Microalgae Biomass as a Potential Feedstock for the Carboxylate Platform
Abstract
1. Introduction
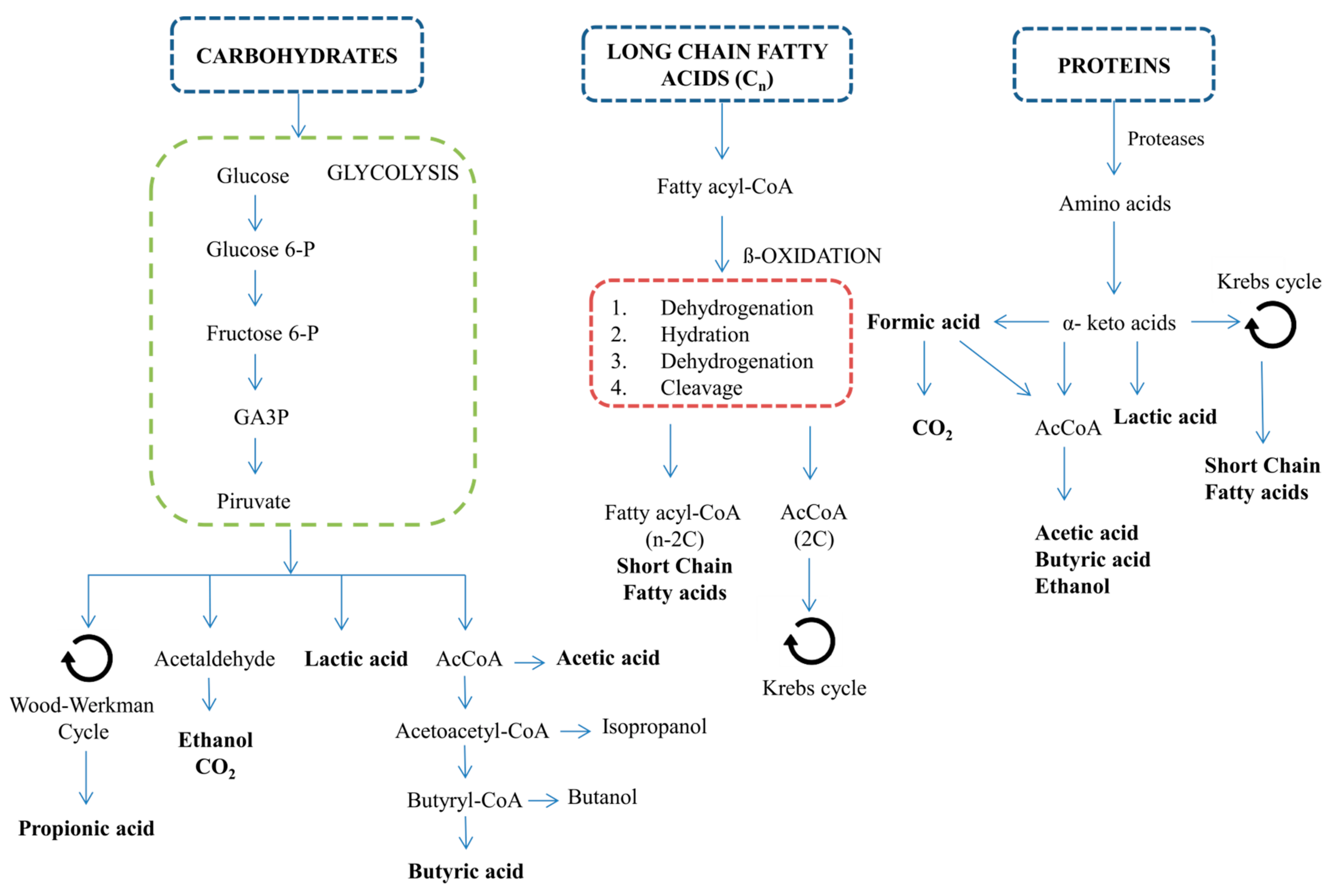
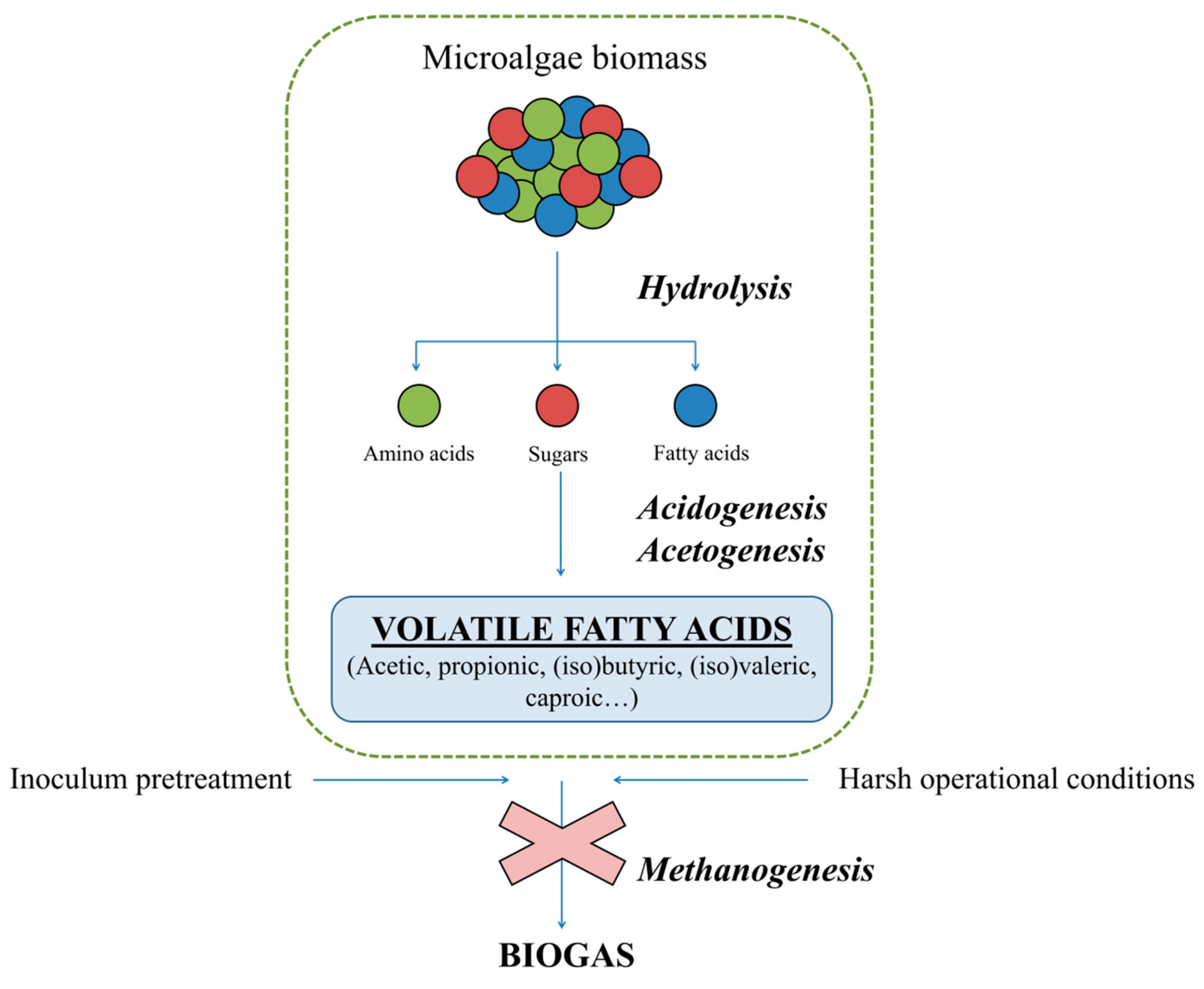
2. Volatile Fatty Acid Production by Means of Anaerobic Fermentation
2.1. Microalgae Biomass as a Substrate for VFA Production
2.2. Operational Conditions for VFA Production
2.2.1. Inoculum
2.2.2. pH
2.2.3. Temperature
2.2.4. Organic Loading Rate (OLR)
2.2.5. Hydraulic Retention Time (HRT)
3. Microbial Populations Involved in VFAs Productions
4. VFAs As Building Blocks for the Industry
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- European Commission. Report from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions on the Implementation of the Circular Economy Action Plan. 2019. Available online: https://ec.europa.eu/environment/circular-economy/pdf/report_implementation_54_actions.pdf (accessed on 2 December 2019).
- Holtzapple, M.T.; Granda, C.B. Carboxylate platform: The MixAlco process part 1: Comparison of three biomass conversion platforms. Appl. Biochem. Biotechnol. 2009, 156, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Agler, M.T.; Wrenn, B.A.; Zinder, S.H.; Angenent, L.T. Waste to bioproduct conversion with undefined mixed cultures: The carboxylate platform. Trends Biotechnol. 2011, 29, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-based volatile fatty acid production and recovery from waste streams: Current status and future challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Calt, E.A. Products Produced from Organic Waste Using Managed Ecosystem Fermentation. J. Sustain. Dev. 2015, 8, 43. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, B.; Wong, J.W.C.; Zhang, Y. Enhanced volatile fatty acids production from anaerobic fermentation of food waste: A mini-review focusing on acidogenic metabolic pathways. Bioresour. Technol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Davis, R.; Aden, A.; Pienkos, P.T. Techno-economic analysis of autotrophic microalgae for fuel production. Appl. Energy 2011, 88, 3524–3531. [Google Scholar] [CrossRef]
- Magdalena, J.; Ballesteros, M.; González-Fernandez, C. Efficient Anaerobic Digestion of Microalgae Biomass: Proteins as a Key Macromolecule. Molecules 2018, 23, 1098. [Google Scholar] [CrossRef]
- Passos, F.; Carretero, J.; Ferrer, I. Comparing pretreatment methods for improving microalgae anaerobic digestion: Thermal, hydrothermal, microwave and ultrasound. Chem. Eng. J. 2015, 279, 667–672. [Google Scholar] [CrossRef]
- Arslan, D.; Steinbusch, K.J.J.; Diels, L.; Hamelers, H.V.M.; Strik, D.P.B.T.B.; Buisman, C.J.N.; de Wever, H. Selective short-chain carboxylates production: A review of control mechanisms to direct mixed culture fermentations. Crit. Rev. Environ. Sci. Technol. 2016, 46, 592–634. [Google Scholar] [CrossRef]
- Khan, M.A.; Ngo, H.H.; Guo, W.S.; Liu, Y.; Nghiem, L.D.; Hai, F.I.; Deng, L.J.; Wang, J.; Wu, Y. Optimization of process parameters for production of volatile fatty acid, biohydrogen and methane from anaerobic digestion. Bioresour. Technol. 2016, 219, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-J.; Lim, S.-J.; Chang, H.N. Volatile Fatty Acid Platform: Concept and Application Concept of Volatile Fatty Acid Platform Platforms for Biofuel Production. Emerg. Areas Bioeng. 2018, 173–201. [Google Scholar] [CrossRef]
- Sengun, I.Y.; Karabiyikli, S. Importance of acetic acid bacteria in food industry. Food Control. 2011, 22, 647–656. [Google Scholar] [CrossRef]
- Ahmadi, N.; Khosravi-Darani, K.; Mortazavian, A.M. An overview of biotechnological production of propionic acid: From upstream to downstream processes. Electron. J. Biotechnol. 2017, 28, 67–75. [Google Scholar] [CrossRef]
- Dwidar, M.; Park, J.-Y.; Mitchell, R.J.; Sang, B.-I. The Future of Butyric Acid in Industry. Sci. World J. 2012, 2012, 471417. [Google Scholar] [CrossRef]
- Passos, F.; Uggetti, E.; Carrère, H.; Ferrer, I. Pretreatment of microalgae to improve biogas production: A review. Bioresour. Technol. 2014, 172, 403–412. [Google Scholar] [CrossRef]
- Oh, S.-E.; van Ginkel, S.; Logan, B.E. The Relative Effectiveness of pH Control and Heat Treatment for Enhancing Biohydrogen Gas Production. Environ. Sci. Technol. 2003, 37, 5186–5190. [Google Scholar] [CrossRef]
- Horiuchi, J.-I.; Shimizu, T.; Tada, K.; Kanno, T.; Kobayashi, M. Selective production of organic acids in anaerobic acid reactor by pH control. Bioresour. Technol. 2002, 82, 209–213. [Google Scholar] [CrossRef]
- Xin, C.; Addy, M.M.; Zhao, J.; Cheng, Y.; Cheng, S.; Mu, D.; Liu, Y.; Ding, R.; Chen, P.; Ruan, R. Comprehensive techno-economic analysis of wastewater-based algal biofuel production: A case study. Bioresour. Technol. 2016, 211, 584–593. [Google Scholar] [CrossRef]
- Carrere, H.; Antonopoulou, G.; Affes, R.; Passos, F.; Battimelli, A.; Lyberatos, G.; Ferrer, I. Review of feedstock pretreatment strategies for improved anaerobic digestion: From lab-scale research to full-scale application. Bioresour. Technol. 2016, 199, 386–397. [Google Scholar] [CrossRef]
- Sialve, B.; Bernet, N.; Bernard, O. Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechnol. Adv. 2009, 27, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Mahdy, A.; Ballesteros, M.; González-Fernández, C. Enzymatic pretreatment of Chlorella vulgaris for biogas production: Influence of urban wastewater as a sole nutrient source on macromolecular profile and biocatalyst efficiency. Bioresour. Technol. 2016, 199, 319–325. [Google Scholar] [CrossRef]
- Yenigün, O.; Demirel, B. Ammonia inhibition in anaerobic digestion: A review. Process. Biochem. 2013, 48, 901–911. [Google Scholar] [CrossRef]
- Tian, H.; Fotidis, I.A.; Mancini, E.; Treu, L.; Mahdy, A.; Ballesteros, M.; González-Fernández, C.; Angelidaki, I. Acclimation to extremely high ammonia levels in continuous biomethanation process and the associated microbial community dynamics. Bioresour. Technol. 2018, 247, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Mahdy, A.; Mendez, L.; Ballesteros, M.; González-Fernández, C. Protease pretreated Chlorella vulgaris biomass bioconversion to methane via semi-continuous anaerobic digestion. Fuel 2015, 158, 35–41. [Google Scholar] [CrossRef]
- Angelidaki, I.; Sanders, W. Assessment of the anaerobic biodegradability of macropollutants. Re/Views Environ. Sci. Bio/Technol. 2004, 3, 117–129. [Google Scholar] [CrossRef]
- Kim, D.; Kim, S.; Han, J.I.; Yang, J.W.; Chang, Y.K.; Ryu, B.G. Carbon balance of major volatile fatty acids (VFAs) in recycling algal residue via a VFA-platform for reproduction of algal biomass. J. Environ. Manage. 2019, 237, 228–234. [Google Scholar] [CrossRef]
- Magdalena, J.A.; Tomás-Pejó, E.; Ballesteros, M.; González-Fernandez, C. Volatile fatty acids production from protease pretreated Chlorella biomass via anaerobic digestion. Biotechnol. Prog. 2018, 34, 1363–1369. [Google Scholar] [CrossRef]
- Xie, J.; Chen, Y.; Duan, X.; Feng, L.; Yan, Y.; Wang, F.; Zhang, X.; Zhang, Z.; Zhou, Q. Activated carbon promotes short-chain fatty acids production from algae during anaerobic fermentation. Sci. Total Environ. 2019, 658, 1131–1138. [Google Scholar] [CrossRef]
- Sun, C.; Xia, A.; Liao, Q.; Fu, Q.; Huang, Y.; Zhu, X.; Wei, P.; Lin, R.; Murphy, J.D. Improving production of volatile fatty acids and hydrogen from microalgae and rice residue: Effects of physicochemical characteristics and mix ratios. Appl. Energy 2018, 230, 1082–1092. [Google Scholar] [CrossRef]
- Jankowska, E.; Chwialkowska, J.; Stodolny, M.; Oleskowicz-Popiel, P. Volatile fatty acids production during mixed culture fermentation–The impact of substrate complexity and pH. Chem. Eng. J. 2017, 326, 901–910. [Google Scholar] [CrossRef]
- Xia, A.; Jacob, A.; Tabassum, M.R.; Herrmann, C.; Murphy, J.D. Production of hydrogen, ethanol and volatile fatty acids through co-fermentation of macro- and micro-algae. Bioresour. Technol. 2016, 205, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.U.; Kim, Y.M.; Choi, Y.-N.; Kim, H.G.; Park, J.M. Influence of temperature on volatile fatty acid production and microbial community structure during anaerobic fermentation of microalgae. Bioresour. Technol. 2015, 191, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Suresh, A.; Seo, C.; Chang, H.N.; Kim, Y.-C. Improved volatile fatty acid and biomethane production from lipid removed microalgal residue (LRμAR) through pretreatment. Bioresour. Technol. 2013, 149, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Gruhn, M.; Frigon, J.-C.; Guiot, S.R. Acidogenic fermentation of Scenedesmus sp.-AMDD: Comparison of volatile fatty acids yields between mesophilic and thermophilic conditions. Bioresour. Technol. 2016, 200, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Magdalena, J.A.; Llamas, M.; Tomás-Pejó, E.; González-Fernández, C. Semi-Continuous anaerobic digestion of protease pretreated Chlorella Biomass for volatile fatty acids production. J. Chem. Technol. Biotechnol. 2019, 94, 1861–1869. [Google Scholar] [CrossRef]
- Tamis, J.; Joosse, B.M.; van Loosdrecht, M.C.M.; Kleerebezem, R. High-rate volatile fatty acid (VFA) production by a granular sludge process at low pH. Biotechnol. Bioeng. 2015, 112, 2248–2255. [Google Scholar] [CrossRef]
- Mottet, A.; Habouzit, F.; Steyer, J.P. Anaerobic digestion of marine microalgae in different salinity levels. Bioresour. Technol. 2014, 158, 300–306. [Google Scholar] [CrossRef]
- Schwede, S.; Rehman, Z.-U.; Gerber, M.; Theiss, C.; Span, R. Effects of thermal pretreatment on anaerobic digestion of Nannochloropsis salina biomass. Bioresour. Technol. 2013, 143, 505–511. [Google Scholar] [CrossRef]
- Roberts, K.P.; Heaven, S.; Banks, C.J. Semi-continuous anaerobic digestion of the marine micro-algal species I. galbana and D. salina grown under low and high sulphate conditions. Algal Res. 2019, 41, 101564. [Google Scholar] [CrossRef]
- Han, S.-K.; Shin, H.-S. Biohydrogen production by anaerobic fermentation of food waste. Int. J. Hydrogen Energy 2004, 29, 569–577. [Google Scholar] [CrossRef]
- Tao, Y.; Chen, Y.; Wu, Y.; He, Y.; Zhou, Z. High hydrogen yield from a two-step process of dark- and photo-fermentation of sucrose. Int. J. Hydrogen Energy 2007, 32, 200–206. [Google Scholar] [CrossRef]
- Magdalena, J.A.; González-Fernández, C. Archaea inhibition: Strategies for the enhancement of volatile fatty acids production from microalgae. Waste Manag. 2020, 102, 222–230. [Google Scholar] [CrossRef]
- Luo, G.; Xie, L.; Zou, Z.; Wang, W.; Zhou, Q. Evaluation of pretreatment methods on mixed inoculum for both batch and continuous thermophilic biohydrogen production from cassava stillage. Bioresour. Technol. 2010, 101, 959–964. [Google Scholar] [CrossRef]
- Pham, T.N.; Nam, W.J.; Jeon, Y.J.; Yoon, H.H. Volatile fatty acids production from marine macroalgae by anaerobic fermentation. Bioresour. Technol. 2012, 124, 500–503. [Google Scholar] [CrossRef]
- Bengtsson, S.; Hallquist, J.; Werker, A.; Welander, T. Acidogenic fermentation of industrial wastewaters: Effects of chemostat retention time and pH on volatile fatty acids production. Biochem. Eng. J. 2008, 40, 492–499. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J.; Dewil, R.; De heyder, B. Advanced sludge treatment affects extracellular polymeric substances to improve activated sludge dewatering. J. Hazard. Mater. 2004, 106, 83–92. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, S.; Yuan, H.; Zhou, Q.; Gu, G. Hydrolysis and acidification of waste activated sludge at different pHs. Water Res. 2007, 41, 683–689. [Google Scholar] [CrossRef]
- González-Fernández, C.; Sialve, B.; Bernet, N.; Steyer, J.P. Effect of organic loading rate on anaerobic digestion of thermally pretreated Scenedesmus sp. biomass. Bioresour. Technol. 2013, 129, 219–223. [Google Scholar] [CrossRef]
- De Groof, V.; Coma, M.; Arnot, T.; Leak, D.J.; Lanham, A.B. Medium chain carboxylic acids from complex organic feedstocks by mixed culture fermentation. Molecules 2019, 24, 398. [Google Scholar] [CrossRef] [PubMed]
- Magdalena, J.A.; Greses, S.; González-Fernández, C. Impact of Organic Loading Rate in Volatile Fatty Acids Production and Population Dynamics Using Microalgae Biomass as Substrate. Sci. Rep. [CrossRef]
- Henze, M.; van Loosdrecht, M.C.M.; Ekama, G.A.; Brdjanovic, D. Biological Wastewater Treatment; IWA Publishing: London, UK, 2008; ISBN 9781843391883. [Google Scholar]
- Campanaro, S.; Treu, L.; Rodriguez, R.L.M.; Kovalovszki, A.; Ziels, R.M.; Maus, I.; Zhu, X.; Kougias, P.G.; Basile, A.; Luo, G. The anaerobic digestion microbiome: A collection of 1600 metagenome-assembled genomes shows high species diversity related to methane production. bioRxiv 2019, 680553. [Google Scholar] [CrossRef]
- Jaenicke, S.; Ander, C.; Bekel, T.; Bisdorf, R.; Droge, M.; Gartemann, K.-H.; Junemann, S.; Kaiser, O.; Krause, L.; Tille, F.; et al. Comparative and joint analysis of two metagenomic datasets from a biogas fermenter obtained by 454-pyrosequencing. PLoS ONE 2011, 6, e14519. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.U.; Kim, Y.M.; Park, J.M. Changes in microbial communities during volatile fatty acid production from cyanobacterial biomass harvested from a cyanobacterial bloom in a river. Chemosphere 2018, 202, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Seo, C.; Kim, W.; Chang, H.N.; Han, J.I.; Kim, Y.C. Comprehensive study on volatile fatty acid production from Ettlia sp. residue with molecular analysis of the microbial community. Algal Res. 2016, 17, 161–167. [Google Scholar] [CrossRef]
- Xu, K.; Liu, H.; Chen, J. Effect of classic methanogenic inhibitors on the quantity and diversity of archaeal community and the reductive homoacetogenic activity during the process of anaerobic sludge digestion. Bioresour. Technol. 2010, 101, 2600–2607. [Google Scholar] [CrossRef]
- Patnaik, P.R. Perspectives in the modeling and optimization of PHB production by pure and mixed cultures. Crit. Rev. Biotechnol. 2005, 25, 153–171. [Google Scholar] [CrossRef]
- Pagliano, G.; Ventorino, V.; Panico, A.; Pepe, O. Integrated systems for biopolymers and bioenergy production from organic waste and by-products: A review of microbial processes. Biotechnol. Biofuels 2017, 10, 113. [Google Scholar] [CrossRef]
- Albuquerque, M.G.E.; Eiroa, M.; Torres, C.; Nunes, B.R.; Reis, M.A.M. Strategies for the development of a side stream process for polyhydroxyalkanoate (PHA) production from sugar cane molasses. J. Biotechnol. 2007, 130, 411–421. [Google Scholar] [CrossRef]
- Colombo, B.; Pepè Sciarria, T.; Reis, M.; Scaglia, B.; Adani, F. Polyhydroxyalkanoates (PHAs) production from fermented cheese whey by using a mixed microbial culture. Bioresour. Technol. 2016, 218, 692–699. [Google Scholar] [CrossRef]
- Kourmentza, C.; Placido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A.M. Recent Advances and Challenges towards Sustainable Polyhydroxyalkanoate (PHA) Production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef] [PubMed]
- Mohanakrishna, G.; Venkata Mohan, S.; Sarma, P.N. Utilizing acid-rich effluents of fermentative hydrogen production process as substrate for harnessing bioelectricity: An integrative approach. Int. J. Hydrogen Energy 2010, 35, 3440–3449. [Google Scholar] [CrossRef]
- Rabaey, I.; Ossieur, W.; Verhaege, M.; Verstraete, W. Continuous microbial fuel cells convert carbohydrates to electricity. Water Sci. Technol. 2005, 52, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cheng, S.; Logan, B.E. Production of Electricity from Acetate or Butyrate Using a Single-Chamber Microbial Fuel Cell. Environ. Sci. Technol. 2005, 39, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Freguia, S.; Teh, E.H.; Boon, N.; Leung, K.M.; Keller, J.; Rabaey, K. Microbial fuel cells operating on mixed fatty acids. Bioresour. Technol. 2010, 101, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.-X.; Tong, Z.-H.; Li, W.-W.; Wang, S.-G.; Sheng, G.-P.; Shi, X.-Y.; Liu, X.-W.; Yu, H.-Q. Electricity generation from mixed volatile fatty acids using microbial fuel cells. Appl. Microbiol. Biotechnol. 2010, 87, 2365–2372. [Google Scholar] [CrossRef]
- Roghair, M.; Liu, Y.; Strik, D.P.B.T.B.; Weusthuis, R.A.; Bruins, M.E.; Buisman, C.J.N. Development of an Effective Chain Elongation Process From Acidified Food Waste and Ethanol Into n-Caproate. Front. Bioeng. Biotechnol. 2018, 6, 50. [Google Scholar] [CrossRef]
- Reddy, M.V.; Mohan, S.V.; Chang, Y.C. Medium-Chain Fatty Acids (MCFA) Production Through Anaerobic Fermentation Using Clostridium kluyveri: Effect of Ethanol and Acetate. Appl. Biochem. Biotechnol. 2018, 185, 594–605. [Google Scholar] [CrossRef]
- Llamas, M.; Magdalena, J.A.; González-Fernández, C.; Tomás-Pejó, E. Volatile fatty acids as novel building blocks for oil based chemistry via oleaginous yeasts fermentation. Biotechnol. Bioeng. 2019, 1–13. [Google Scholar] [CrossRef]
- Sitepu, I.R.; Sestric, R.; Ignatia, L.; Levin, D.; German, J.B.; Gillies, L.A.; Almada, L.A.G.; Boundy-Mills, K.L. Manipulation of culture conditions alters lipid content and fatty acid profiles of a wide variety of known and new oleaginous yeast species. Bioresour. Technol. 2013, 144, 360–369. [Google Scholar] [CrossRef]
- Fei, Q.; Chang, H.N.; Shang, L.; Choi, J.d.-r.; Dal rae Kim, N.J.; Kang, J.W. The effect of volatile fatty acids as a sole carbon source on lipid accumulation by Cryptococcus albidus for biodiesel production. Bioresour. Technol. 2011, 102, 2695–2701. [Google Scholar] [CrossRef] [PubMed]
| BATCH MODE | ||||||||||||||
| Strain | Temperature (°C) | pH | Pretreatment | Composition | % DW | Ac | Pr | But | IBut | Val | IVal | Cap | COD-VFAs/CODin (%) | Reference |
| 15 | 6.4 | Carbohydrates | 47.5 | 70 | 30 | 0 | - | - | - | - | 17.37 | [29] | ||
| Chlorella vulgaris | 35 | - | Proteins | 20.4 | 70 | 20 | 10 | - | - | - | - | 38.17 | ||
| 55 | Fatty acids | 0.9 | 70 | 10 | 20 | - | - | - | - | 40.47 | ||||
| 15 | 6.4 | Carbohydrates | 28.6 | 70 | 30 | 0 | - | - | - | - | 9.44 | [29] | ||
| C. vulgaris | 35 | - | Proteins | 56.8 | 50 | 40 | 10 | - | - | - | - | 33.40 | ||
| 55 | Fatty acids | 0.004 | 60 | 10 | 30 | - | - | - | - | 42.03 | ||||
| 25 | Carbohydrates | 25 | 41 | 28 | 7 | 9 | 7 | 8 | - | 47.7 | [30] | |||
| Chlorella sp. | 35 | 5.5 | Enzymatic (proteases) | Proteins | 64 | 26 | 35 | 9 | 12 | 8 | 9 | - | 39.1 | |
| 50 | Lipids | 10 | 33 | 11 | 15 | 14 | - | 27 | - | 34.5 | ||||
| 25 | Carbohydrates | 25 | 54 | 21 | 6 | 6 | 6 | 7 | - | 45.1 | [30] | |||
| Chlorella sp. | 35 | 7.5 | Enzymatic (proteases) | Proteins | 64 | 57 | 21 | 6 | 8 | 1 | 7 | - | 48.3 | |
| 50 | Lipids | 10 | 46 | 17 | 12 | 15 | - | 9 | - | 37.1 | ||||
| Control | Carbohydrates | 6 | 38 | 14 | 36 | 12 | - | 13.09 | [31] | |||||
| Microcystis | 25 | 10 | 0.5 Activated carbon (g/L) | Proteins | 63 | 50 | 13 | 21.1 | 15.9 | - | 31.50 | |||
| Lipids | 4 | |||||||||||||
| Carbohydrates | 34 | [32] | ||||||||||||
| Chlorella Pyrenoidosa | 35 | 6.5 | - | Proteins | 48 | 52 | 11 | 35 | - | - | - | 3 | 4.28 | |
| 140 °C, 10 min 1% H2SO4 | Lipids | 18 | 41 | 14 | 37 | - | - | - | 8 | 9.14 | ||||
| Scenedesmus quadricadua and C.vulgaris | 35 | 5 | Non pretreated | NA | 40 | 20 | 5 | 20 | 15 | - | - | 0.25 | [33] | |
| 7.4 | 50 | 20 | 5 | 10 | 10 | 5 | - | 5.42 | ||||||
| Carbohydrates | 19 | [34] | ||||||||||||
| Arthrospira Platensis | 37 | 6 | 2.5% dilute H2SO4 at 135 °C for 15 min | Proteins | 76 | 40 | 5 | 28 | 5 | 5 | 9 | 8 | NA | |
| Lipids | 5 | |||||||||||||
| 35 | 6.9 | Carbohydrates | NA | 86 | 2 | 2 | 0 | 0 | 10 | 0 | 20.0 | [35] | ||
| Desmodesmus sp., Scenedesmus sp., and Chlamydomonas sp | 45 | - | Proteins | NA | 74 | 8 | 5 | 2 | 0 | 10 | 0 | 33.0 | ||
| 55 | Lipids | NA | 66 | 15 | 5 | 1 | 2 | 11 | 0 | 50.0 | ||||
| Carbohydrates | 45.5 | [36] | ||||||||||||
| Ettlia sp | 35 | 7.2 | 1% NaOH + ultrasound | Proteins | 35 | 64 | 25 | 11 | - | - | - | - | 25.25 | |
| Lipids | 5.5 | |||||||||||||
| SEMI-CONTINUOUS MODE | ||||||||||||||
| Strain | Temperature (°C) | Operational conditions | Composition (%) DW | Ac | Pr | But | Ibut | Val | Ival | Cap | COD-VFAs/CODin | Reference | ||
| Scenedesmus sp. Frozen | 35 | HRT 15 days OLR 2.5 VS/Ld | Carbohydrates | 45 | 11 | 3 | 57 | 18 | 3 | 3 | 4 | 0.171 g COD-VFAs/g VSin | [37] | |
| 55 | Proteins | 44 | 14 | 1 | 48 | 2 | 0 | 0 | 0 | 0.088 g COD-VFAs/g VSin | ||||
| Lipids | 4 | |||||||||||||
| 35 | HRT 10 days OLR 1.5 g COD/Ld | 14 | 36 | 10 | 11 | 11 | 18 | - | 25.6 | [38] | ||||
| 35 | HRT 10 days OLR 3 g COD/Ld | Carbohydrates | 21.6 | 18 | 32 | 12 | 10 | 11 | 17 | - | 25.8 | |||
| C. vulgaris Enzymatic pretreatment | 25 | HRT 10 days OLR 1.5 g COD/Ld | Proteins | 57.9 | 20 | 17 | 9 | 17 | 15 | 12 | 9 | 35.4 | ||
| 25 | HRT 12 days OLR 1.5 g COD/Ld | Lipids | 13.4 | 24 | 16 | 8 | 20 | 14 | 18 | 13 | 38.0 | |||
| 25 | HRT 8 days OLR 1.5 g COD/Ld | 24 | 15 | 8 | 20 | 14 | 18 | 12 | 39.8 | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magdalena, J.A.; González-Fernández, C. Microalgae Biomass as a Potential Feedstock for the Carboxylate Platform. Molecules 2019, 24, 4404. https://doi.org/10.3390/molecules24234404
Magdalena JA, González-Fernández C. Microalgae Biomass as a Potential Feedstock for the Carboxylate Platform. Molecules. 2019; 24(23):4404. https://doi.org/10.3390/molecules24234404
Chicago/Turabian StyleMagdalena, Jose Antonio, and Cristina González-Fernández. 2019. "Microalgae Biomass as a Potential Feedstock for the Carboxylate Platform" Molecules 24, no. 23: 4404. https://doi.org/10.3390/molecules24234404
APA StyleMagdalena, J. A., & González-Fernández, C. (2019). Microalgae Biomass as a Potential Feedstock for the Carboxylate Platform. Molecules, 24(23), 4404. https://doi.org/10.3390/molecules24234404







