Synthesis and Structure of Methylsulfanyl Derivatives of Nickel Bis(Dicarbollide) †
Abstract
1. Introduction
2. Results and Discussion
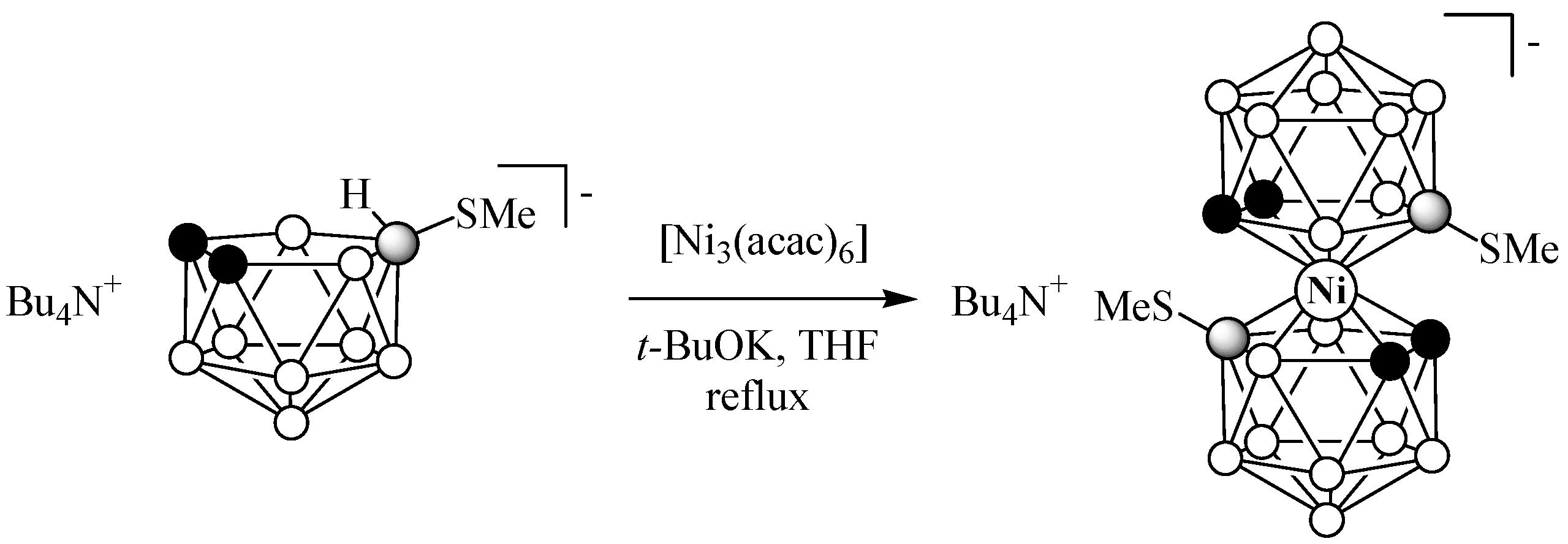
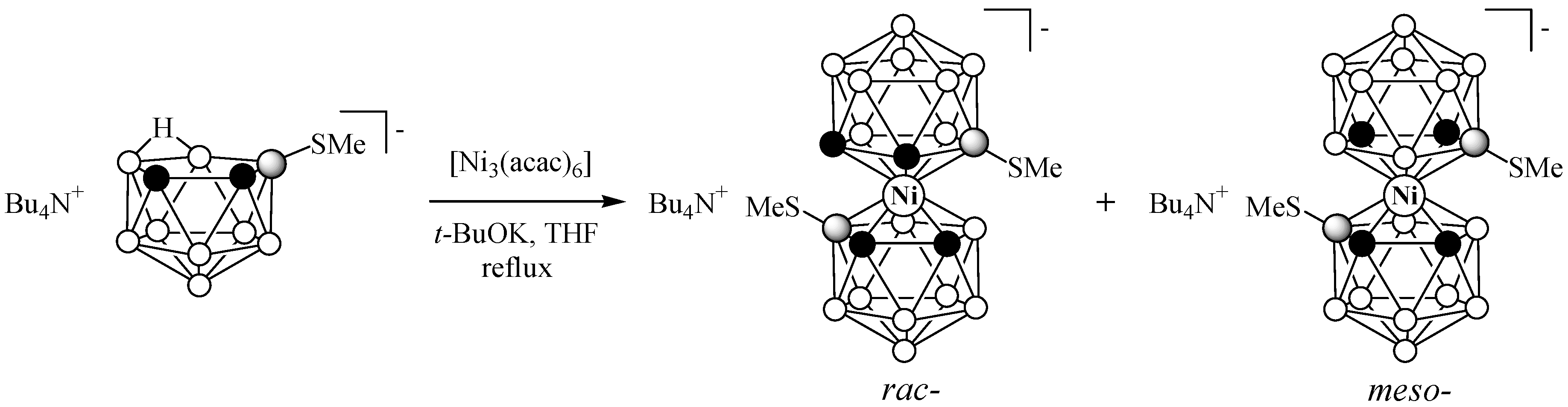
2.1. Synthesis
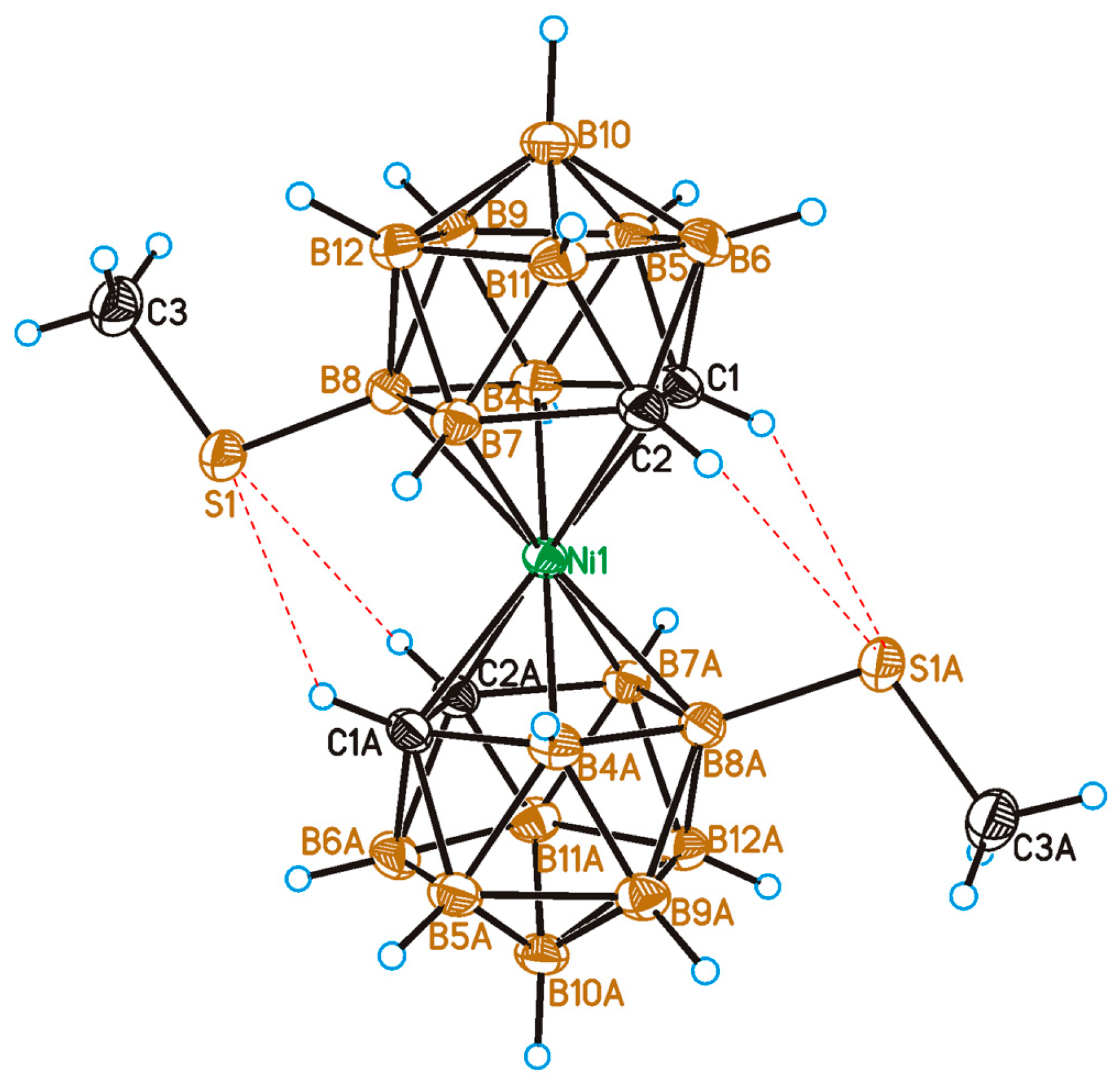
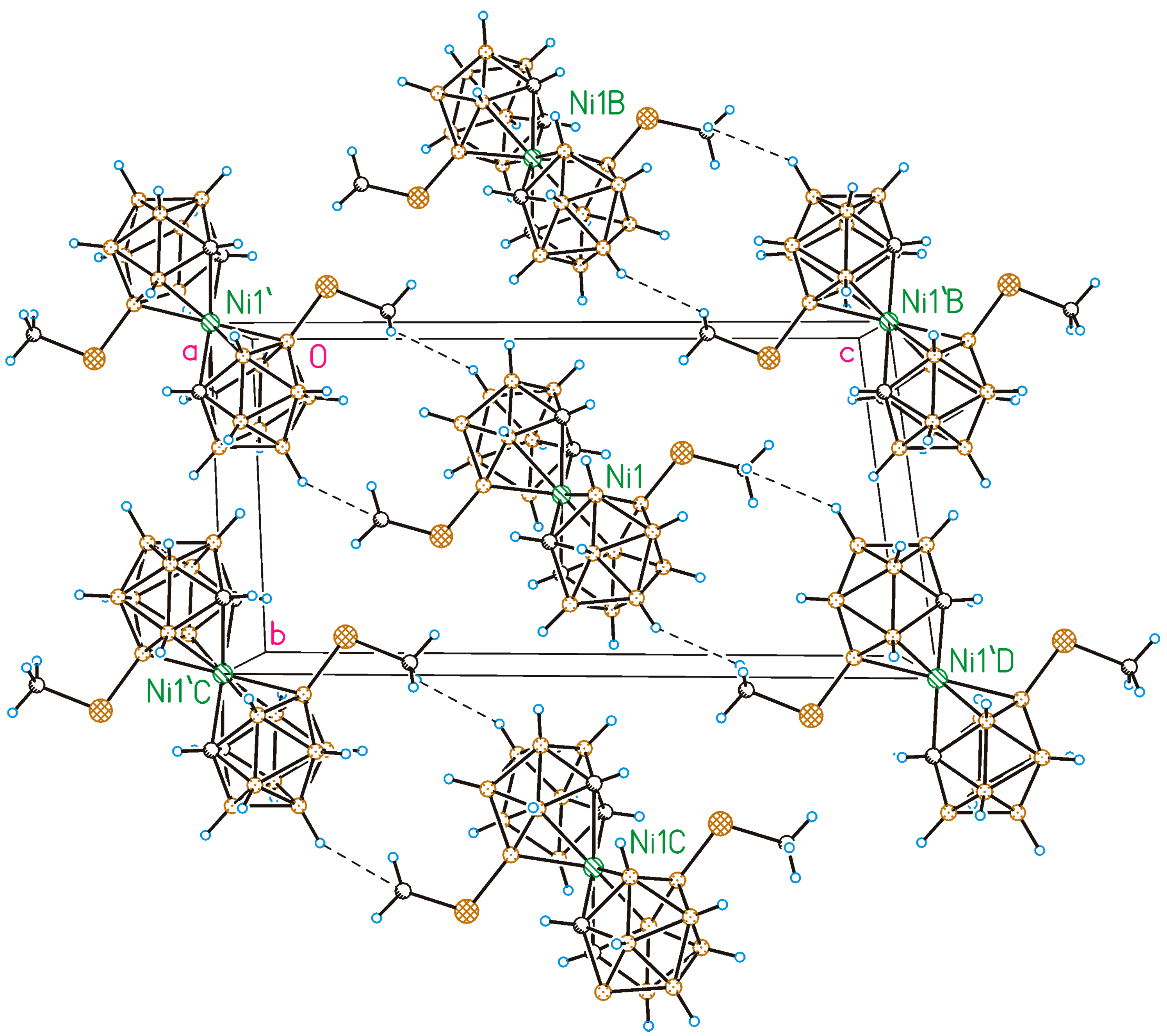
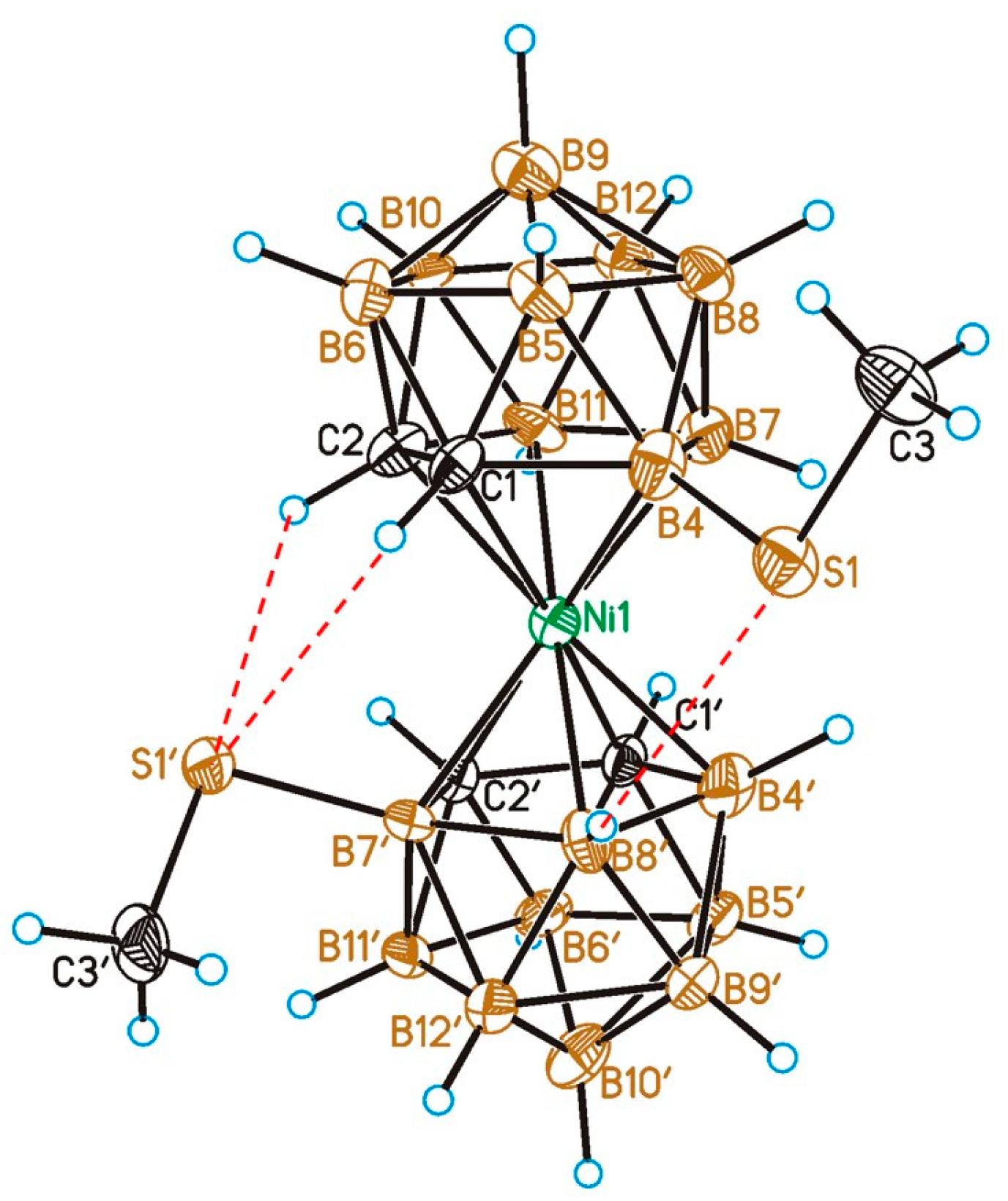
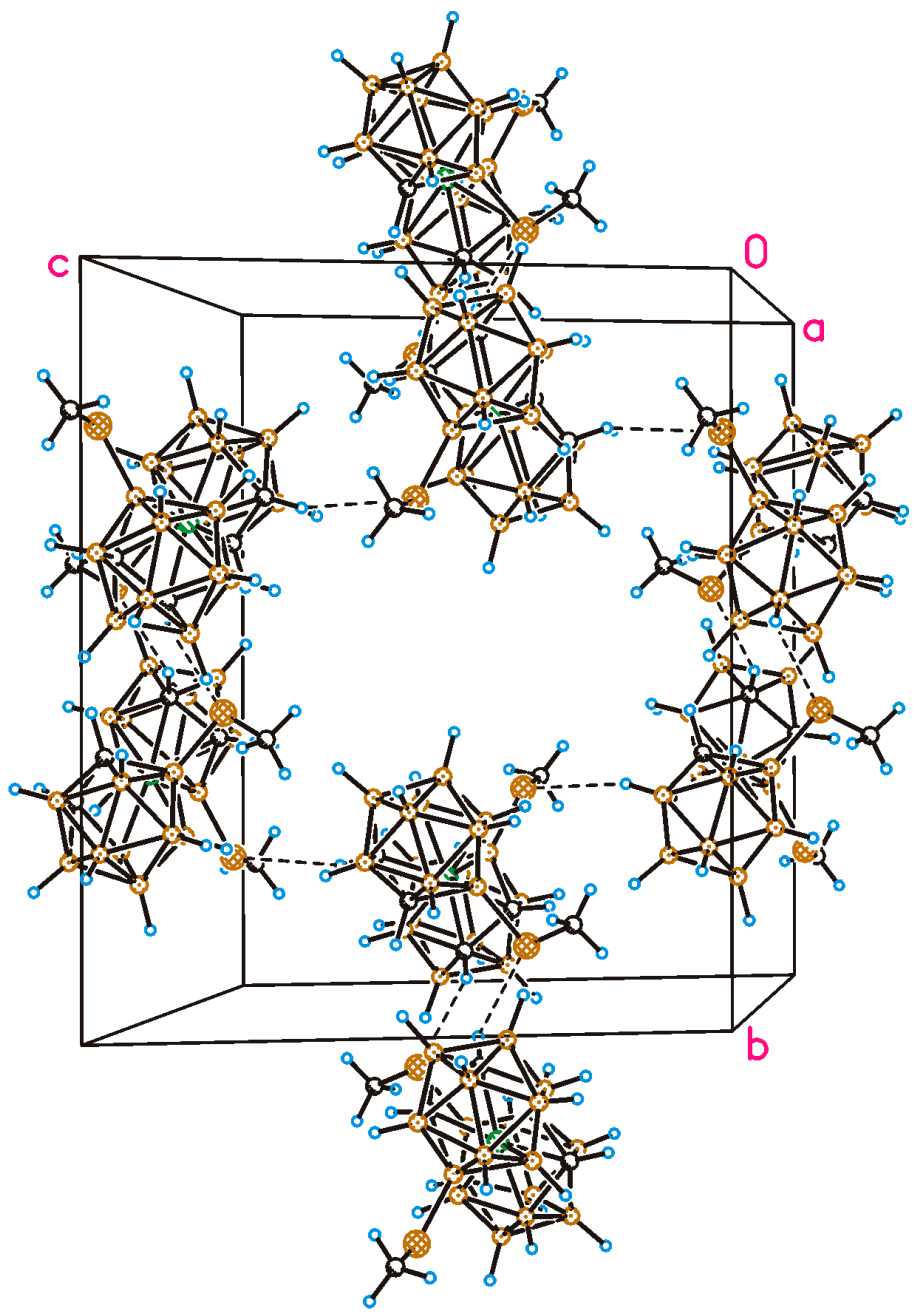
2.2. X-Ray Diffraction Study
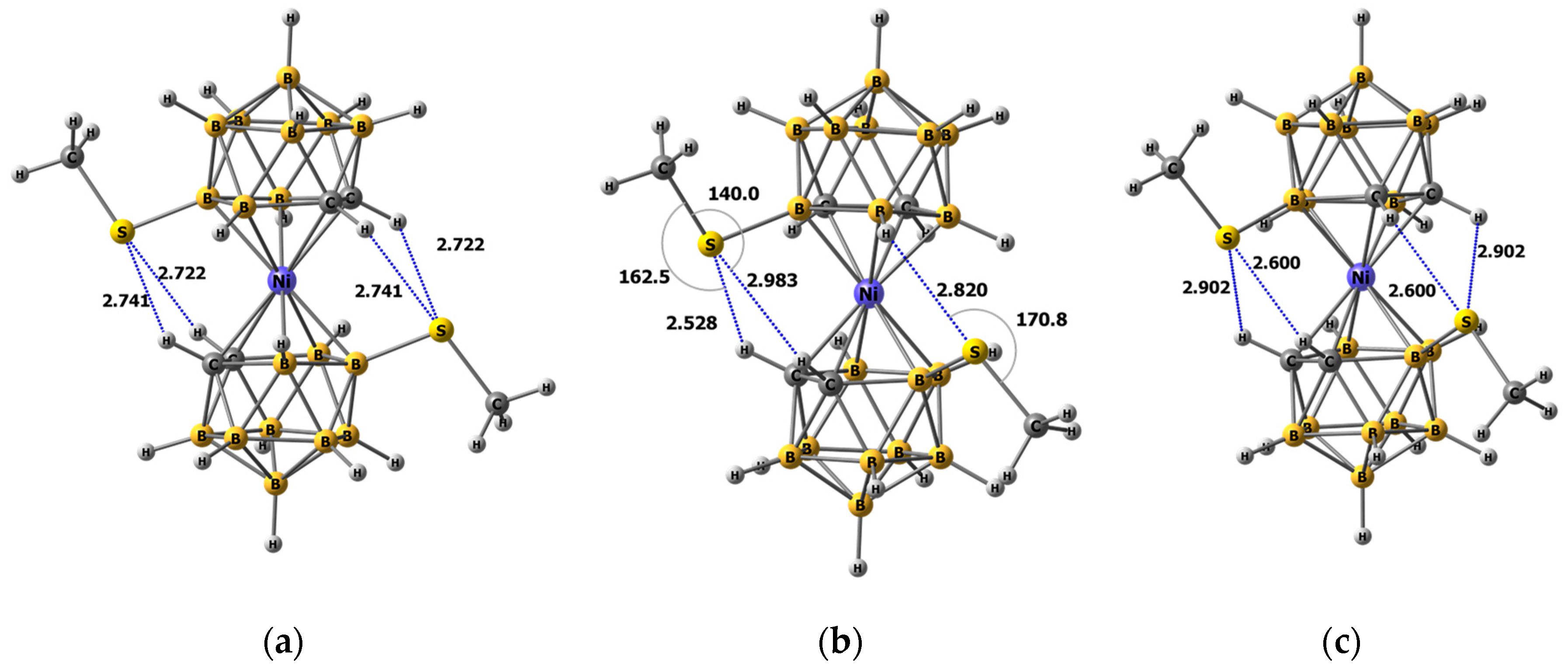
2.3. Quantum Chemical Calculations
3. Materials and Methods
3.1. General Considerations
3.2. Synthesis of Methylsulfanyl Derivatives of Nickel(III) Bis(Dicarbollide)
3.2.1. Synthesis of (Bu4N)[8,8′-(MeS)2-3,3′-Ni(1,2-C2B9H10)2]
3.2.2. Synthesis of (Bu4N)[4,4′-(MeS)2-3,3′-Ni(1,2-C2B9H10)2] (rac-isomer) and (Bu4N)[4,7′-(MeS)2-3,3′- Ni(1,2-C2B9H10)2] (meso-isomer)
3.3. X-Ray Diffraction Study
3.4. Quantum Chemical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Feringa, B.L.; Browne, W.R. Molecular Switches, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2011; 792p. [Google Scholar]
- Karnati, C.; Ji, H.-F. Molecular logic gates. In Micromanufacturing and Nanotechnology; Mahalik, N.P., Ed.; Springer-Verlag: Berlin, Germany, 2006; 468p. [Google Scholar]
- Katz, E. (Ed.) Molecular and Supramolecular Information Processing. From Molecular Switches to Logic. Systems; Wiley-VCH: Weinheim, Germany, 2012; 364p. [Google Scholar]
- de Silva, A.P. Molecular Logic.-based Computation; RSC Publishing: Cambridge, UK, 2013; 398p. [Google Scholar]
- Andreasson, I.; Pischel, U. Molecules with a Sense of Logic: A Progress report. Chem. Soc. Rev. 2015, 44, 1053–1069. [Google Scholar] [CrossRef] [PubMed]
- Erbas-Cakmak, S.; Kolemen, S.; Sedgwick, A.C.; Gunnlaugsson, T.; James, T.D.; Yoon, J.; Akkaya, E.U. Molecular Logic Gates: The Past, Present and Future. Chem. Soc. Rev. 2018, 47, 2228–2248. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, M.F.; Zink, J.I.; Skelton, J.M.; Bayer, M.B.; Liu, C.; Livshits, E.; Baer, R.; Neuhauser, D. Electrical or Photocontrol of the Rotary Motion of a Metallacarborane. Science 2004, 303, 1849–1852. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, M.F.; Ramachandran, B.M.; Kennedy, R.D.; Knobler, C.B. Approaches to Rotary Molecular Motors. Pure Appl. Chem. 2006, 78, 1299–1304. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I. Chemistry of Nickel and Iron Bis(dicarbollides). A Review. J. Organomet. Chem. 2000, 614–615, 27–36. [Google Scholar] [CrossRef]
- Bühl, M.; Holub, J.; Hnyk, D.; Macháček, J. Computational Studies of Structures and Properties of Metallaboranes. 2. Transition-Metal Dicarbollide Complexes. Organometallics 2006, 25, 2173–2181. [Google Scholar] [CrossRef]
- Safronov, A.V.; Shlyakhtina, N.I.; Everett, T.A.; VanGordon, M.R.; Sevryugina, Y.V.; Jalisatgi, S.S.; Hawthorne, M.F. Direct Observation of Bis(dicarbollyl)nickel Conformers in Solution by Fluorescence Spectroscopy: An Approach to Redox-Controlled Metallacarborane Molecular Motors. Inorg. Chem. 2014, 53, 10045–10053. [Google Scholar] [CrossRef]
- Shlyakhtina, N.I.; Safronov, A.V.; Sevryugina, Y.V.; Jalisatgi, S.S.; Hawthorne, M.F. Synthesis, Characterization, and Preliminary Fluorescence Study of a Mixed Ligand Bis(dicarbollyl)nickel Complex bearing a Tryptophan-BODIPY FRET Couple. J. Organomet. Chem. 2015, 798, 234–244. [Google Scholar] [CrossRef]
- Warren, L.F.; Hawthorne, M.F. Chemistry of the Bis[π.-(3)-1,2-dicarbollyl] Metalates of Nickel and Palladium. J. Am. Chem. Soc. 1970, 92, 1157–1173. [Google Scholar] [CrossRef]
- Andreichuk, E.P.; Anisimov, A.A.; Shmalko, A.V.; Suponitsky, K.Y.; Sivaev, I.B.; Bregadze, V.I. Stability of Nickel Bis(dicarbollide) Complexes. Mendeleev Commun. 2019, 29, 534–536. [Google Scholar] [CrossRef]
- Sivaev, I.B. Ferrocene and Transition Metal Bis(dicarbollides) as Platform for Design of Rotatory Molecular Switches. Molecules 2017, 22, 2201. [Google Scholar] [CrossRef] [PubMed]
- Bühl, M.; Hnyk, D.; Macháček, J. Computational Study of Structures and Properties of Metallaboranes: Cobalt Bis(dicarbollide). Chem. Eur. J. 2005, 11, 4109–4120. [Google Scholar] [CrossRef] [PubMed]
- Zaulet, A.; Teixidor, F.; Bauduin, P.; Diat, O.; Hirva, P.; Ofori, A.; Viñas, C. Deciphering the Role of the Cation in Anionic Cobaltabisdicarbollide Clusters. J. Organomet. Chem. 2018, 865, 214–225. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Erokhina, S.A.; Suponitsky, K.Y.; Godovikov, I.A.; Filippov, O.A.; Fabrizi de Biani, F.; Corsini, M.; Chizhov, A.O.; Sivaev, I.B. Methylsulfanyl-Stabilized Rotamers of Cobalt Bis(dicarbollide). Eur. J. Inorg. Chem. 2017, 38–39, 4444–4451. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Erokhina, S.A.; Suponitsky, K.Y.; Anisimov, A.A.; Laskova, J.N.; Godovikov, I.A.; Fabrizi de Biani, F.; Corsini, M.; Sivaev, I.B.; Bregadze, V.I. Synthesis and Structure of Bis(methylsulfanyl) Derivatives of Iron Bis(dicarbollide). J. Organomet. Chem. 2018, 865, 239–246. [Google Scholar] [CrossRef]
- Timofeev, S.V.; Anufriev, S.A.; Sivaev, I.B.; Bregadze, V.I. Synthesis of Cobalt Bis(8-methylthio-1,2-Dicarbollide)-Pentacarbonyltungsten Complexes. Russ. Chem. Bull. 2018, 67, 570–572. [Google Scholar] [CrossRef]
- Sivaev, I.B. Intramolecular Non-Covalent Interactions in Carboranes and Metallacarboranes. In Proceedings of the 8th European Conference on Boron Chemistry (EUROBORON-8), Montpellier, France, 24–27 June 2019. KL2. [Google Scholar]
- Hawthorne, M.F.; Young, D.C.; Andrews, T.D.; Howe, D.V.; Pilling, R.L.; Pitts, A.D.; Reintjes, M.; Warren, L.F.; Wegner, P.A. π-Dicarbollyl Derivatives of the Transition Metals. Metallocene Analogs. J. Am. Chem. Soc. 1968, 90, 879–896. [Google Scholar] [CrossRef]
- Spokoyny, A.M.; Li, T.C.; Farha, O.K.; Machan, C.W.; She, C.; Stern, C.L.; Marks, T.J.; Hupp, J.T.; Mirkin, C.A. Electronic Tuning of Nickel-Based Bis(dicarbollide) Redox Shuttles in Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 2010, 49, 5339–5343. [Google Scholar] [CrossRef]
- Shmal’ko, A.V.; Anufriev, S.A.; Anisimov, A.A.; Stogniy, M.Y.; Sivaev, I.B.; Bregadze, V.I. Synthesis of Cobalt and Nickel 6,6’-Diphenyl Bis(dicarbollides). Russ. Chem. Bull. 2019, 68, 1239–1247. [Google Scholar] [CrossRef]
- Kennedy, R.D.; Knobler, C.B.; Hawthorne, M.F. Toward Unidirectional Motion in Nickelacarboranes: Characterization of Diastereomeric Nickel Bis(dicarbollide) Complexes Derived from the [nido-7-CH3-7,8-C2B9H11]− Anion. Inorg. Chem. 2009, 48, 9377–9384. [Google Scholar] [CrossRef]
- Pennanen, T.O.; Macháček, J.; Taubert, S.; Vaara, J.; Hnyk, D. Ferrocene-Like Iron Bis(dicarbollide), [3-FeIII-(1,2-C2B9H11)2]−. The First Experimental and Theoretical Refinement of a Paramagnetic 11B NMR Spectrum. Phys. Chem. Chem. Phys. 2010, 12, 7018–7025. [Google Scholar] [CrossRef] [PubMed]
- Stogniy, M.Y.; Erokhina, S.A.; Kosenko, I.D.; Semioshkin, A.A.; Sivaev, I.B. Dimethyloxonium and Methoxy Derivatives of nido-Carborane and Metal Complexes Thereof. Inorganics 2019, 7, 46. [Google Scholar] [CrossRef]
- Petřiček, V.; Maly, K.; Petřina, A.; Baše, K.; Linek, A. The Crystal and Molecular Structure of (s-1,4′,2,1′)- 3,3′-commo-Bis [8-Methoxy-1,2-Dicarba-3-Nickela-closo-Dodecaborane(11)], [8-CH3O-1,2-C2B9H10]2Ni. Z. Kristallogr. 1984, 166, 1–10. [Google Scholar] [CrossRef]
- Nuñez, R.; Tutusaus, O.; Teixidor, F.; Viñas, C.; Sillanpää, R.; Kivekäs, R. Highly Stable Neutral and Positively Charged Dicarbollide Sandwich Complexes. Chem. Eur. J. 2005, 11, 5637–5647. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Erokhina, S.A.; Anisimov, A.A.; Suponitsky, K.Y.; Sivaev, I.B.; Bregadze, V.I. 10- NCCH2CH2OCH2CH2C≡N-7,8-C2B9H11: Synthesis and Reactions with Various Nucleophiles. Polyhedron 2019, 174, 114170. [Google Scholar] [CrossRef]
- Sheremetev, A.B.; Palysaeva, N.V.; Struchkova, M.I.; Suponitsky, K.Y.; Antipin, M.Y. Copper-Catalyzed C-N Coupling Reactions of Nitrogen-Rich Compounds-Reaction of Iodofurazans with s-Tetrazinylamines. Eur. J. Org. Chem. 2012, 2266–2272. [Google Scholar] [CrossRef]
- Suponitsky, K.Y.; Lyssenko, K.A.; Ananyev, I.V.; Kozeev, A.M.; Sheremetev, A.B. Role of Weak Intermolecular Interactions in the Crystal Structure of Tetrakis-Furazano[3,4-c:3′,4′-g:3″,4″-k:3‴,4‴-o][1,2,5,6,9,10,13,14]octaazacyclohexadecine and its Solvates. Cryst. Growth Des. 2014, 14, 4439–4449. [Google Scholar] [CrossRef]
- Database of Ionic Radii, Imperial College, London. Available online: http://abulafia.mt.ic.ac.uk/shannon/ptable.php (accessed on 15 November 2019).
- Murray, J.S.; Lane, P.; Politzer, P. Expansion of the σ-Hole Concept. J. Mol. Model. 2009, 15, 723–729. [Google Scholar] [CrossRef]
- Mahmudov, K.T.; Kopylovich, M.N.; da Silva, M.F.C.G.; Pombeiro, A.J.L. Chalcogen Bonding in Synthesis, Catalysis and Design of Materials. Dalton Trans. 2017, 46, 10121–10138. [Google Scholar] [CrossRef]
- Scilabra, P.; Terraneo, G.; Resnati, G. The Chalcogen Bond in Crystalline Solids: A World Parallel to Halogen Bond. Acc. Chem. Res. 2019, 52, 1313–1324. [Google Scholar] [CrossRef]
- Aakeroy, C.B.; Bryce, D.L.; Desiraju, G.R.; Frontera, A.; Legon, A.C.; Nicotra, F.; Rissanen, K.; Scheiner, S.; Terraneo, G.; Metrangolo, P.; et al. Definition of the Chalcogen Bond (IUPAC Recommendations 2019). Pure Appl. Chem. 2019, 91, 1889–1892. [Google Scholar] [CrossRef]
- Fanfrlík, J.; Hnyk, D.; Hobza, P. Chalcogen Bonding due to the Exo-Substitution of Icosahedral Dicarbaborane. Molecules 2019, 24, 2657. [Google Scholar] [CrossRef] [PubMed]
- Esrafili, M.D.; Mohammadian-Sabet, F.; Baneshi, M.M. An ab Initio Investigation of Chalcogen-Hydride Interactions Involving HXeH as a Chalcogen Bond Acceptor. Struct. Chem. 2016, 27, 785–792. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Jena, S.; Dutta, J.; Rana, A.; Biswal, H.S. Nature and Strength of M-H···S and M-H···Se (M = Mn, Fe, & Co) Hydrogen Bond. J. Phys. Chem. A 2019, 123, 2227–2236. [Google Scholar] [CrossRef]
- Ma, N.-N.; Li, S.-J.; Yan, L.-K.; Qiu, Y.-Q.; Su, Z.-M. Switchable NLO Response Induced by Rotation of Metallacarboranes [NiIII/IV(C2B9H11)2]−/0 and C-,B-Functionalized Derivatives. Dalton Trans. 2014, 43, 5069–5075. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Sivaev, I.B.; Suponitsky, K.Y.; Bregadze, V.I. Practical Synthesis of 9-Methylthio-7,8-nido-Carborane [9-MeS-7,8-C2B9H11]-. Some Evidences of BH···X Hydride-Halogen Bonds in 9-XCH2(Me)S-7,8-C2B9H11 (X = Cl, Br, I). J. Organomet. Chem. 2017, 849–850, 315–323. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Sivaev, I.B.; Suponitsky, K.Y.; Godovikov, I.A.; Bregadze, V.I. Synthesis of 10-Methylsulfide and 10-Alkylmethylsulfonium nido-Carborane Derivatives: B-H···π Interactions between the B-H-B Hydrogen Atom and Alkyne Group in 10-RC≡CCH2S(Me)-7,8-C2B9H11. Eur. J. Inorg. Chem. 2017, 38–39, 4436–4443. [Google Scholar] [CrossRef]
- Wielandt, J.W.; Ruckerbauer, D. Hexakis(acetylacetonato)trinickel(II). Inorg. Synth. 2010, 35, 121–122. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals; Butterworth-Heinemann: Burlington, MA, USA, 2009; 790p. [Google Scholar]
- APEX2 and SAINT; Bruker AXS Inc.: Madison, WI, USA, 2014.
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-Functional Approximation for the Correlation-Energy of the Inhomogeneous Electron-Gas. Phys. Rev. B Condens. Matter 1986, 33, 8822–8824. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic-Behavior. Phys Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Dunning, T.H. Gaussian-Basis Sets for Use in Correlated Molecular Calculations. 1. The Atoms Boron through Neon and Hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Woon, D.E.; Dunning, T.H. Gaussian-Basis Sets for Use in Correlated Molecular Calculations. 3. The Atoms Aluminum through Argon. J. Chem. Phys. 1993, 98, 1358–1371. [Google Scholar] [CrossRef]
- Balabanov, N.B.; Peterson, K.A. Systematically Convergent Basis Sets for Transition Metals. I. All-Electron Correlation Consistent Basis Sets for the 3d Elements Sc-Zn. J. Chem. Phys. 2005, 123, 064107. [Google Scholar] [CrossRef]
- Balabanov, N.B.; Peterson, K.A. Basis Set Limit Electronic Excitation Energies, Ionization Potentials, and Electron Affinities for the 3d Transition Metal Atoms: Coupled Cluster and Multireference Methods. J. Chem. Phys. 2006, 125, 074110. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Keith, T.A. AIMAll (Version 15.05.18); TK Gristmill Software: Overland Park, KS, USA, 2015. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput Chem 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Espinosa, E.; Alkorta, I.; Rozas, I.; Elguero, J.; Molins, E. About the Evaluation of the Local Kinetic, Potential and Total Energy Densities in Closed-Shell Interactions. Chem. Phys. Lett. 2001, 336, 457–461. [Google Scholar] [CrossRef]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen Bond Strengths Revealed by Topological Analyses of Experimentally Observed Electron Densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Dmitrienko, A.O.; Karnoukhova, V.A.; Potemkin, A.A.; Struchkova, M.I.; Kryazhevskikh, I.A.; Suponitsky, K.Y. The Influence of Halogen type on Structural Features of Compounds Containing α-Halo-α,α-Dinitroethyl Moieties. Chem. Heterocycl. Comp. 2017, 53, 532–539. [Google Scholar] [CrossRef]
- Suponitsky, K.Y.; Burakov, N.I.; Kanibolotsky, A.L.; Mikhailov, V.A. Multiple Noncovalent Bonding in Halogen Complexes with Oxygen Organics. I. Tertiary Amides. J. Phys. Chem. A. 2016, 120, 4179–4190. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| M = Fe | M = Co | M = Ni | |
|---|---|---|---|
| C(1)H(1)···S(1′) distance, Å | 2.75 | 2.71(3) | 2.68 |
| C(2)H(2)···S(1′) distance, Å | 2.69 | 2.70(3) | 2.83 |
| S(1′)Me group rotation angle, º | 27.9(2) | 29.59(14) | 30.1(9) |
| M···ligand plane distance, Å | 1.530(3) | 1.474(2) | 1.555(10) |
| B(8′)H(8′)···S(1) distance, Å | 2.61 | 2.70(3) | 2.53 |
| S(1)Me group rotation angle, º | 6.5(2) | 6.66(13) | 10.9(9) |
| M···ligand plane distance, Å | 1.538(2) | 1.482(2) | 1.582(9) |
| ligand rotation angle, º | 102.4(3) | 104.2(2) | 105.0(11) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anufriev, S.A.; Suponitsky, K.Y.; Filippov, O.A.; Sivaev, I.B. Synthesis and Structure of Methylsulfanyl Derivatives of Nickel Bis(Dicarbollide). Molecules 2019, 24, 4449. https://doi.org/10.3390/molecules24244449
Anufriev SA, Suponitsky KY, Filippov OA, Sivaev IB. Synthesis and Structure of Methylsulfanyl Derivatives of Nickel Bis(Dicarbollide). Molecules. 2019; 24(24):4449. https://doi.org/10.3390/molecules24244449
Chicago/Turabian StyleAnufriev, Sergey A., Kyrill Yu. Suponitsky, Oleg A. Filippov, and Igor B. Sivaev. 2019. "Synthesis and Structure of Methylsulfanyl Derivatives of Nickel Bis(Dicarbollide)" Molecules 24, no. 24: 4449. https://doi.org/10.3390/molecules24244449
APA StyleAnufriev, S. A., Suponitsky, K. Y., Filippov, O. A., & Sivaev, I. B. (2019). Synthesis and Structure of Methylsulfanyl Derivatives of Nickel Bis(Dicarbollide). Molecules, 24(24), 4449. https://doi.org/10.3390/molecules24244449








