.R.V.; Fernandez-Lafuente, R.; Freire, D.M.G. The Protagonism of Biocatalysis in Green Chemistry and Its Environmental Benefits. Catalysts 2017, 7, 9. [Google Scholar] [CrossRef]
- Wohlgemuth, R. Biocatalysis—Key to sustainable industrial chemistry. Curr. Opin. Biotechnol. 2010, 21, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.S.; Brustad, E.M.; Kannan, A.; Arnold, F.H. Olefin Cyclopropanation via Carbene Transfer Catalyzed by Engineered Cytochrome P450 Enzymes. Science 2013, 339, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Renata, H.; Lewis, R.D.; Sweredoski, M.J.; Moradian, A.; Hess, S.; Wang, Z.J.; Arnold, F.H. Identification of Mechanism-Based Inactivation in P450-Catalyzed Cyclopropanation Facilitates Engineering of Improved Enzymes. J. Am. Chem. Soc. 2016, 138, 12527–12533. [Google Scholar] [CrossRef]
- Waldman, A.J.; Balskus, E.P. Discovery of a Diazo-Forming Enzyme in Cremeomycin Biosynthesis. J. Org. Chem. 2018, 83, 7539–7546. [Google Scholar] [CrossRef]
- Mittmann, E.; Hu, Y.; Peschke, T.; Rabe, K.S.; Niemeyer, C.M.; Bräse, S. Chemoenzymatic Synthesis of O-Containing Heterocycles from α-Diazo Esters. ChemCatChem 2019, 11, 5519–5523. [Google Scholar]
- Costin, T.A.; Dutra, L.G.; Bortoluzzi, A.J.; Sá, M.M. Amine-mediated synthesis of amides from 1,3-dicarbonyl compounds through a domino diazo transfer/aminolysis process. Tetrahedron 2017, 73, 4549–4559. [Google Scholar] [CrossRef]
- Stampfer, W.; Kosjek, B.; Moitzi, C.; Kroutil, W.; Faber, K. Biocatalytic asymmetric hydrogen transfer. Angew. Chem. Int. Ed. 2002, 41, 1014–1017. [Google Scholar] [CrossRef]
- Peters, J.; Minuth, T.; Kula, M.-R. A novel NADH-dependent carbonyl reductase with an extremely broad substrate range from Candida parapsilosis: Purification and characterization. Enzyme Microb. Technol. 1993, 11, 950–958. [Google Scholar] [CrossRef]
- Wolberg, M.; Hummel, W.; Wandrey, C.; Müller, M. Highly Regio- and Enantioselective Reduction of 3,5-Dioxocarboxylates. Angew. Chem. Int. Ed. 2000, 39, 4306–4308. [Google Scholar] [CrossRef]
- Leuchs, S.; Greiner, L. Alcohol Dehydrogenase from Lactobacillus brevis: A Versatile Robust Catalyst for Enantioselective Transformations. Chem. Biochem. Eng. Q. 2011, 2, 267–281. [Google Scholar]
- Lavandera, I.; Kern, A.; Ferreira-Silva, B.; Glieder, A.; de Wildeman, S.; Kroutil, W. Stereoselective Bioreduction of Bulky-Bulky Ketones by a Novel ADH from Ralstonia sp. J. Org. Chem. 2008, 15, 6003–6005. [Google Scholar] [CrossRef] [PubMed]
- Man, H.; Kędziora, K.; Kulig, J.; Frank, A.; Lavandera, I.; Gotor-Fernández, V.; Röther, D.; Hart, S.; Turkenburg, J.P.; Grogan, G. Structures of Alcohol Dehydrogenases from Ralstonia and Sphingobium spp. Reveal the Molecular Basis for Their Recognition of ‘Bulky-Bulky’ Ketones. Top. Catal. 2014, 5, 356–365. [Google Scholar] [CrossRef]
- Lavandera, I.; Oberdofer, G.; Gross, J.; de Wildeman, S.; Kroutil, W. Stereocomplementary Asymmetric Reduction of Bulky–Bulky Ketones by Biocatalytic Hydrogen Transfer. Eur. J. Org. Chem. 2008, 2539–2543. [Google Scholar] [CrossRef]
- Lavandera, I.; Kern, A.; Resch, V.; Ferreira-Silva, B.; Glieder, A.; Fabian, W.M.F.; de Wildeman, S.; Kroutil, W. One-Way Biohydrogen Transfer for Oxidation of sec-Alcohols. Org. Lett. 2008, 11, 2155–2158. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.; Laivenieks, M.; Zeikus, J.G.; Phillips, R.S. Mutation of cysteine-295 to alanine in secondary alcohol dehydrogenase from Thermoanaerobacter ethanolicus affects the enantioselectivity and substrate specificity of ketone reductions. Bioorg. Med. Chem. 2001, 7, 1659–1666. [Google Scholar] [CrossRef]
- Kroutil, W.; Velikonge, S. Method for the Preparation of Substituted Pyrazines via Enzymatic Catalysis. Patent No. 3,569,594,A1, 20 11 2019. [Google Scholar]
- Könst, P.; Merkens, H.; Kara, S.; Kochius, S.; Vogel, A.; Zuhse, R.; Holtmann, D.; Arends, I.W.C.E.; Hollmann, F. Enantioselective oxidation of aldehydes catalyzed by alcohol dehydrogenase. Angew. Chem. Int. Ed. 2012, 51, 9914–9917. [Google Scholar] [CrossRef]
- Wang, W.; Shen, K.; Hu, X.; Wang, J.; Liu, X.; Feng, X. Highly Enantioselective Synthesis of α-Diazo-β-hydroxy Esters Using a Bifunctional Titanium Complex. Synlett 2009, 1655–1658. [Google Scholar] [CrossRef]
- Méndez-Sánchez, D.; Mourelle-Insua, Á.; Gotor-Fernández, V.; Lavandera, I. Synthesis of α-Alkyl-β-Hydroxy Amides through Biocatalytic Dynamic Kinetic Resolution Employing Alcohol Dehydrogenases. Adv. Synth. Catal. 2019, 361, 2706–2712. [Google Scholar] [CrossRef]
- Kato, T.; Sato, M.; Kimura, H. Studies on Keten and Its Derivatives. Part 89. Ethyl 4-Substituted Acetoacetates: Synthesis and Reaction with Diketen. J. Chem. Soc. Perkin Trans. 1 1979, 529–532. [Google Scholar] [CrossRef]
- Chandrashaker, V.; Ptaszek, M.; Taniguchi, M.; Lindsey, J.S. Synthesis of diverse acyclic precursors to pyrroles for studies of prebiotic routes to tetrapyrrole macrocycles. New J. Chem. 2016, 40, 8786–8808. [Google Scholar] [CrossRef]
- Padwa, A.; Sá, M.M.; Weingarten, M.D. Metal substituted diazo esters as substrates for cross coupling reaction. Tetrahedron 1997, 53, 2371–2386. [Google Scholar] [CrossRef]
- Bagley, M.C.; Buck, R.T.; Hind, S.L.; Moody, C.J. Synthesis of functionalised oxazoles and bis-oxazoles. J. Chem. Soc. Perkin Trans. 1 1998, 591–600. [Google Scholar] [CrossRef]
- Dar’in, D.; Kantin, G.; Krasavin, M. A ‘sulfonyl-azide-free’ (SAFE) aqueous-phase diazo transfer reaction for parallel and diversity-oriented synthesis. Chem. Commun. 2019, 55, 5239–5242. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xie, Y.; Xiao, X.; Li, G.; Deng, Y.; Jiang, H.; Zeng, W. Rh(III)-catalyzed chelation-assisted intermolecular carbenoid functionalization of α-imino Csp3–H bonds. Chem. Commun. 2015, 51, 15328–15331. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

| Entry | Compound | R1 | R2 | Keto Ester 2 (%) a | Hydroxy Ester 3 (%) a |
|---|---|---|---|---|---|
| 1 | a | N3CH2 | CH3CH2 | 86 b | 71 |
| 2 | b | ClCH2 | CH3CH2 | 96 b | 75 |
| 3 | c | ClCH2 | CH3 | 90 | 69 |
| 4 | d | CH3 | CH3 | 81 | 62 |
| 5 | e | CH3OCH2 | CH3 | 92 | 78 |
| 6 | f | C6H5 | CH3CH2 | 95 | 85 |
| 7 | g | CH3 | C6H5CH2 | 90 | 78 |
| 8 | h | BrCH2 | CH3CH2 | 94 | 71 |
| 9 | i | NCSCH2 | CH3CH2 | 88 | 77 |

| Entry | ADH/KRED | Conversion (%) a | Alcohol 3a ee (%) b |
|---|---|---|---|
| 1 | Ras-ADH | n.m.c | - |
| 2 | LB-ADH | <5 | - |
| 3 | Sy-ADH | 6 | 50 |
| 4 | Tes-ADH | <5 | - |
| 5 | ADH-T | <5 | - |
| 6 | ADH-A | 5 | >99 |
| 7 | evo-1.1.200 | 6 | rac |
| 8 | KRED-P1-A04 | 5 | 40 (S) |
| 9 | KRED-P1-A12 | 49 | 94 (S) |
| 10 | KRED-P1-B02 | 42 | 95 (S) |
| 11 | KRED-P1-B05 | 14 | 96 (S) |
| 12 | KRED-P1-B10 | 64 | 96 (S) |
| 13 | KRED-P1-B12 | 83 | 99 (S) |
| 14 | KRED-P1-C01 | 81 | 99 (S) |
| 15 | KRED-P1-H08 | 86 | 99 (S) |
| 16 | KRED-P2-B02 | 73 | 86 (S) |
| 17 | KRED-P2-C02 | 84 | 74 (S) |
| 18 | KRED-P2-D03 | 77 | 98 (S) |
| 19 | KRED-P2-D11 | 23 | 97 (S) |
| 20 | KRED-P2-D12 | 89 | 98 (S) |
| 21 | KRED-P2-G03 | 38 | 78 (S) |
| 22 | KRED-P2-H07 | 7 | 7 (S) |
| 23 | KRED-P3-B03 | <5 | - |
| 24 | KRED-P3-G09 | 7 | 76 (R) |
| Entry | Substrate | R1 | R2 | ADH/KRED | Conversion (%) a | ee 3 (%) a |
|---|---|---|---|---|---|---|
| 1 | 2a | N3CH2 | CH3CH2 | KRED-P1-B12 | 83 | 99 (S) |
| 2 | 2a | N3CH2 | CH3CH2 | KRED-P1-C01 | 81 | 99 (S) |
| 3 | 2a | N3CH2 | CH3CH2 | KRED-P1-H08 | 86 | 99 (S) |
| 4 | 2a | N3CH2 | CH3CH2 | KRED-P2-D12 | 89 | 98 (S) |
| 5 | 2b | ClCH2 | CH3CH2 | KRED-P2-D11 | 99 | 99 (R) |
| 6 | 2b | ClCH2 | CH3CH2 | KRED-P2-D12 | >99 | 98 (R) |
| 7 | 2b | ClCH2 | CH3CH2 | KRED-P2-G03 | 99 | 98 (R) |
| 8 | 2b | ClCH2 | CH3CH2 | LB-ADH | 71 | 99 (R) |
| 9 | 2c | ClCH2 | CH3 | KRED-P1-A12 | >99 | >99 (R) |
| 10 | 2c | ClCH2 | CH3 | KRED-P2-D11 | 99 | 96 (R) |
| 11 | 2c | ClCH2 | CH3 | KRED-P2-D12 | 99 | 99 (R) |
| 12 | 2c | ClCH2 | CH3 | KRED-P2-G03 | 99 | 98 (R) |
| 13 | 2c | ClCH2 | CH3 | LB-ADH | 71 | 98 (R) |
| 14 | 2d | CH3 | CH3 | KRED-P1-C01 | 60 | 85 (S) |
| 15 | 2d | CH3 | CH3 | KRED-P2-B02 | 50 | 67 (S) |
| 16 | 2e | CH3OCH2 | CH3 | KRED-P1-C01 | 82 | 99 (R) |
| 17 | 2e | CH3OCH2 | CH3 | KRED-P2-B02 | 98 | 81 (R) |
| 18 | 2f | C6H5 | CH3CH2 | KRED-P1-B02 | 93 | 99 (S) |
| 19 | 2f | C6H5 | CH3CH2 | KRED-P1-B05 | 84 | 98 (S) |
| 20 | 2f | C6H5 | CH3CH2 | KRED-P2-D11 | 67 | 97 (S) |
| 21 | 2f | C6H5 | CH3CH2 | KRED-P2-G03 | 73 | 59 (R) |
| 22 | 2g | CH3 | C6H5CH2 | KRED-P1-C01 | 40 | 96 (S) |
| 23 | 2g | CH3 | C6H5CH2 | KRED-P2-B02 | 60 | 95 (S) |
| 24 | 2g | CH3 | C6H5CH2 | KRED-P2-C02 | 50 | 96 (S) |
| 25 | 2h | BrCH2 | CH3CH2 | KRED-P2-D11 | 98 | 98 (R) |
| 26 | 2h | BrCH2 | CH3CH2 | KRED-P2-D12 | 99 | 91 (R) |
| 27 | 2h | BrCH2 | CH3CH2 | KRED-P2-G03 | 99 | 90 (R) |
| 28 | 2i | NCSCH2 | CH3CH2 | KRED-P2-D11 | 92 | 97 (R) |
| 29 | 2i | NCSCH2 | CH3CH2 | KRED-P2-D12 | 99 | 98 (R) |
| 30 | 2i | NCSCH2 | CH3CH2 | KRED-P2-G03 | 99 | 97 (R) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Granda, S.; Costin, T.A.; Sá, M.M.; Gotor-Fernández, V. Stereoselective Bioreduction of α-diazo-β-keto Esters. Molecules 2020, 25, 931. https://doi.org/10.3390/molecules25040931
González-Granda S, Costin TA, Sá MM, Gotor-Fernández V. Stereoselective Bioreduction of α-diazo-β-keto Esters. Molecules. 2020; 25(4):931. https://doi.org/10.3390/molecules25040931
Chicago/Turabian StyleGonzález-Granda, Sergio, Taíssa A. Costin, Marcus M. Sá, and Vicente Gotor-Fernández. 2020. "Stereoselective Bioreduction of α-diazo-β-keto Esters" Molecules 25, no. 4: 931. https://doi.org/10.3390/molecules25040931
APA StyleGonzález-Granda, S., Costin, T. A., Sá, M. M., & Gotor-Fernández, V. (2020). Stereoselective Bioreduction of α-diazo-β-keto Esters. Molecules, 25(4), 931. https://doi.org/10.3390/molecules25040931








