Harvest Age Effect on Phytochemical Content of White and Black Glutinous Rice Cultivars
Abstract
:1. Introduction
2. Results
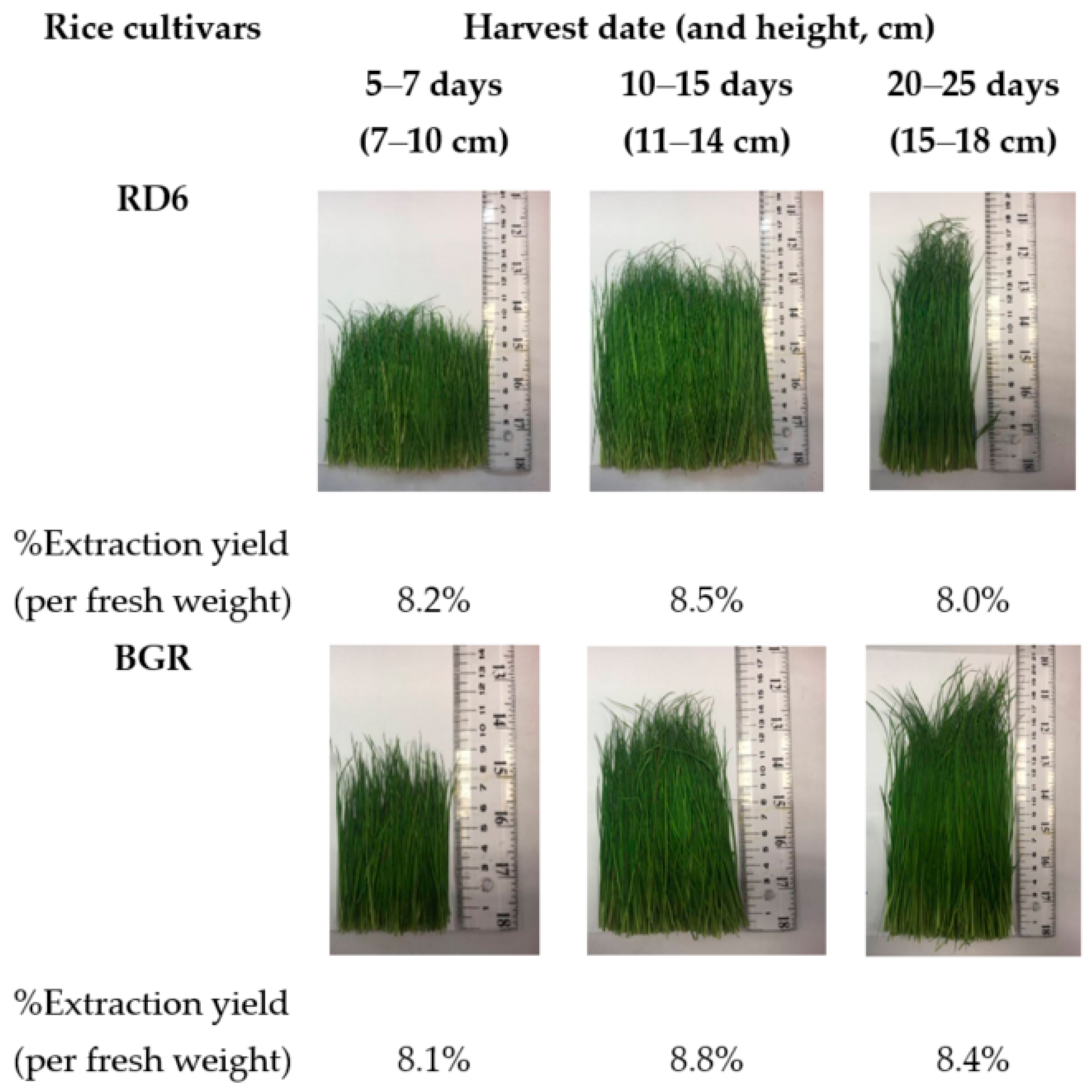
2.1. Extraction Yield
2.2. HPLC Analysis for Phenolic and Flavonoid Contents
2.3. Total Phenolic Content (TPC)
2.4. Total Chlorophyll Content (TCC)
2.5. Total Anthocyanin Content (TAC)
2.6. Total Flavonoid Content (TFC)
2.7. Proximate
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material and Extractions
4.3. Identification of Phenolics and Flavonoids by Using HPLC
4.4. Determination of Total Phenolic Contents
4.5. Determination of Total Chlorophyll Contents
4.6. Determination of Total Anthocyanins Content
4.7. Determination of Total Flavonoid Content
4.8. Proximate Analysis
4.8.1. Determination of Total Ash
4.8.2. Determination of Moisture
4.8.3. Determination of Fat
4.8.4. Determination of Protein
4.8.5. Determination of Carbohydrate
4.8.6. Determination of Energy
4.8.7. Determination of Dietary Fiber
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). Rice Market Monitor: April 2018. Available online: http://www.fao.org/3/I9243EN/i9243en.pdf (accessed on 13 November 2019).
- Mir, S.A.; Shah, M.A.; Bosco, S.J.D. Variations in brown rice quality among cultivars. In Brown Rice; Manickavasagan, A., Santhakumar, C., Venkatachalapathy, N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 25, pp. 25–44. [Google Scholar]
- Food Standards Agency. Food and Climate Change: A Review of the Effects of Climate Change on Food within the Remit of the Food Standards Agency. Available online: http://www.fao.org/3/I9243EN/i9243en.pdf (accessed on 13 November 2019).
- Food and Agriculture Organization of the United Nations (FAO). Country Fact Sheet on Food and Agriculture Policy Trends (Thailand): February 2018. Available online: http://www.fao.org/3/I8683EN/i8683en.pdf (accessed on 13 November 2019).
- Beidaghy Dizaji, H.; Zeng, T.; Hartmann, I.; Enke, D.; Schliermann, T.; Lenz, V.; Bidabadi, M. Generation of high quality biogenic silica by combustion of rice husk and rice straw combined with pre- and post-treatment strategies—A review. Appl. Sci. 2019, 9, 1083. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Sengupta, B.; Dasgupta, D.; Mandal, T.; Datta, S. Recovery of value added products from rice husk ash to explore an economic way for recycle and reuse of agricultural waste. Rev. Environ. Sci. Biotechnol. 2016, 15, 47–65. [Google Scholar] [CrossRef]
- Ciulu, M.; Cádiz–Gurrea, M.L.; Segura–Carretero, A. Extraction and analysis of phenolic compounds in rice: A review. Molecules 2018, 23, 2890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodie, A.R.; Micciche, A.C.; Atungulu, G.G.; Rothrock, M.J.; Ricke, S.C. Current trends of rice milling byproducts for agricultural applications and alternative food production systems. Front. Sustain. Food Syst. 2019, 3. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Lourith, N.; Chaikul, P. Jasmine rice panicle: A safe and efficient natural ingredient for skin aging treatments. J. Ethnopharmacol. 2016, 193, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.; Micalos, P.; Pak, S. A study protocol to evaluate a fermented rice bran supplement and resistance training on immune function and muscle performance in healthy older people. J. Sci. Med. Sport. 2019, 22, 79. [Google Scholar] [CrossRef]
- Lourith, N.; Kanlayavattanakul, M. Appraisal of Thai glutinous rice husk for health promotion products. J. Cereal Sci. 2013, 57, 343–347. [Google Scholar] [CrossRef]
- Papillo, V.A.; Locatelli, M.; Travaglia, F.; Bordiga, M.; Garino, C.; Arlorio, M.; Coïsson, J.D. Spray–dried polyphenolic extract from Italian black rice (Oryza sativa L., var. Artemide) as new ingredient for bakery products. Food Chem. 2018, 269, 603–609. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Basak, J.; Kar, M.; Mandal, S.; Mukhopadhyay, A. The role of iron chelation activity of wheat grass juice in patients with myelodysplastic syndrome. J. Clin. Oncol. 2009, 27, 7012. [Google Scholar]
- Padalia, S.; Drabu, S.; Raheja, I.; Gupta, A.; Dhamija, M. Multitude potential of wheatgrass juice (green blood): An overview. Chron. Young Sci. 2010, 1, 23–28. [Google Scholar]
- Singhal, V.K.; Singhal, A.K.; Jagatheesh, K.; Padmavathi, K.; Elangoran, N.; Bangr, O.P.; Sil, R. Multifunctional role of green blood therapy to cure for many diseases. Chron. Young Sci. 2012, 3, 12–16. [Google Scholar] [CrossRef] [Green Version]
- Yi, B.; Kasai, H.; Lee, H.S.; Kang, Y.; Park, J.Y.; Yang, M. Inhibition by wheat sprout (Triticum aestivum) juice of bisphenol A-induced oxidative stress in young women. Mutat. Res. Toxicol. Environ. Mutagen. 2011, 724, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Ben–Arye, E.; Golden, E.; Wengrower, D.; Stamper, A.; Kohn, R.; Berry, E. Wheat grass juice in the treatment of active distal ulcerative colitis: A randomized double–blind placebo–controlled trial. Nutr. Cancer. 2002, 37, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Shyam, R.; Singh, S.N.; Vats, P.; Singh, V.K.; Bajaj,, R.; Singh, S.B.; Banerjee, P.K. Wheat grass supplementation decreases oxidative stress in healthy subjects: A comparative study with spirulina. J. Altern. Complement. Med. 2007, 13, 789–791. [Google Scholar] [CrossRef] [PubMed]
- Marawaha, R.K.; Bansal, D.; Kaur, S.; Trehan, A. Wheat grass juice reduces transfusion requirement in patients with thalassemia major: A pilot study. Indian Pediatr. 2004, 41, 716–720. [Google Scholar] [PubMed]
- Singh, K.; Pannu, M.S.; Singh, P.; Singh, J. Effect of wheat grass tablets on the frequency of blood transfusions in thalassemia major. Indian J. Pediatr. 2010, 77, 90–91. [Google Scholar] [CrossRef]
- Khanthapok, P.; Muangprom, A.; Sukrong, S. Antioxidant activity and DNA protective properties of rice grass juices. ScienceAsia 2015, 41, 119. [Google Scholar] [CrossRef] [Green Version]
- Özkan, G.; Bilek, S.E. Enzyme–assisted extraction of stabilized chlorophyll from spinach. Food Chem. 2015, 176, 152–157. [Google Scholar] [CrossRef]
- Aher, R.; Kumar, B.; Sudalai, A. One–pot synthesis of cyclic carbonates from aldehydes, sulfur ylide, and CO2. Synlett 2013, 25, 97–101. [Google Scholar]
- Moongngarm, A.; Daomukda, N.; Khumpika, S. Chemical compositions, phytochemicals, and antioxidant capacity of rice bran, rice bran layer, and rice germ. APCBEE Procedia 2012, 2, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Wangcharoen, W.; Phimphilai, S. Chlorophyll and total phenolic contents, antioxidant activities and consumer acceptance test of processed grass drinks. J. Food Sci. Technol. 2016, 53, 4135–4140. [Google Scholar] [CrossRef] [PubMed]
- Song, B.H.; Lee, D.H.; Kim, B.C.; Ku, S.H.; Park, E.J.; Kwon, I.H.; Kim, K.H.; Kim, K.J. Photodynamic therapy using chlorophyll–a in the treatment of acne vulgaris: A randomized, single–blind, split–face study. J. Am. Acad. Dermatol. 2014, 71, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Wink, M. Chlorophyll enhances oxidative stress tolerance in Caenorhabditis elegans and extends its lifespan. Peer J. 2016, 4, 1879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel–Aal, E.S.M.; Young, J.C.; Rabalski, I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J. Agric. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef] [PubMed]
- Rattanachaisit, P.; Kongkiattikajorn, J. Antioxidative activities of bran extracts from pigmented rice cultivars. Isan J. Pharm. Sci. 2015, 10, 33–42. [Google Scholar]
- Roland, B.; Bernard, G.C.; Fernand, G.; Coffi, A.A. Phytochemical screening and quantitative variation of some secondary metabolites in five cultivated rice cultivars. J. Appl. Biosci. 2017, 113, 11146–11157. [Google Scholar]
- Putri, A.P.; Sukanta, E.A. Proximate analysis of rice bran as alternative comestible. In Proceedings of the Fourth Gruber–Soedigdo Lecture Conference, Groningen, The Netherlands, 25–28 September 2012. [Google Scholar]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef]
- Yao, S.L.; Xu, Y.; Zhang, Y.Y.; Lu, Y.H. Black rice and anthocyanins induce inhibition of cholesterol absorption in vitro. Food Funct. 2013, 4, 1602–1608. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, Y.; Bao, J.; Beta, T. Phenolic compounds and antioxidant properties of breeding lines between the white and black rice. Food Chem. 2015, 172, 630–639. [Google Scholar] [CrossRef]
- Semaming, Y.; Pannengpetch, P.; Chattipakorn, S.C.; Chattipakorn, N. Pharmacological properties of protocatechuic acid and its potential roles as complementary medicine. Evid. Based Complement. Alternat. Med. 2015, 2015, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Krzysztoforska, K.; Mirowska-Guzel, D.; Widy-Tyszkiewicz, E. Pharmacological effects of protocatechuic acid and its therapeutic potential in neurodegenerative diseases: Review on the basis of in vitro and in vivo studies in rodents and humans. Nutr. Neurosci. 2019, 22, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Masella, R.; Santangelo, C.; D’Archivio, M.; LiVolti, G.; Giovannini, C.; Galvano, F. Protocatechuic acid and human disease prevention: biological activities and molecular mechanisms. Curr. Med. Chem. 2012, 19, 2901–2917. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.K.; Rashid, R.; Fatima, N.; Mahmood, S.; Mir, S.; Khan, S.; Jabeen, N.; Murtaza, G. Pharmacological activities of protocatechuic acid. Acta Pol. Pharm. 2015, 72, 643–650. [Google Scholar] [PubMed]
- Pietta, P.G.; Simonetti, P.; Gardana, C.; Brusamolino, A.; Morazzoni, P.; Bombardelli, E. Catechin metabolites after intake of green tea infusions. BioFactors 1998, 8, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Kullu, M.; Sachan, A.; Vidyarthi, A.S.; Sachan, S.G. Bioconversion of ferulic acid to vanillic acid by Paenibacillus lactis SAMS–2001. Ann. Microbiol. 2016, 66, 875–882. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, M.C.; Um, J.Y.; Hong, S.H. The beneficial effect of vanillic acid on ulcerative colitis. Molecules 2010, 15, 7208–7217. [Google Scholar] [CrossRef]
- Kim, M.C.; Kim, S.J.; Kim, D.S.; Jeon, Y.D.; Park, S.J.; Lee, H.S.; Um, J.Y.; Hong, S.H. Vanillic acid inhibits inflammatory mediators by suppressing NF–κB in lipopolysaccharide–stimulated mouse peritoneal macrophages. Immunopharmacol. Immunotoxicol. 2011, 33, 525–532. [Google Scholar] [CrossRef]
- Brimson, J.M.; Onlamoon, N.; Tencomnao, T.; Thitilertdecha, P. Clerodendrum petasites S. Moore: The therapeutic potential of phytochemicals, hispidulin, vanillic acid, verbascoside, and apigenin. Biomed. Pharmacother. 2019, 118, 109319. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [Green Version]
- Kamalakkannan, N.; Prince, P.S.M. Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic wistar rats. Basic Htmlent Glyphamp Asciiamp Clin. 2006, 98, 97–103. [Google Scholar] [CrossRef]
- Butsat, S.; Weerapreeyakul, N.; Siriamornpun, S. Changes in phenolic acids and antioxidant activity in Thai rice husk at five growth stages during grain development. J. Agric. Food Chem. 2009, 57, 4566–4571. [Google Scholar] [CrossRef] [PubMed]
- Kaisoon, O.; Siriamornpun, S.; Weerapreeyakul, N.; Meeso, N. Phenolic compounds and antioxidant activities of edible flowers from Thailand. J. Funct. Foods 2011, 3, 88–99. [Google Scholar] [CrossRef]
- Shao, Y.; Xu, F.; Sun, X.; Bao, J.; Beta, T. Phenolic acids, anthocyanins, and antioxidant capacity in rice (Oryza Sativa L.) grains at four stages of development after flowering. Food Chem. 2014, 143, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Tananuwong, K.; Tewaruth, W. Extraction and application of antioxidants from black glutinous rice. LWT - Food Sci. Technol. 2010, 43, 476–481. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists [AOAC]. Official Methods of Analysis, 20th ed.; AOAC: Washington, DC, USA, 2016. [Google Scholar]
- Department of Medical Sciences. Compendium of Method for Food Analysis, 1st ed.; National Bureau of Agricultural Commodity and Food Standards: Nonthaburi, Thailand, 2003; pp. 2–18.
Sample Availability: Not available. |
| Standard Compounds | Retention Time (min) | ||
|---|---|---|---|
| 280 nm | 320 nm | 370 nm | |
| Hydroxybenzoic Acids | |||
| Gallic acid (1) | 5.74 ± 0.01 | 5.74 ± 0.01 | ND |
| Protocatechuic acid (2) | 8.58 ± 0.08 | 8.58 ± 0.08 | ND |
| p-Hydroxybenzoic acid (3) | 12.06 ± 0.03 | ND | ND |
| Vanillic acid (5) | 14.89 ± 0.02 | ND | ND |
| Syringic acid (7) | 17.19 ± 0.01 | ND | ND |
| Hydroxycinnamic Acids | |||
| Chlorogenic acid (4) | 13.71 ± 0.03 | 13.71 ± 0.03 | 13.71 ± 0.03 |
| Caffeic acid (6) | 15.56 ± 0.03 | 15.64 ± 0.03 | 15.57 ± 0.03 |
| p-Coumaric acid (8) | 23.10 ± 0.07 | 23.10 ± 0.07 | not detected |
| Ferulic acid (9) | 27.29 ± 0.02 | 27.29 ± 0.02 | 27.24 ± 0.02 |
| Flavonoids | |||
| Rutin (10) | 32.89 ± 0.06 | 32.89 ± 0.06 | 32.89 ± 0.06 |
| Compound (Peak Number) | Detected Amount (mg/g) and Harvest Date | |||||
|---|---|---|---|---|---|---|
| RD6 | BGR | |||||
| 5−7 Days | 10−15 Days | 20−25 Days | 5−7 Days | 10−15 Days | 20−25 Days | |
| 7–10 cm | 11–14 cm | 15–18 cm | 7–10 cm | 11–14 cm | 15–18 cm | |
| Hydroxybenzoic Acids | ||||||
| Protocatechuic acid (2) | 1.58 ± 0.12 a,A | 1.31 ± 0.08 b,B | 0.38 ± 0.03 d,C | 1.44 ± 0.12 ab,B | 0.90 ± 0.10 c,B | 0.56 ± 0.01 d,B |
| Vanillic acid (5) | 1.45 ± 0.12 cd,A | 0.65 ± 0.06 d,C | 1.56 ± 0.14 c,A | 7.69 ± 0.58 a,A | 4.39 ± 0.48 b,A | 1.28 ± 0.01 cd,A |
| Total | 3.04 ± 0.24 | 1.96 ± 0.14 | 1.94 ± 0.17 | 9.12 ± 0.7 | 5.29 ± 0.57 | 1.84 ± 0.03 |
| Flavonoids | ||||||
| Rutin (10) | 1.68 ± 0.11 a,A | 1.60 ± 0.10 a,A | 0.98 ± 0.08 c,B | 0.47 ± 0.04 d,C | 1.25 ± 0.08 b,B | 1.32 ± 0.01 b,A |
| Total | 1.68 ± 0.11 | 1.60 ± 0.10 | 0.98 ± 0.08 | 0.47 ± 0.04 | 1.25 ± 0.08 | 1.32 ± 0.01 |
| Phytochemical or Component (Units) | RD6 | BGR | ||||
|---|---|---|---|---|---|---|
| 5–7 Days | 10–15 Days | 20–25 Days | 5–7 Days | 10–15 Days | 20–25 Days | |
| 7–10 cm | 11–14 cm | 15–18 cm | 7–10 cm | 11–14 cm | 15–18 cm | |
| Phytochemical content (mg) per g of dry extract (DE) | ||||||
| TPC (mg GAE) | 44.8 ± 1.6 a | 41.5 ± 2.2 b | 29.1 ± 1.4 c,d | 28.1 ± 0.6 d | 31.9 ± 1.1 c | 24.9 ± 1.3 e |
| TCC (mg) | 40.5 ± 2.2 a | 34.6 ± 2.1 c | 35.9 ± 1.9 b | 19.3 ± 1.8 d | 40.8 ± 0.8 a | 40.6 ± 0.8 a |
| TAC (mg C3GE) | 0.06 ± 0.001 c | 0.01 ± 0.001 c | 0.15 ± 0.001 c | 22.13 ± 0.002 a | 0.45 ± 0.001 b,c | 0.71 ± 0.001 b |
| TFC (mg QE) | 34.2 ± 3.4 a | 32.6 ± 0.3 a,b | 16.3 ± 0.5 d | 21.5 ± 1.4 c | 30.3 ± 0.3 b | 29.2 ± 0.7 b |
| Proximate composition (g) per 100 g fresh weight | ||||||
| Carbohydrate | 12.22 | 12.68 | 15.34 | 16.39 | 24.54 | 15.09 |
| Protein | 5.99 | 4.96 | 4.81 | 5.32 | 3.76 | 3.38 |
| Fat | 0.75 | 0.32 | 1.01 | 0.39 | 1.20 | 0.78 |
| Ash | 2.86 | 2.93 | 3.65 | 3.31 | 5.78 | 4.02 |
| Moisture | 78.18 | 79.11 | 75.19 | 74.59 | 64.72 | 76.73 |
| Energy | 79.59 | 73.44 | 89.69 | 90.35 | 124.00 | 80.90 |
| Dietary fiber | nd | 11.98 | nd | nd | 16.92 | nd |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamprasit, K.; Weerapreeyakul, N.; Sutthanut, K.; Thukhammee, W.; Wattanathorn, J. Harvest Age Effect on Phytochemical Content of White and Black Glutinous Rice Cultivars. Molecules 2019, 24, 4432. https://doi.org/10.3390/molecules24244432
Tamprasit K, Weerapreeyakul N, Sutthanut K, Thukhammee W, Wattanathorn J. Harvest Age Effect on Phytochemical Content of White and Black Glutinous Rice Cultivars. Molecules. 2019; 24(24):4432. https://doi.org/10.3390/molecules24244432
Chicago/Turabian StyleTamprasit, Kawintra, Natthida Weerapreeyakul, Khaetthareeya Sutthanut, Wipawee Thukhammee, and Jintanaporn Wattanathorn. 2019. "Harvest Age Effect on Phytochemical Content of White and Black Glutinous Rice Cultivars" Molecules 24, no. 24: 4432. https://doi.org/10.3390/molecules24244432