Production and Application of Biosurfactant Produced by Bacillus licheniformis Ali5 in Enhanced Oil Recovery and Motor Oil Removal from Contaminated Sand
Abstract
1. Introduction
2. Results and Discussions
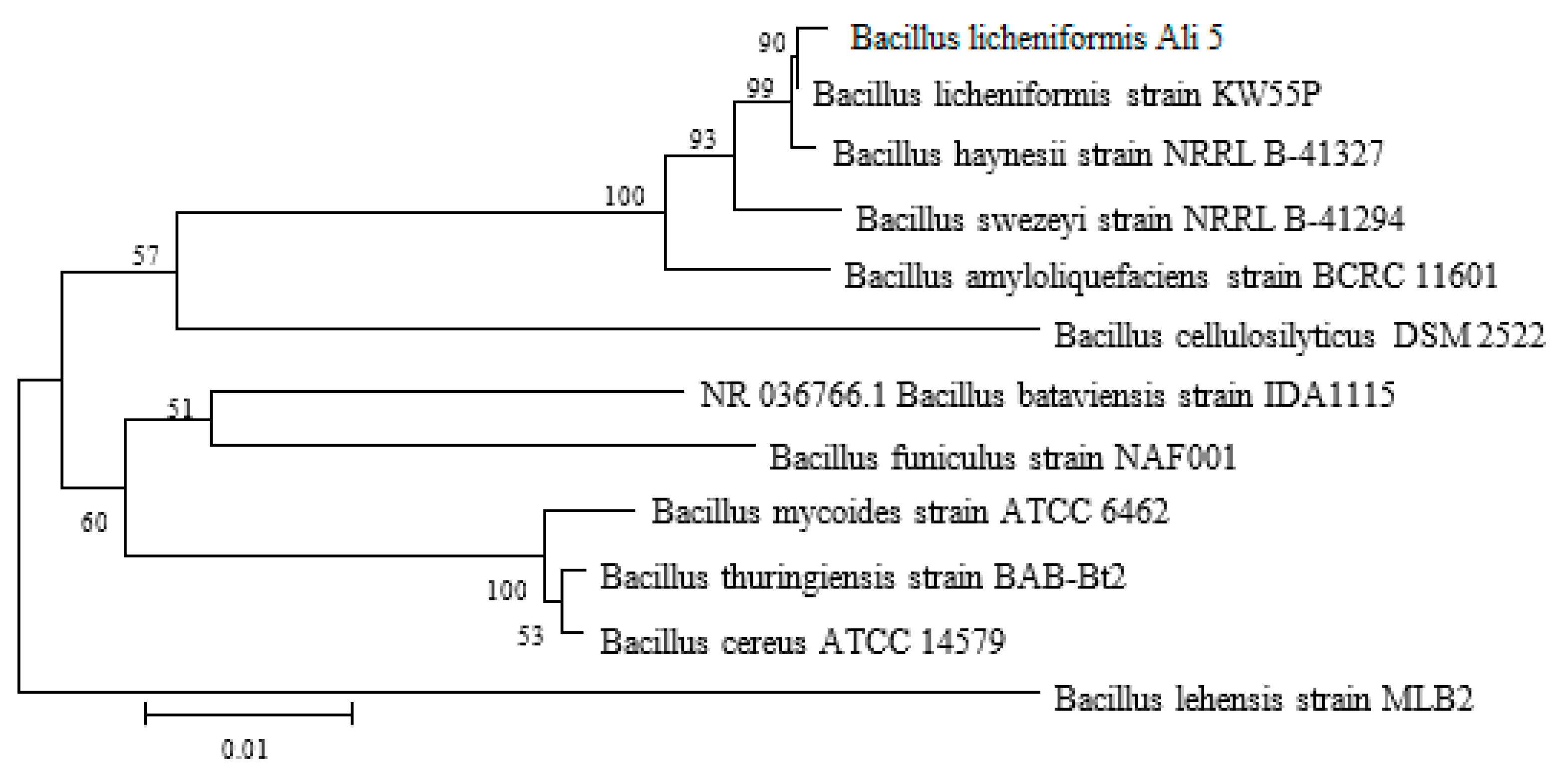
2.1. Screening and Identification of the Bacterial Isolate
2.2. Media and Carbon Source Optimization
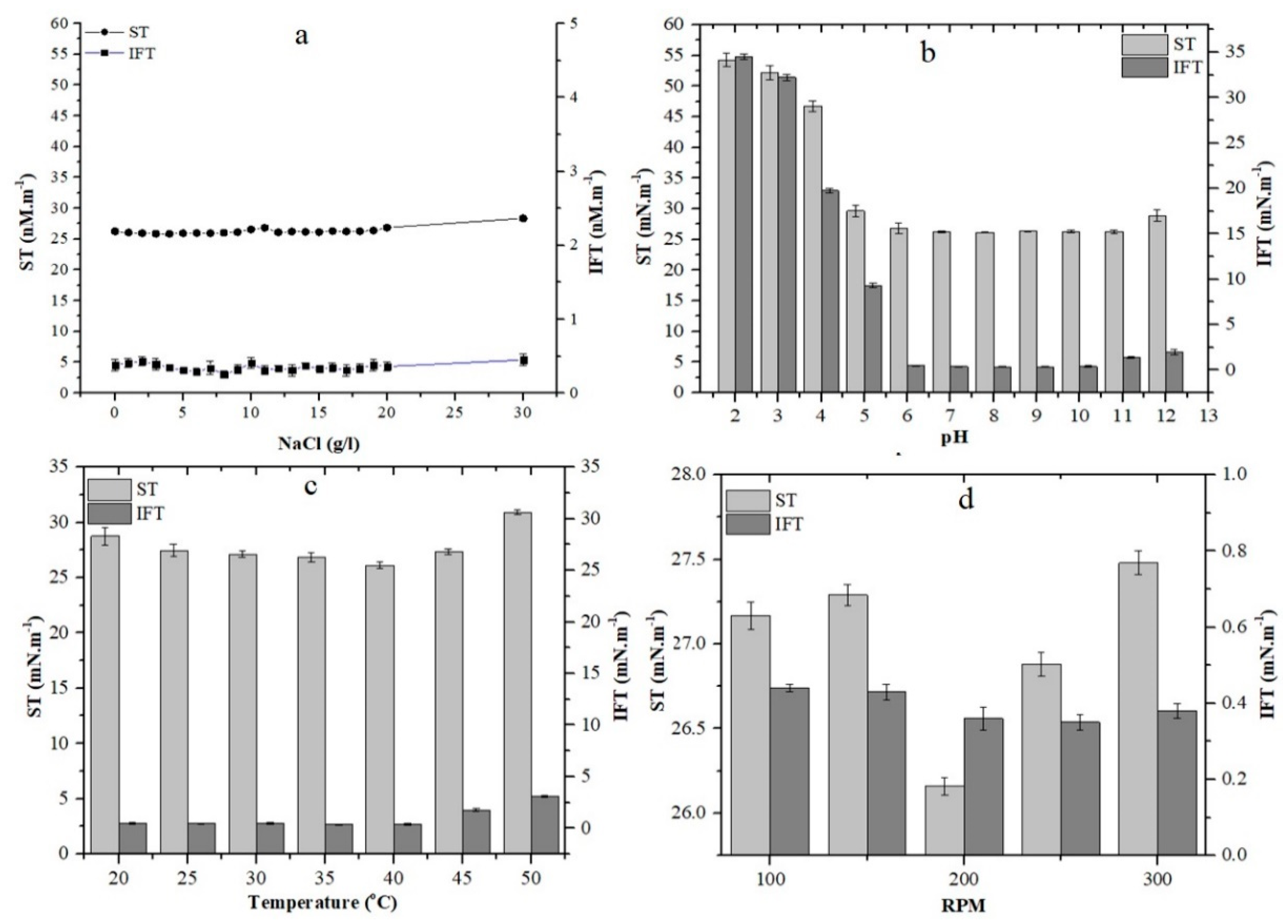
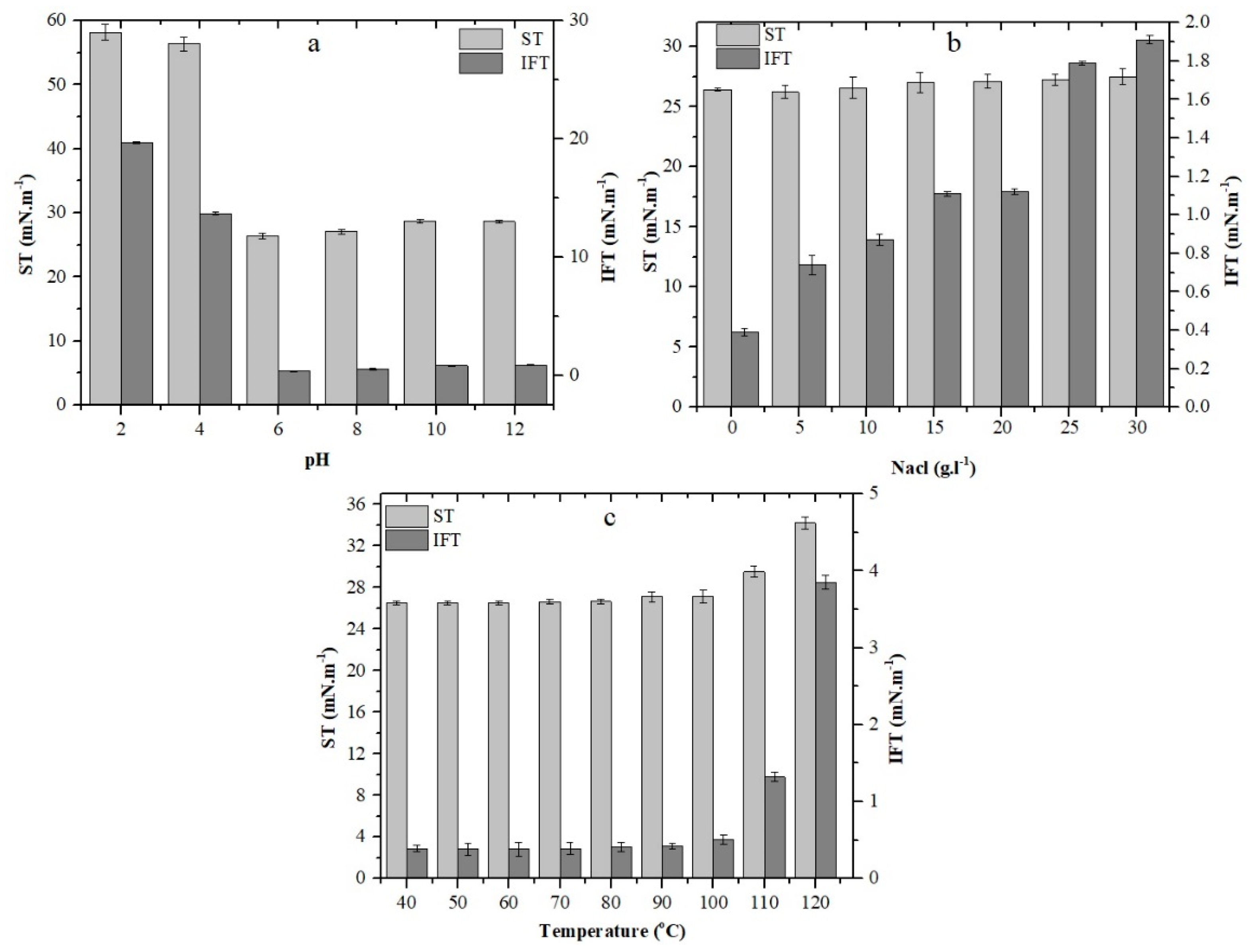
2.3. Effect of Environmental Conditions on Growth and Stability
2.4. Determination of Emulsification Index E24
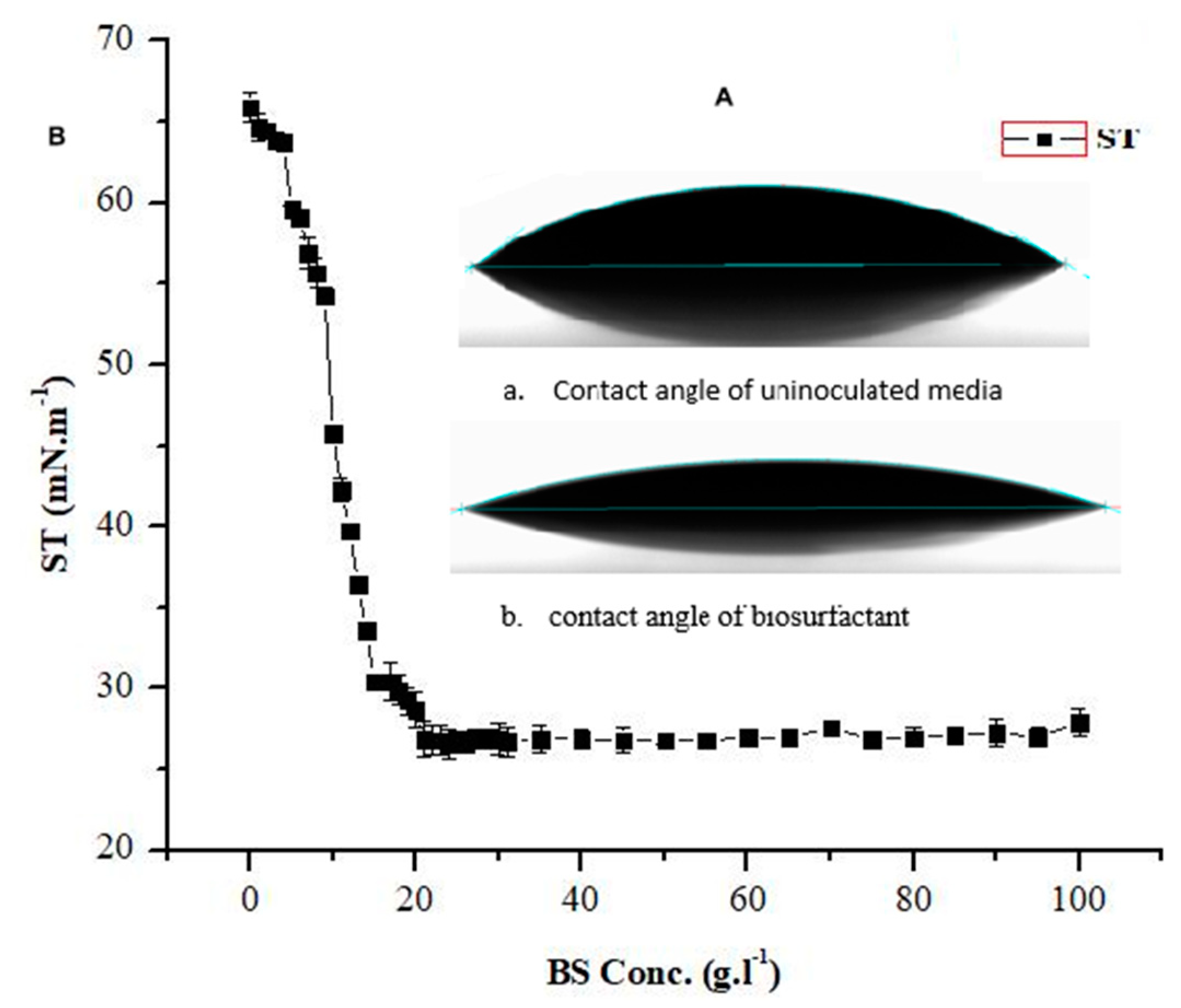
2.5. Critical Micelle Concentration (CMC) Determination
2.6. Surface Wettability Alteration
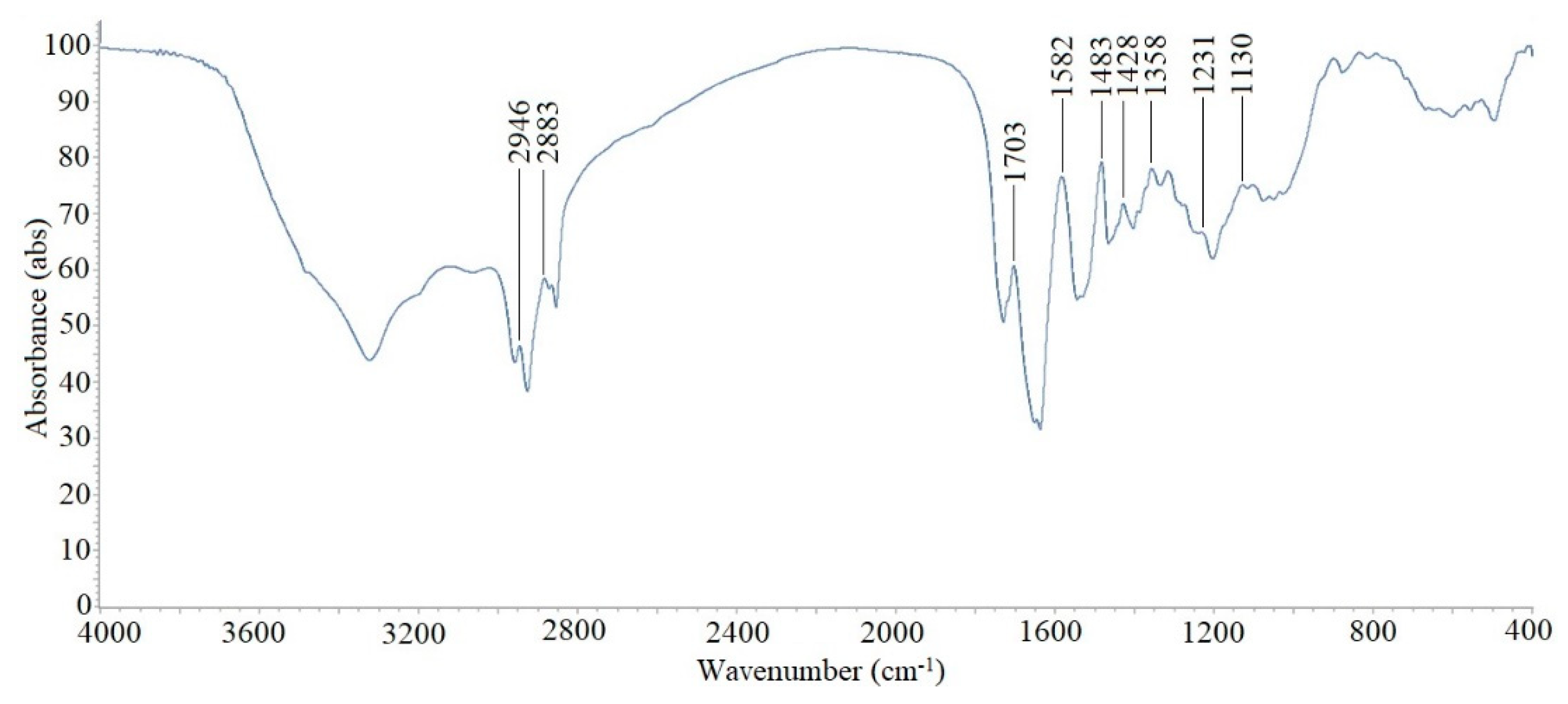
2.7. Partial Structural Identification Using Fourier Transform Infrared Spectroscopy (FTIR)
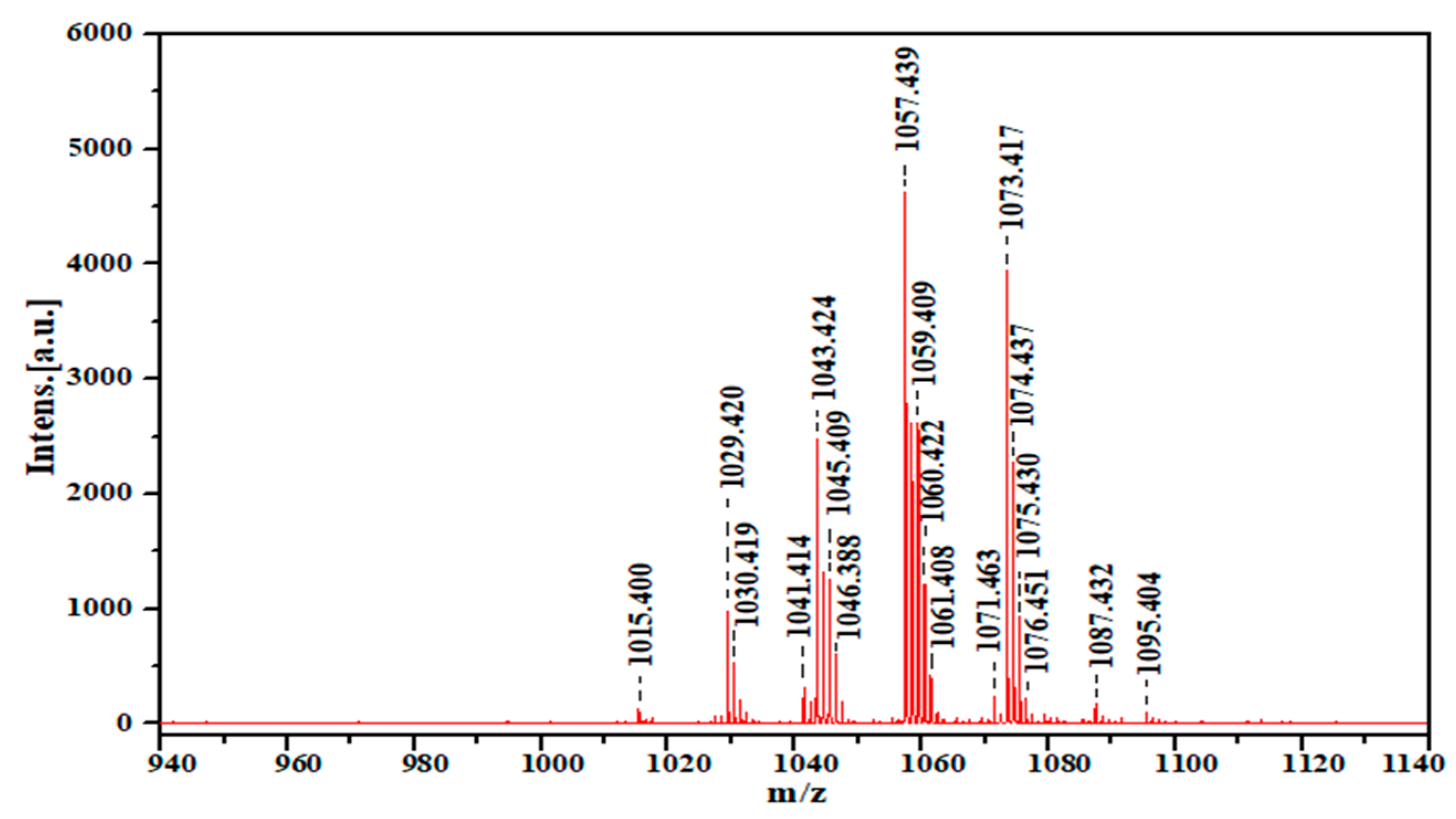
2.8. MALDI TOF-MS of Produced Biosurfactant
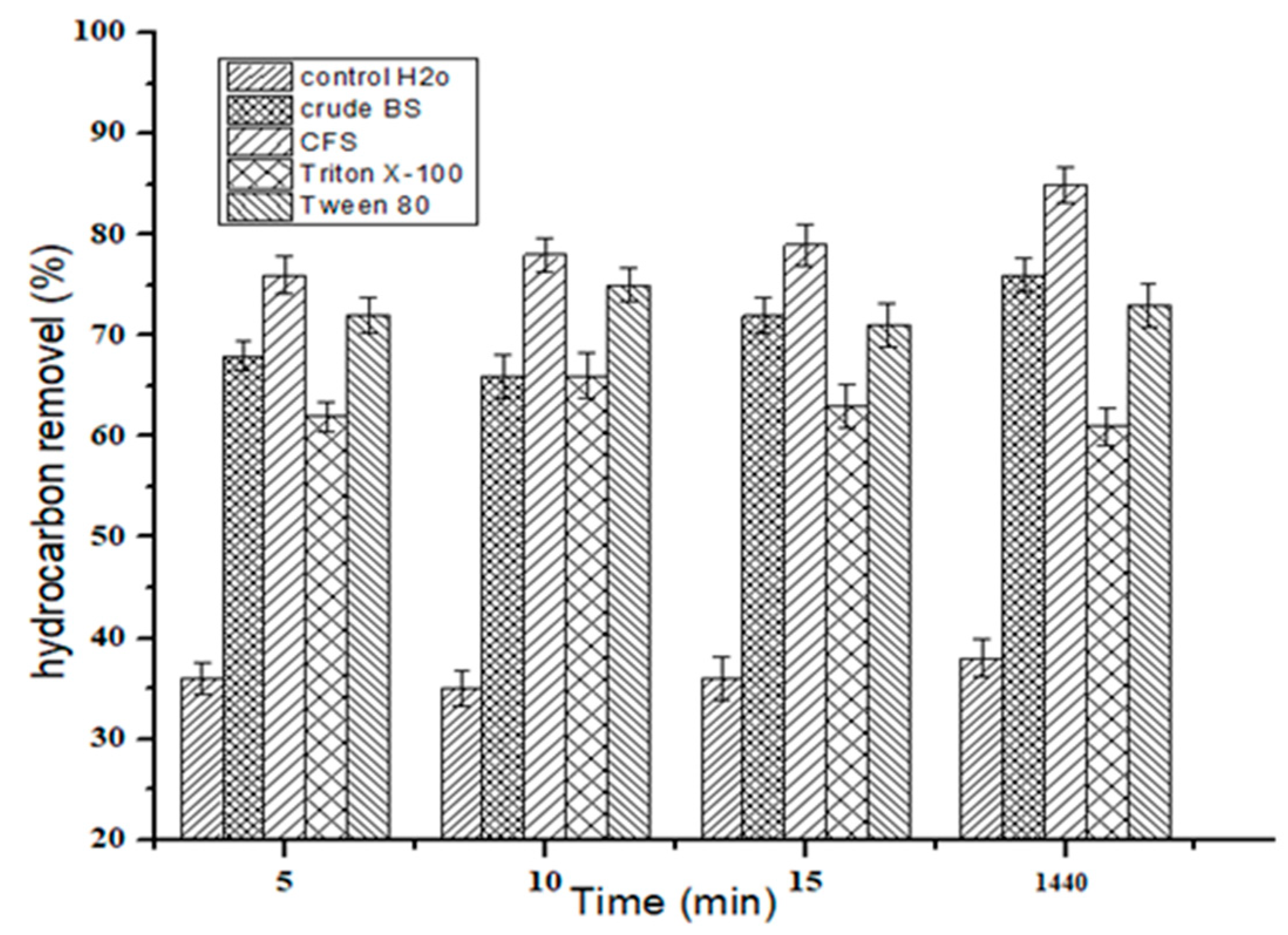
2.9. Biosurfactant Application in Hydrocarbon Removal from Contaminated Sand
2.10. Enhanced Oil Recovery Using Sand-Packed Column Test
3. Materials and Methods
3.1. Chemicals/Reagents and Isolation of Biosurfactant-Producing Bacteria
3.2. Screening Assays for Biosurfactant Producers
3.2.1. Oil-Spreading Method
3.2.2. Drop-Collapsing Test
3.2.3. Emulsification Index (E24) Measurement
3.3. Identification of Selected Bacterial Isolate
3.4. Media Optimization
Carbon Source Optimization
3.5. Determination of Surface Tension, Interfacial Tension and Alteration in Wettability
3.6. Biosurfactant Extraction and Partial Purification
3.7. Critical Micelle Concentration (CMC) of Biosurfactant
3.8. Effect of Environmental Factors on Production and Stability of Biosurfactant
3.9. Fourier Transform Infrared Spectroscopy (FT-IR)
3.10. Molecular Mass Determination of the Biosurfactant
3.11. Hydrocarbon (Motor Oil) Removal from Soil
3.12. Enhanced Oil Recovery at Laboratory Scale
- (i)
- Column saturation with brine: To ensure the removal of gases, nitrogen gas for 5 min was passed from the column and a vacuum was held for 5 min. Brine was then flooded at 7 kg·cm−2 air pressure, pore volume was calculated as the brine volume required for 100% saturation of the column. Three pore volume (PV) of brine was required for the column saturation.
- (ii)
- Oil saturation of column: Light crude oil (Xinjiang crude oil, China) density 850 kg/m3 was passed through the sand pack column under pressure, as did earlier with brine until remaining brine saturation is reached. As oil passed in the column it displaced brine which was collected at the bottom end, Soi or initial saturation of oil was calculated by measuring the brine displaced by oil, also called OOIP (original oil in place).
- (iii)
- Column flooding with brine: The column was flooded with brine again and the displaced oil was collected until there was no oil discharge from effluent. The PV of brine flooding was calculated to be about 6 to 9, termed as (Sor). The amount of oil retained was volumetrically determined by measuring the oil displaced. The residue oil saturation (Sor) was measured by assessing the displaced oil volume.
- (iv)
- Biosurfactant flooding: Sand-packed columns were flooded with biosurfactant as described earlier for brine and oil, with 0.6 pore volumes of biosurfactant (CFS), flow rate was set to 3 mL/min. The biosurficant was passed through the columns, and the columns were incubated for 24 h following brine flooding. Column discharges were collected and the oil recovered using CFS flooding was measured. The oil recovery percentage was calculated using following formula,
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- He, L.; Lin, F.; Li, X.; Sui, H.; Xu, Z. Interfacial sciences in unconventional petroleum production: From fundamentals to applications. Chem. Soc. Rev. 2015, 44, 5446–5494. [Google Scholar] [CrossRef] [PubMed]
- Rudyk, S.; Spirov, P.; Samuel, P.; Joshi, S.J. Vaporization of Crude Oil by Supercritical CO2 at Different Temperatures and Pressures: Example from Gorm Field in the Danish North Sea. Energy Fuels 2017, 31, 6274–6283. [Google Scholar] [CrossRef]
- Al-Sulaimani, H.; Al-Wahaibi, Y.; Al-Bahry, S.; Elshafle, A.; Al-Bemani, A.; Joshi, S.; Zargari, S. Optimization and partial characterization of biosurfactants produced by bacillus species and their potential for ex-situ enhanced oil recovery. SPE J. 2011, 16, 672–682. [Google Scholar] [CrossRef]
- Al-Wahaibi, Y.; Al-Hadrami, H.; Al-Bahry, S.; Elshafie, A.; Al-Bemani, A.; Joshi, S. Injection of biosurfactant and chemical surfactant following hot water injection to enhance heavy oil recovery. Pet. Sci. 2016, 13, 100–109. [Google Scholar] [CrossRef]
- Bryant, R.S.; Donaldson, E.C.; Yen, T.F.; Chilingarian, G.V. Microbial Enhanced Oil Recovery. Dev. Pet. Sci. 1989, 17, 423–450. [Google Scholar] [CrossRef]
- Mulligan, C.N. Recent advances in the environmental applications of biosurfactants. Curr. Opin. Colloid Interface Sci. 2009, 14, 372–378. [Google Scholar] [CrossRef]
- Santos, D.K.F.; Meira, H.M.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Biosurfactant production from Candida lipolytica in bioreactor and evaluation of its toxicity for application as a bioremediation agent. Process Biochem. 2017, 54, 20–27. [Google Scholar] [CrossRef]
- Luna, J.M.; Filho, A.S.S.; Rufino, R.D.; Sarubbo, L.A. Production of biosurfactant from Candida bombicola URM 3718 for environmental applications. Chem. Eng. Trans. 2016, 49, 583–588. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, X.; Chen, C.; Zhu, L. Enhanced soil washing of phenanthrene by a plant-derived natural biosurfactant, Sapindus saponin. Colloids Surf. A Physicochem. Eng. Asp. 2013, 425, 122–128. [Google Scholar] [CrossRef]
- Jha, S.S.; Joshi, S.J.; Geetha, S.J. Lipopeptide production by Bacillus subtilis R1 and its possible applications. Braz. J. Microbiol. 2016, 47, 955–964. [Google Scholar] [CrossRef]
- Simpson, D.R.; Natraj, N.R.; McInerney, M.J.; Duncan, K.E. Biosurfactant-producing Bacillus are present in produced brines from Oklahoma oil reservoirs with a wide range of salinities. Appl. Microbiol. Biotechnol. 2011, 91, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, M.; Pastore, G.M. Production and properties of a surfactant obtained from Bacillus subtilis grown on cassava wastewater. Bioresour. Technol. 2006, 97, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Juang, R.S.; Wei, Y.H. Applications of a lipopeptide biosurfactant, surfactin, produced by microorganisms. Biochem. Eng. J. 2015, 103, 158–169. [Google Scholar] [CrossRef]
- Verma, N. Biosurfactant Production and Biodesulphurization: Integrated Approach for Fuel Processing. Adv. Biotechnol. Microbiol. 2017, 4, 9–13. [Google Scholar] [CrossRef]
- De Almeida, D.G.; Soares Da Silva, R.D.C.F.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Banat, I.M.; Sarubbo, L.A. Biosurfactants: Promising molecules for petroleum biotechnology advances. Front. Microbiol. 2016, 7, 1718. [Google Scholar] [CrossRef] [PubMed]
- Pacwa-Płociniczak, M.; Płaza, G.A.; Piotrowska-Seget, Z.; Cameotra, S.S. Environmental applications of biosurfactants: Recent advances. Int. J. Mol. Sci. 2011, 12, 633–654. [Google Scholar] [CrossRef] [PubMed]
- Adelowo, O.O.; Alagbe, S.O.; Ayandele, A.A. Time-dependent stability of used engine oil degradation by cultures of Pseudomonas fragi and Achromobacter aerogenes. Afr. J. Biotechnol. 2006, 5, 2476–2479. [Google Scholar]
- Chandankere, R.; Yao, J.; Cai, M.; Masakorala, K.; Jain, A.K.; Choi, M.M.F. Properties and characterization of biosurfactant in crude oil biodegradation by bacterium Bacillus methylotrophicus USTBa. Fuel 2014, 122, 140–148. [Google Scholar] [CrossRef]
- Urum, K.; Grigson, S.; Pekdemir, T.; McMenamy, S. A comparison of the efficiency of different surfactants for removal of crude oil from contaminated soils. Chemosphere 2006, 62, 1403–1410. [Google Scholar] [CrossRef]
- Lai, C.C.; Huang, Y.C.; Wei, Y.H.; Chang, J.S. Biosurfactant-enhanced removal of total petroleum hydrocarbons from contaminated soil. J. Hazard. Mater. 2009, 167, 609–614. [Google Scholar] [CrossRef]
- Osunde, J.E.; Balogun, S.A. Micro-organisms in enhanced oil recovery. Ife J. Sci. 2013, 15, 617–630. [Google Scholar]
- Varjani, S.J.; Pollution, G.; Board, C.; Bateja, S.; Gas, N.; Limited, C.; Upasani, V.N. Screening and identification of biosurfactant (bioemulsifier) producing bacteria from crude oil contaminated sites of Gujarat, India. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3297, 9205–9213. [Google Scholar]
- Joshi, S.J.; Al-Wahaibi, Y.M.; Al-Bahry, S.N.; Elshafie, A.E.; Al-Bemani, A.S.; Al-Bahri, A.; Al-Mandhari, M.S. Production, characterization, and application of bacillus licheniformis W16 biosurfactant in enhancing oil recovery. Front. Microbiol. 2016, 7, 1853. [Google Scholar] [CrossRef] [PubMed]
- Purwasena, I.A.; Astuti, D.I.; Syukron, M.; Amaniyah, M.; Sugai, Y. Stability test of biosurfactant produced by Bacillus licheniformis DS1 using experimental design and its application for MEOR. J. Pet. Sci. Eng. 2019, 183. [Google Scholar] [CrossRef]
- De Faria, A.F.; Teodoro-Martinez, D.S.; De Oliveira Barbosa, G.N.; Gontijo Vaz, B.; Serrano Silva, Í.; Garcia, J.S.; Tótola, M.R.; Eberlin, M.N.; Grossman, M.; Alves, O.L.; et al. Production and structural characterization of surfactin (C 14/Leu7) produced by Bacillus subtilis isolate LSFM-05 grown on raw glycerol from the biodiesel industry. Process Biochem. 2011, 46, 1951–1957. [Google Scholar] [CrossRef]
- Al-Wahaibi, Y.; Joshi, S.; Al-Bahry, S.; Elshafie, A.; Al-Bemani, A.; Shibulal, B. Biosurfactant production by Bacillus subtilis B30 and its application in enhancing oil recovery. Colloids Surf. B Biointerfaces 2014, 114, 324–333. [Google Scholar] [CrossRef]
- Banat, I.M.; Satpute, S.K.; Cameotra, S.S.; Patil, R.; Nyayanit, N.V. Cost effective technologies and renewable substrates for biosurfactants’ production. Front. Microbiol. 2014, 5, 697. [Google Scholar] [CrossRef]
- Joshi, S.J.; Geetha, S.J.; Desai, A.J. Characterization and Application of Biosurfactant Produced by Bacillus licheniformis R2. Appl. Biochem. Biotechnol. 2015, 177, 346–361. [Google Scholar] [CrossRef]
- Dubey, K.V.; Charde, P.N.; Meshram, S.U.; Shendre, L.P.; Dubey, V.S.; Juwarkar, A.A. Surface-active potential of biosurfactants produced in curd whey by Pseudomonas aeruginosa strain-PP2 and Kocuria turfanesis strain-J at extreme environmental conditions. Bioresour. Technol. 2012, 126, 368–374. [Google Scholar] [CrossRef]
- Darvishi, P.; Ayatollahi, S.; Mowla, D.; Niazi, A. Biosurfactant production under extreme environmental conditions by an efficient microbial consortium, ERCPPI-2. Colloids Surf. B Biointerfaces 2011, 84, 292–300. [Google Scholar] [CrossRef]
- Bognolo, G. Biosurfactants as emulsifying agents for hydrocarbons. Colloids Surf. A Physicochem. Eng. Asp. 1999, 152, 41–52. [Google Scholar] [CrossRef]
- Elshafie, A.E.; Joshi, S.J.; Al-Wahaibi, Y.M.; Al-Bemani, A.S.; Al-Bahry, S.N.; Al-Maqbali, D.; Banat, I.M. Sophorolipids production by Candida bombicola ATCC 22214 and its potential application in microbial enhanced oil recovery. Front. Microbiol. 2015, 6, 1324. [Google Scholar] [CrossRef] [PubMed]
- Sajna, K.V.; Sukumaran, R.K.; Gottumukkala, L.D.; Pandey, A. Crude oil biodegradation aided by biosurfactants from Pseudozyma sp. NII 08165 or its culture broth. Bioresour. Technol. 2015, 191, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.F.B.; Gudiña, E.J.; Costa, R.; Vitorino, R.; Teixeira, J.A.; Coutinho, J.A.P.; Rodrigues, L.R. Optimization and characterization of biosurfactant production by Bacillus subtilis isolates towards microbial enhanced oil recovery applications. Fuel 2013, 111, 259–268. [Google Scholar] [CrossRef]
- Mikkola, R.; Kolari, M.; Andersson, M.A.; Helin, J.; Salkinoja-Salonen, M.S. Toxic lactonic lipopeptide from food poisoning isolates of Bacillus licheniformis. Eur. J. Biochem. 2000, 267, 4068–4074. [Google Scholar] [CrossRef]
- Vater, J.; Kablitz, B.; Wilde, C.; Franke, P.; Mehta, N.; Cameotra, S.S. Matrix-assisted laser desorption ionization-time of flight mass spectrometry of lipopeptide biosurfactants in whole cells and culture filtrates of Bacillus subtilis C-1 isolated from petroleum sludge. Appl. Environ. Microbiol. 2002, 68, 6210–6219. [Google Scholar] [CrossRef]
- De Franҫa, Í.W.L.; Lima, A.P.; Lemos, J.A.M.; Lemos, C.G.F.; Melo, V.M.M.; De Sant’ana, H.B.; Gonҫalves, L.R.B. Production of a biosurfactant by Bacillus subtilis ICA56 aiming bioremediation of impacted soils. Catal. Today 2015, 255, 10–15. [Google Scholar] [CrossRef]
- Silva, S.N.R.L.; Farias, C.B.B.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Glycerol as substrate for the production of biosurfactant by Pseudomonas aeruginosa UCP0992. Colloids Surf. B Biointerfaces 2010, 79, 174–183. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Lin, J.; Wang, W.; Li, S. High-Yield Di-Rhamnolipid Production by Pseudomonas aeruginosa YM4 and its Potential Application in MEOR. Molecules 2019, 24, 1433. [Google Scholar] [CrossRef]
- Suthar, H.; Hingurao, K.; Desai, A.; Nerurkar, A. Evaluation of bioemulsifier mediated Microbial Enhanced Oil Recovery using sand pack column. J. Microbiol. Methods 2008, 75, 225–230. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Pereira, J.F.B.; Costa, R.; Coutinho, J.A.P.; Teixeira, J.A.; Rodrigues, L.R. Biosurfactant-producing and oil-degrading Bacillus subtilis strains enhance oil recovery in laboratory sand-pack columns. J. Hazard. Mater. 2013, 261, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Hu, J.; Wang, Z.; Li, X.; Wang, W. Dynamic investigation of microbial activity in microbial enhanced oil recovery (MEOR). Pet. Sci. Technol. 2018, 36, 1265–1271. [Google Scholar] [CrossRef]
- Reddy, C.O. Isolation and characterization of biosurfactant producing bacteria from groundnut oil cake dumping site for the control of foodborne pathogens. Grain Oil Sci. Technol. 2019, 2, 15–20. [Google Scholar] [CrossRef]
- Alvarez, V.M.; Jurelevicius, D.; Marques, J.M.; de Souza, P.M.; de Araújo, L.V.; Barros, T.G.; de Souza, R.O.M.A.; Freire, D.M.G.; Seldin, L. Bacillus amyloliquefaciens TSBSO 3.8, a biosurfactant-producing strain with biotechnological potential for microbial enhanced oil recovery. Colloids Surf. B Biointerfaces 2015, 136, 14–21. [Google Scholar] [CrossRef]
- San Keskin, N.O.; Han, D.; Devrim Ozkan, A.; Angun, P.; Onarman Umu, O.C.; Tekinay, T. Production and structural characterization of biosurfactant produced by newly isolated staphylococcus xylosus STF1 from petroleum contaminated soil. J. Pet. Sci. Eng. 2015, 133, 689–694. [Google Scholar] [CrossRef]
- Datta, P.; Tiwari, P.; Pandey, L.M. Isolation and Characterization of Biosurfactant Producing and Oil Degrading Bacillus Subtilis MG495086 from Formation Water of Assam Oil Reservoir and its Suitability for Enhanced Oil Recovery; Elsevier: Amsterdam, The Netherlands, 2018; Volume 270, ISBN 9136125822. [Google Scholar] [CrossRef]
- Joshi, S.; Bharucha, C.; Desai, A.J. Production of biosurfactant and antifungal compound by fermented food isolate Bacillus subtilis 20B. Bioresour. Technol. 2008, 99, 4603–4608. [Google Scholar] [CrossRef]
- Yadav, S.; Nerurkar, A.; Desai, A.J. Statistical Optimization of Medium Components for the Production of Biosurfactant by Bacillus licheniformis K51. J. Microbiol. Biotechnol. 2007, 17, 313–319. [Google Scholar]
- Landy, M.; Warren, G.H.; Rosenmanm, S.B.; Colio, L.G. Bacillomycin: An Antibiotic from Bacillus subtilis Active against Pathogenic Fungi. Proc. Soc. Exp. Biol. Med. 1948, 67, 539–541. [Google Scholar] [CrossRef]
- Jenny, K.; Kiippeli, O.; Fiechter, A. Applied Microbiology Biotechnology Biosurfactants from Bacillus licheniformis : Structural analysis and characterization. Appl. Microbiol. Biotechnol. 1991, 36, 5–13. [Google Scholar] [CrossRef]
- Youssef, N.; Simpson, D.R.; Duncan, K.E.; McInerney, M.J.; Folmsbee, M.; Fincher, T.; Knapp, R.M. In Situ Biosurfactant Production by Bacillus Strains Injected into a Limestone Petroleum Reservoir. Appl. Environ. Microbiol. 2007, 73, 1239–1247. [Google Scholar] [CrossRef]
- Cooper, D.G.; Macdonald, C.R.; Duff, S.J.B.; Kosaric, N. Enhanced production of surfactin from Bacillus subtilis by continuous product removal and metal cation additions. Appl. Environ. Microbiol. 1981, 42, 408–412. [Google Scholar] [PubMed]
- Mukherjee, S.; Das, P.; Sen, R. Rapid quantification of a microbial surfactant by a simple turbidometric method. J. Microbiol. Methods 2009, 76, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.J.; Desai, A.J. Bench-Scale Production of Biosurfactants and their Potential in Ex-Situ MEOR Application. Soil Sediment Contam. 2013, 22, 701–715. [Google Scholar] [CrossRef]
- Sharma, D.; Saharan, B.S.; Chauhan, N.; Procha, S.; Lal, S. Isolation and functional characterization of novel biosurfactant produced by Enterococcus faecium. Springerplus 2015, 4, 4. [Google Scholar] [CrossRef]
- Hentati, D.; Chebbi, A.; Hadrich, F.; Frikha, I.; Rabanal, F.; Sayadi, S.; Manresa, A.; Chamkha, M. Production, characterization and biotechnological potential of lipopeptide biosurfactants from a novel marine Bacillus stratosphericus strain FLU5. Ecotoxicol. Environ. Saf. 2019, 167, 441–449. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds Lichenysin are not available from the authors. |
| Parameters | Time | Media 1 | Media 2 | Media 3 | Media 4 | Media 5 | Media 6 | Media 7 |
|---|---|---|---|---|---|---|---|---|
| Growth(OD660) | 0 h | 0.07 ± 0.02 | 0.00 ± 0.0 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.20 ± 0.06 | 0.03 ± 0.04 | 0.06 ± 0.03 |
| 24 h | 1.42 ± 0.12 | 1.77 ± 0.04 | 0.77 ± 0.13 | 0.77 ± 0.13 | 1.45 ± 0.07 | 1.41 ± 0.08 | 1.64 ± 0.13 | |
| 48 h | 1.56 ± 0.08 | 2.16 ± 0.07 | 0.85 ± 0.45 | 0.85 ± 0.45 | 1.52 ± 0.15 | 1.96 ± 0.21 | 2.15 ± 0.11 | |
| 72 h | 1.53 ± 0.11 | 2.11 ± 0.03 | 0.94 ± 0.03 | 0.94 ± 0.03 | 1.48 ± 0.08 | 1.87 ± 0.08 | 2.11 ± 0.08 | |
| pH | 0 h | 7.25 ± 0.07 | 7.04 ± 0.04 | 7.08 ± 0.11 | 7.08 ± 0.11 | 7.04 ± 0.03 | 7.13 ± 0.09 | 7.28 ± 0.12 |
| 24 h | 7.18 ± 0.05 | 7.14 ± 0.04 | 5.95 ± 0.03 | 5.95 ± 0.03 | 6.64 ± 0.19 | 7.04 ± 0.24 | 8.06 ± 0.06 | |
| 48 h | 7.05 ± 0.03 | 7.15 ± 0.10 | 5.75 ± 0.24 | 5.75 ± 0.24 | 6.63 ± 0.27 | 6.83 ± 0.19 | 8.86 ± 0.23 | |
| 72 h | 7.05 ± 0.13 | 7.25 ± 0.03 | 4.80 ± 0.13 | 4.80 ± 0.13 | 6.71 ± 0.13 | 6.63 ± 0.20 | 9.46 ± 0.46 | |
| ST mN·m−1 | 0 h | 71.13 ± 1.12 | 69.37 ± 0.37 | 68.92 ± 0.85 | 68.92 ± 0.85 | 69.34 ± 1.08 | 72.11 ± 0.65 | 73.06 ± 1.03 |
| 24 h | 48.48 ± 0.42 | 27.33 ± 0.51 | 40.64 ± 1.18 | 40.64 ± 1.18 | 36.45 ± 0.94 | 34.43 ± 0.61 | 26.37 ± 0.31 | |
| 48 h | 37.44 ± 0.56 | 28.45 ± 0.71 | 50.67 ± 0.32 | 50.67 ± 0.32 | 31.70 ± 0.40 | 40.00 ± 0.84 | 26.63 ± 0.81 | |
| 72 h | 35.57 ± 0.36 | 27.50 ± 0.29 | 57.48 ± 0.40 | 57.48 ± 0.40 | 31.04 ± 0.69 | 33.70 ± 1.17 | 26.55 ± 0.57 | |
| IFT mN·m−1 | 0 h | 46.82 ± 0.23 | 39.52 ± 0.10 | 39.91 ± 0.35 | 39.91 ± 0.35 | 38.77 ± 0.37 | 46.53 ± 0.46 | 47.39 ± 0.11 |
| 24 h | 19.81 ± 0.18 | 2.60 ± 0.74 | 16.11 ± 0.23 | 16.11 ± 0.23 | 16.44 ± 0.28 | 9.6 ± 0.22 | 0.78 ± 0.13 | |
| 48 h | 9.26 ± 0.28 | 3.72 ± 0.36 | 23.38 ± 0.69 | 23.38 ± 0.69 | 9.37 ± 0.24 | 14.72 ± 0.21 | 0.83 ± 0.16 | |
| 72 h | 9.04 ± 0.52 | 3.49 ± 0.13 | 35.45 ± 0.20 | 35.45 ± 0.20 | 12.90 ± 0.16 | 10.63 ± 0.29 | 0.85 ± 0.20 | |
| Viscosity | 0 h | 1.14 ± 0.06 | 1.10 ± 0.03 | 1.19 ±0.07 | 1.19 ± 0.07 | 1.24 ± 0.04 | 1.09 ± 0.03 | 1.12 ± 0.05 |
| 24 h | 1.25 ± 0.03 | 1.26 ± 0.07 | 1.17 ± 0.06 | 1.17 ± 0.06 | 1.28 ± 0.05 | 1.38 ± 0.06 | 1.36 ± 0.04 | |
| 48 h | 1.38 ± 0.04 | 1.36 ± 0.04 | 1.18 ± 0.08 | 1.18 ± 0.08 | 1.18 ± 0.08 | 1.44 ± 0.05 | 1.31 ± 0.07 | |
| 72 h | 1.35 ± 0.07 | 1.29 ± 0.05 | 1.30 ± 0.05 | 1.30 ± 0.05 | 1.10 ± 0.06 | 1.32 ± 0.08 | 1.39 ± 0.03 |
| Parameters | Time | M2 (Glucose) | M2 (Sucrose) | M2 (Starch) | M7 (Glucose) | M7 (Sucrose) | M7 (Starch) |
|---|---|---|---|---|---|---|---|
| Growth (OD660) | 0 h | 00 ± 0.00 | 0.05 ± 0.03 | 0.05 ± 0.05 | 0.30 ± 0.07 | 0.03 ± 0.02 | 0.07 ± 0.05 |
| 24 h | 1.71 ± 0.04 | 1.66 ± 0.05 | 1.33 ± 0.05 | 1.64 ± 0.05 | 1.84 ± 0.02 | 1.46 ± 0.13 | |
| 48 h | 2.11 ± 0.02 | 1.81 ± 0.06 | 1.51 ± 0.04 | 2.19 ± 0.06 | 2.16 ± 0.04 | 1.91 ± 0.12 | |
| 72 h | 2.08 ± 0.03 | 1.74 ± 0.02 | 1.61 ± 0.08 | 2.13 ± 0.01 | 2.11 ± 0.03 | 1.73 ± 0.05 | |
| pH | 0 h | 7.04 ± 0.04 | 7.09 ± 0.10 | 7.06 ± 0.07 | 7.28 ± 0.08 | 7.08 ± 0.36 | 7.11 ± 0.06 |
| 24 h | 7.12 ± 0.20 | 7.22 ± 0.06 | 7.28 ± 0.19 | 7.39 ± 0.13 | 7.22 ± 0.22 | 7.50 ± 0.24 | |
| 48 h | 7.15 ± 0.17 | 7.28 ± 0.50 | 7.39 ± 0.25 | 8.49 ± 0.28 | 7.37 ± 0.04 | 8.48 ± 0.10 | |
| 72 h | 7.43 ± 0.10 | 7.65 ± 0.10 | 7.33 ± 0.09 | 9.01 ± 0.12 | 8.76 ± 0.43 | 8.89 ± 0.28 | |
| ST (mN·m−1) | 0 h | 70.26 ± 0.29 | 71.63 ± 0.55 | 72.83 ± 0.36 | 72.24 ± 0.40 | 72.87± 0.48 | 71.72 ± 0.28 |
| 24 h | 27.66 ± 0.25 | 27.63 ± 0.35 | 29.60 ± 0.36 | 26.21 ± 0.33 | 26.49 ± 0.29 | 26.54 ± 0.34 | |
| 48 h | 28.52 ± 0.55 | 27.70 ± 0.41 | 29.22 ± 0.29 | 26.48 ± 0.42 | 27.57 ± 0.25 | 27.36 ± 0.21 | |
| 72 h | 27.57 ± 0.22 | 27.57 ± 0.14 | 29.40 ± 0.24 | 26.69 ± 0.17 | 26.55 ± 0.31 | 26.53 ± 0.14 | |
| IFT (mN·m−1) | 0 h | 47.86 ± 0.08 | 42.51 ± 0.09 | 47.19 ± 0.57 | 48.66 ± 0.18 | 48.48 ± 0.47 | 46.82 ± 1.39 |
| 24 h | 2.47 ± 0.18 | 1.41 ± 0.07 | 2.31 ± 0.23 | 0.26 ± 0.05 | 0.77 ± 0.02 | 0.56 ± 0.29 | |
| 48 h | 4.61 ± 0.26 | 2.64 ± 0.14 | 2.71 ± 0.20 | 0.35 ± 0.07 | 0.82 ± 0.13 | 0.72 ± 0.14 | |
| 72 h | 3.41 ± 0.14 | 2.10 ± 0.15 | 2.53 ± 0.29 | 0.37 ± 0.04 | 0.86 ± 0.03 | 0.78 ± 0.24 | |
| Viscosity | 0 h | 1.04 ± 0.05 | 1.12 ± 0.05 | 1.17 ± 0.09 | 1.14 ± 0.04 | 1.15 ± 0.02 | 1.18 ± 0.03 |
| 24 h | 1.44 ± 0.19 | 1.59 ± 0.09 | 1.24 ± 0.10 | 1.42 ± 0.07 | 1.32 ± 0.05 | 1.43 ± 0.07 | |
| 48 h | 1.43 ± 0.09 | 1.46 ± 0.08 | 1.27 ± 0.04 | 1.35 ± 0.04 | 1.31 ± 0.04 | 1.31 ± 0.04 | |
| 72 h | 1.33 ± 0.05 | 1.43 ± 0.08 | 1.25 ± 0.09 | 1.39 ± 0.06 | 1.31 ± 0.08 | 1.30 ± 0.05 | |
| Emulsification index (E24) | Diesel | Hexadecane | Heptane | Diesel | Hexadecane | Heptane | |
| 0 h 24 h | 19.8 ± 2.1 51.6 ± 1.0 | 10.6 ± 1.2 51.6 ± 0.6 | 12.7 ± 0.8 50.0 ± 0.9 | 18.3 ± 1.7 53.3 ± 0.7 | 12.8 ± 1.2 52.6 ± 0.3 | 10.4 ± 2.2 51.8 ± 0.6 | |
| 48 h | 52.8 ± 0.7 | 52.5 ± 0.7 | 51.8 ± 1.1 | 53.3 ± 0.8 | 52.8 ± 0.2 | 52.2 ± 1.2 | |
| 72 h | 53.0 ± 1.2 | 52.8 ± 0.5 | 52.7 ± 1.3 | 54.4 ± 0.9 | 53.8 ± 0.5 | 52.6 ± 0.8 | |
| Yield (g·L−1) | 24 h | 0.19 ± 0.2 | 0.22 ± 0.3 | 0.18 ± 0.3 | 0.29 ± 0.2 | 0.30 ± 0.4 | 0.36 ± 0.3 |
| 48 h | 0.45 ± 0.2 | 0.43 ± 0.2 | 0.48 ± 0.1 | 0.57 ± 0.3 | 0.66 ± 0.2 | 0.39 ± 0.2 | |
| 72 h | 0.21 ± 0.1 | 0.48 ± 0.2 | 0.24 ± 0.2 | 1.01 ± 0.2 | 0.98 ± 0.1 | 0.55 ± 0.1 |
| Hydrocarbon | Lichenysin | Triton X-100 | SDS |
|---|---|---|---|
| kerosene | 57.6 ± 0.9 | 51.8 ± 1.4 | 51.2 ± 0.6 |
| hexadecane | 53.8 ± 0.8 | 50.1 ± 0.2 | 50.6 ± 1.4 |
| tridecane | 55.4 ± 1.3 | 51.2 ± 1.6 | 52.3 ± 0.5 |
| tetradecane | 56.5 ± 0.2 | 48.8 ± 0.9 | 51.4 ± 1.6 |
| diesel | 54.5 ± 1.2 | 50.6 ± 0.5 | 53.8 ± 1.9 |
| crude oil | 66.4 ± 1.4 | 48.2 ± 1.0 | 45.2 ± 2.2 |
| pristane | 55.1 ± 1.4 | 49.8 ± 1.2 | 52.1 ± 1.3 |
| heptane | 52.4 ± 1.0 | 47.5 ± 1.3 | 52.4 ± 1.7 |
| Sand-Packed Column Test | ||||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | Biosurfactant Flooding | Control Flooding | ||||||
| SP 1 | SP 2 | SP 3 | Mean ± SD | CT 1 | CT 2 | CT 3 | Mean ± SD | |
| PV (mL) | 59 | 60 | 62 | 60.3 ± 1.52 | 58.0 | 59.0 | 61.0 | 59.3 ± 1.5 |
| OOIP (mL) | 46.4 | 45 | 48 | 46.5 ± 1.50 | 45.5 | 47.0 | 47.5 | 46.6 ± 1.0 |
| Soi (%) | 78.6 | 75 | 77.4 | 77.0 ± 1.85 | 78.4 | 79.6 | 77.8 | 78.6 ± 0.9 |
| Swi (%) | 21.4 | 25 | 22.5 | 23.0 ± 1.86 | 21.5 | 20.3 | 22.1 | 21.3 ± 0.9 |
| Sorwf (mL) | 23.6 | 24.3 | 26.4 | 24.8 ± 1.46 | 22.6 | 20.2 | 23.0 | 21.9 ± 1.5 |
| OOIP-Sorwf mL | 22.8 | 20.7 | 21.6 | 21.7 ± 1.05 | 22.9 | 26.7 | 24.5 | 24.7 ± 1.9 |
| Sor (%) | 49.1 | 46 | 45 | 46.7 ± 2.13 | 50.3 | 57.0 | 51.5 | 52.9 ± 3.6 |
| Sorbf (mL) | 7.5 | 7.9 | 8.5 | 8.0 ± 0.51 | 2.0 | 1.8 | 1.5 | 1.7 ± 0.3 |
| AOR (%) | 31.7 | 32.5 | 32.1 | 32.10 ± 0.4 | 8.7 | 6.7 | 6.1 | 7.1 ± 1.4 |
| Reference | [47] | [48] | [49] | [50] | [51] | [52] | [53] |
|---|---|---|---|---|---|---|---|
| Composition g/L | Media 1 | Media 2 | Media 3 | Media 4 | Media 5 | Media 6 | Media 7 |
| Glucose | 34.0 | 11.0 | 20.0 | 20.0 | 10.0 | 20.0 | - |
| Sucrose | - | - | - | - | - | - | 20.0 |
| KH2PO4 | 6.0 | - | 1.0 | - | - | 4.8 | 0.14 |
| K2HPO4 | - | - | - | - | 2.7 | - | 2.2 |
| Na2HPO4 | 1.0 | - | - | - | - | 7.12 | - |
| NH4NO3 | 1.0 | - | - | - | - | 4.0 | 3.3 |
| NaNO3 | - | 4.4 | - | 2.8 | - | - | - |
| MgSO4·7H2O | 0.1 | 0.8 | 0.5 | 0.20 | 0.25 | 0.2 | 0.6 |
| FeSO4·7H2O | 0.0016 | - | - | - | - | 0.0011 | 0.1 |
| MnSO4·4H2O | 0.0012 | - | 0.005 | - | - | 0.0006 | - |
| CaCl2 | 0.0012 | - | - | - | - | 0.0007 | 0.01 |
| EDTA | 0.0007 | - | - | 0.2 | - | 0.0014 | - |
| KCl | - | 0.4 | - | - | - | - | - |
| H3PO4 (85.4%) | - | 1.0 mL/L | - | 0.5 mL/L | - | - | - |
| C5H8NO4Na | - | - | 5.0 | - | - | - | - |
| CuSO4 | - | - | 0.2 | - | - | - | - |
| (NH4)2SO4 | - | - | - | - | 1.0 | - | - |
| Yeast extract | - | - | 1.0 | - | 1.0 | - | - |
| NaCl | - | - | - | - | 5.0 | - | 0.01 |
| Trace elements | - | 10.0 mL/L | - | 1.0 mL/L | - | - | 0.5 mL/L |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, N.; Wang, F.; Xu, B.; Safdar, B.; Ullah, A.; Naveed, M.; Wang, C.; Rashid, M.T. Production and Application of Biosurfactant Produced by Bacillus licheniformis Ali5 in Enhanced Oil Recovery and Motor Oil Removal from Contaminated Sand. Molecules 2019, 24, 4448. https://doi.org/10.3390/molecules24244448
Ali N, Wang F, Xu B, Safdar B, Ullah A, Naveed M, Wang C, Rashid MT. Production and Application of Biosurfactant Produced by Bacillus licheniformis Ali5 in Enhanced Oil Recovery and Motor Oil Removal from Contaminated Sand. Molecules. 2019; 24(24):4448. https://doi.org/10.3390/molecules24244448
Chicago/Turabian StyleAli, Nawazish, Fenghuan Wang, Baocai Xu, Bushra Safdar, Asad Ullah, Muhammad Naveed, Ce Wang, and Muhammad Tayyab Rashid. 2019. "Production and Application of Biosurfactant Produced by Bacillus licheniformis Ali5 in Enhanced Oil Recovery and Motor Oil Removal from Contaminated Sand" Molecules 24, no. 24: 4448. https://doi.org/10.3390/molecules24244448
APA StyleAli, N., Wang, F., Xu, B., Safdar, B., Ullah, A., Naveed, M., Wang, C., & Rashid, M. T. (2019). Production and Application of Biosurfactant Produced by Bacillus licheniformis Ali5 in Enhanced Oil Recovery and Motor Oil Removal from Contaminated Sand. Molecules, 24(24), 4448. https://doi.org/10.3390/molecules24244448






