Liquid Anaerobic Digestate as a Source of Nutrients for Lipid and Fatty Acid Accumulation by Auxenochlorella Protothecoides
Abstract
1. Introduction
2. Results
2.1. Effect of The Digestate on Growth Kinetics
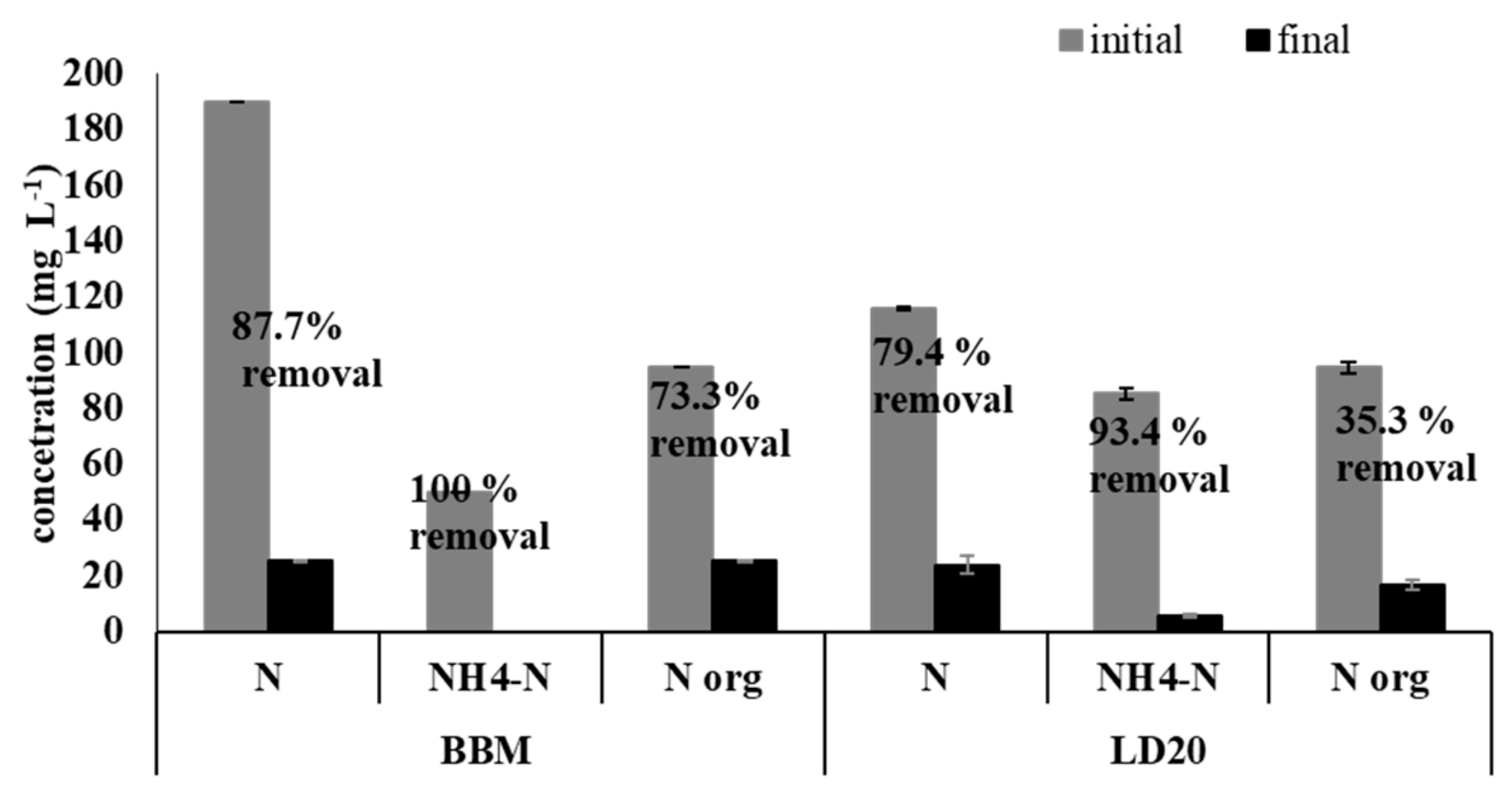
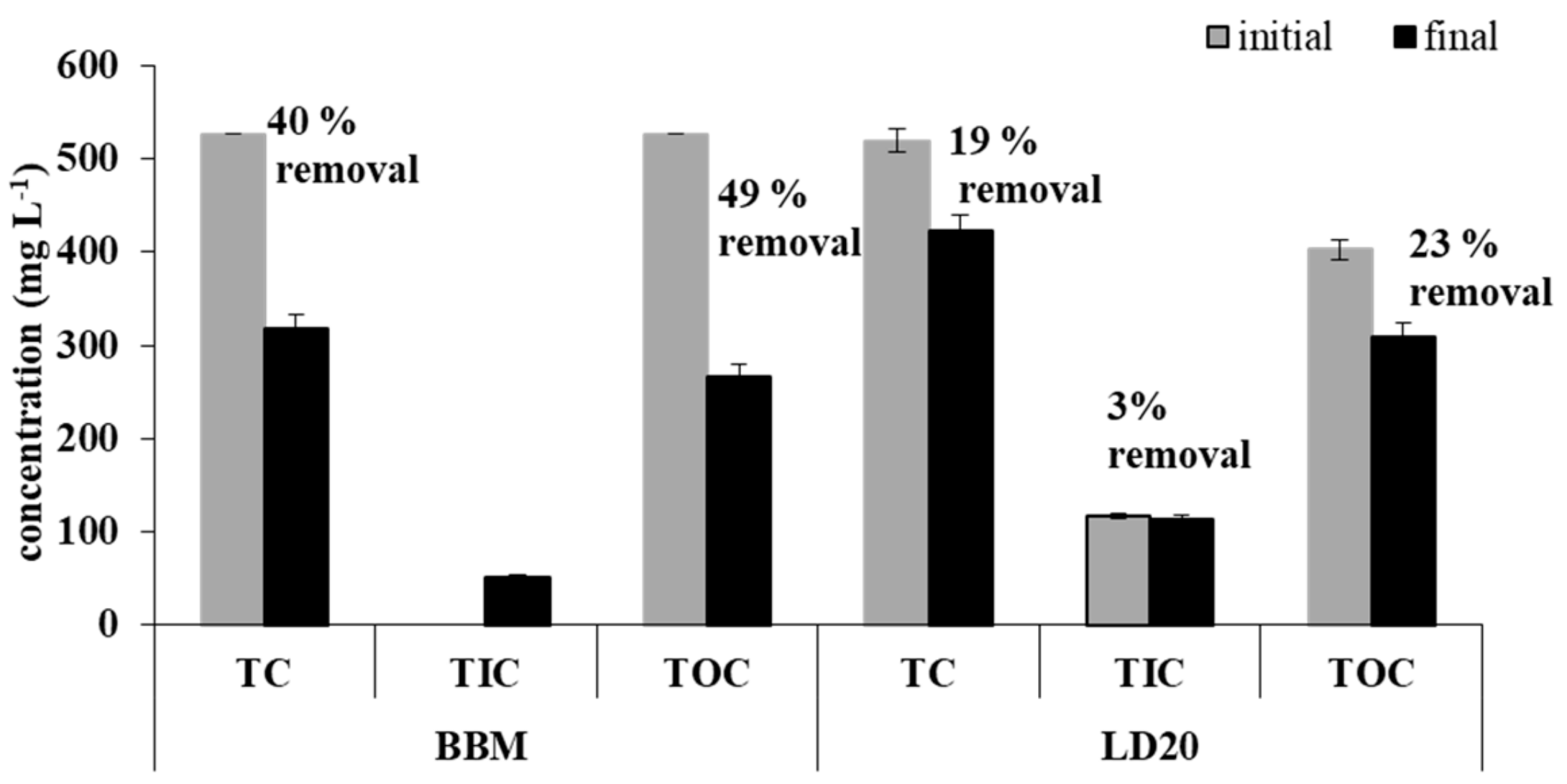
2.2. Characterization of The Digestate and Removal of Macro- and Micronutrients by A. Protothecoides
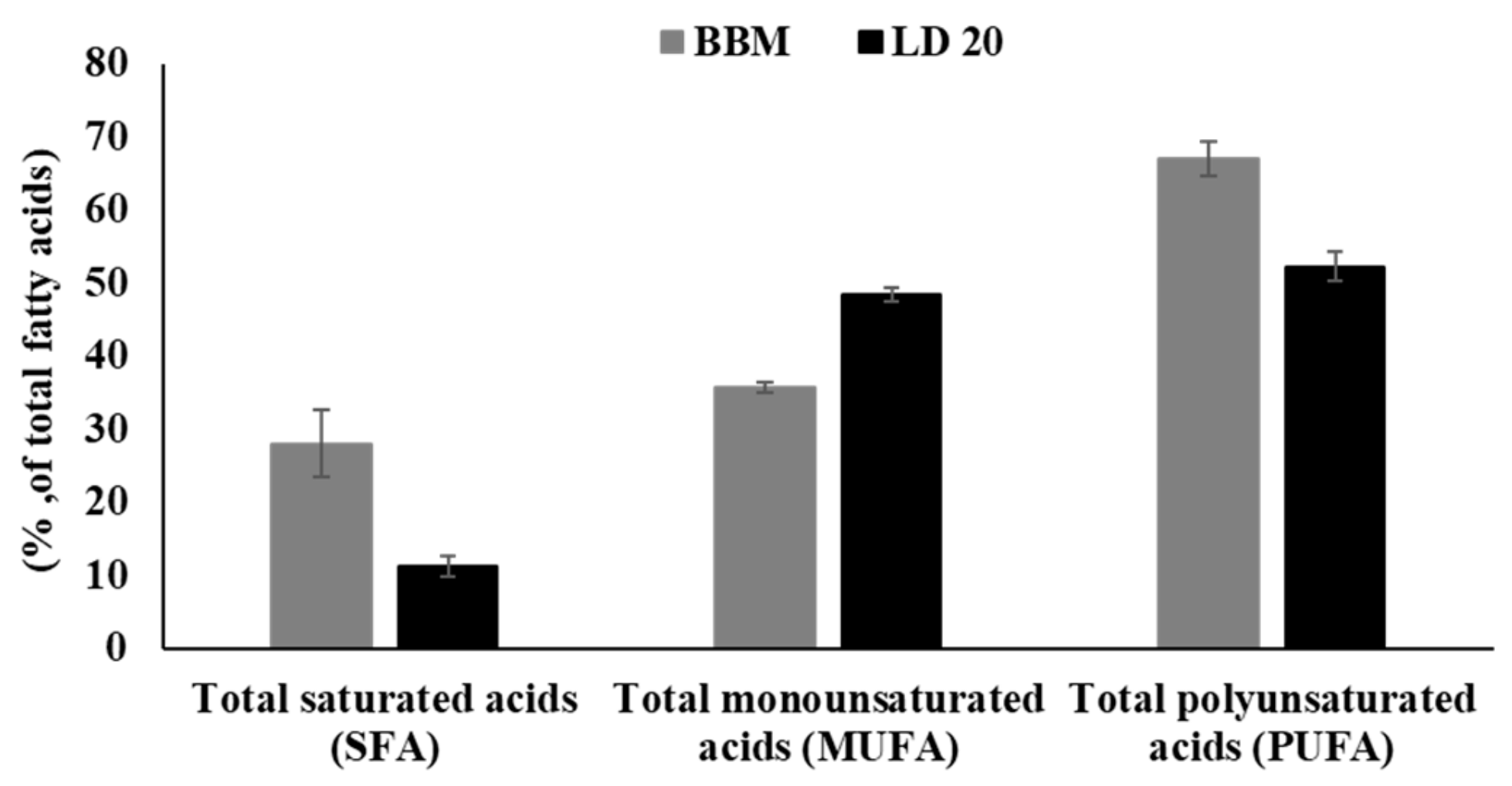
2.3. Lipid Content and Fatty Acid Profile
3. Discussion
4. Materials and Methods
4.1. Culture Conditions
4.2. Physico-Chemical Analysis of The Digestate
4.3. Determination of Algal Growth
4.4. Lipid Extraction
4.5. Analysis of Fatty Acids
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Magierek, E.; Krzemińska, I.; Tys, J. Stimulatory effect of indole-3-acetic acid and continuous illumination on the growth of Parachlorella kessleri. Int. Agrophysics 2017, 31, 483–489. [Google Scholar] [CrossRef][Green Version]
- Grudziński, W.; Krzemińska, I.; Luchowski, R.; Nosalewicz, A.; Gruszecki, W. Strong-light-induced yellowing of green microalgae Chlorella: A study on molecular mechanisms of the acclimation response. Algal Res. 2016, 16, 245–254. [Google Scholar]
- Lam, M.K.; Lee, K.T. Microalgae biofuels: A critical review of issues, problems and the way forward. Biotechnol. Adv. 2012, 30, 673–690. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.B.; Costanza-Robinson, S.M.; Spatafora, G.A. Neochloris oleoabundans grown on anaerobically digested dairy manure for concomitant nutrient removal and biodiesel feedstock production. Biomass Bioenergy 2011, 35, 40–49. [Google Scholar] [CrossRef]
- Krzemińska, I.; Oleszek, M. Glucose supplementation-induced changes in the Auxenochlorella protothecoides fatty acid composition suitable for biodiesel production. Bioresour. Technol. 2016, 218, 1294–1297. [Google Scholar] [CrossRef] [PubMed]
- Darpito, C.; Shin, W.S.; Jeon, S.; Lee, H.; Nam, K.; Kwon, J.H.; Yang, J.W. Cultivation of Chlorella protothecoides in anaerobically treated brewery wastewater for cost-effective biodiesel production. Bioprocess Biosyst. Eng. 2015, 38, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Gonzalez, I.; Parashar, A.; Bressler, D.C. Heterotrophic growth and lipid accumulation of Chlorella protothecoides in whey permeate, a dairy by-product stream, for biofuel production. Bioresour. Technol. 2014, 155, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Walker, T.H.; Bridges, W.C.; Thornton, C.; Gopalakrishnan, K. Biomass and lipid production of Chlorella protothecoides under heterotrophic cultivation on a mixed waste substrate of brewer fermentation and crude glycerol. Bioresour. Technol. 2014, 166, 17–23. [Google Scholar] [CrossRef]
- Ramos Tercero, E.A.; Sforza, E.; Morandini, M.; Bertucco, A. Cultivation of Chlorella protothecoides with Urban Wastewater in Continuous Photobioreactor: Biomass Productivity and Nutrient Removal. Appl. Biochem. Biotechnol. 2014, 172, 1470–1485. [Google Scholar] [CrossRef]
- Wen, Q.; Chen, Z.; Li, P.; Duan, R.; Ren, N. Lipid production for biofuels from hydrolyzate of waste activated sludge by heterotrophic Chlorella protothecoides. Bioresour. Technol. 2013, 143, 695–698. [Google Scholar] [CrossRef]
- Hu, B.; Min, M.; Zhou, W.; Li, Y.; Mohr, M.; Cheng, Y.; Lei, H.; Liu, Y.; Lin, X.; Chen, P.; et al. Influence of Exogenous CO2 on Biomass and Lipid Accumulation of Microalgae Auxenochlorella protothecoides Cultivated in Concentrated Municipal Wastewater. Appl. Biochem. Biotechnol. 2012, 166, 1661–1673. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, W.; Hu, B.; Min, M.; Chen, P.; Ruan, R.R. Integration of algae cultivation as biodiesel production feedstock with municipal wastewater treatment: Strains screening and significance evaluation of environmental factors. Bioresour. Technol. 2011, 102, 10861–10867. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.Y.; Yun, Y.M.; Shin, H.S.; Han, J.I. Cultivation of four microalgae species in the effluent of anaerobic digester for biodiesel production. Bioresour. Technol. 2017, 224, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Xia, A.; Murphy, J.D. Microalgal cultivation in treating liquid digestate from biogas systems. Trends Biotechnol. 2016, 34, 264–275. [Google Scholar] [CrossRef]
- Herrmann, A. Biogas production from maize: current state, challenges ans prospects. 2. Agronomic and environmental aspects. BioEnergy Res. 2013, 6, 372–387. [Google Scholar] [CrossRef]
- Oleszek, M.; Matyka, M. Nitrogen fertilization level and cutting affected lignocellulosic crops properties important for biogas production. BioResources 2017, 12, 8565–8580. [Google Scholar]
- Oleszek, M.; Krzemińska, I. Enhancement of Biogas Production by Co-Digestion of Maize Silage with Common Goldenrod Rich in Biologically Active Compound. BioResources 2017, 12, 704–714. [Google Scholar] [CrossRef][Green Version]
- Yu, Z.; Song, M.; Pei, H.; Han, F.; Jiang, L.; Hou, Q. The growth characteristics and biodiesel production of ten algae strains cultivated in anaerobically digested effluent from kitchen waste. Algal Res. 2017, 24, 265–275. [Google Scholar] [CrossRef]
- Silkina, A.; Zacharof, M.P.; Hery, G.; Nouvel, T.; Lovitt, R.W. Formulation and utilisation of spent anaerobic digestate fluids for the growth and product formation of single cell algal cultures in heterotrophic and autotrophic conditions. Bioresour. Technol. 2017, 244, 1445–1455. [Google Scholar] [CrossRef]
- Mayers, J.J.; Nilsson, A.E.; Albers, E.; Flynnc, K.J. Nutrients from anaerobic digestion effluents for cultivation of the microalga Nannochloropsis sp. -Impact on growth, biochemical composition and the potential for cost and environmental impact savings. Algal Res. 2017, 6, 275–286. [Google Scholar] [CrossRef]
- Massa, M.; Buono, S.; Langellotti, A.L.; Castaldo, L.; Martello, A.; Paduano, A.; Sacchi, R.; Fogliano, V. Evaluation of anaerobic digestates from different feedstocks as growth media for Tetradesmus obliquus, Botryococcus braunii, Phaeodactylum tricornutum and Arthrospira maxima. New Biotechnol. 2017, 36, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, X.; Sun, S.; Zhao, Y.; Hu, C. Effects of influent C/N ratios and treatment technologies on integral biogas upgrading and pollutants removal from synthetic domestic sewage. Sci. Rep. 2017, 7, 10897. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Chen, P.; Min, M.; Chen, Y.; Zhu, J.; Ruan, R.R. Anaerobic digested dairy manure as a nutrient supplement for cultivation of oil-rich green microalgae Chlorella sp. Bioresour. Technol. 2010, 101, 2623–2628. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Vandamme, D.; Muylaert, K. Microalgal and cyanobacterial cultivation: The supply of nutrients. Water Res. 2014, 65, 186–202. [Google Scholar] [CrossRef] [PubMed]
- Grobbelaar, J.U. Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2004; pp. 97–115. [Google Scholar]
- Koutra, E.; Economou, C.N.; Tsafrakidou, P.; Kornaros, M. Bio-Based Products from Microalgae Cultivated in Digestates. Trends Biotechnol. 2018, 36, 819–833. [Google Scholar] [CrossRef]
- Kobayashi, N.; Noel, E.A.; Barnes, A.; Watson, A.; Rosenberg, J.N.; Erickson, G.; Oyler, G.A. Characterization of three Chlorella sorokiniana strains in anaerobic digested effluent from cattle manure. Bioresour. Technol. 2013, 150, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jin, H.F.; Lim, B.R.; Park, K.Y.; Lee, K. Ammonia removal from anaerobic digestion effluent of livestock waste using green alga Scenedesmus sp. Bioresour. Technol. 2010, 101, 8649–8657. [Google Scholar] [CrossRef]
- Sigurnjak, I.; Vaneeckhaute, C.; Michels, E.; Ryckaert, B.; Ghekiere, G.; Tack, F.M.G.; Meers, E. Fertilizer performance of liquid fraction of digestate as synthetic nitrogen substitute in silage maize cultivation for three consecutive years. Sci. Total Environ. 2017, 1885–1894. [Google Scholar] [CrossRef]
- Markou, G.; Georgakakis, D. Cultivation of filamentous cyanobacteria (blue-green algae) in agro-industrial, wastes and wastewaters: A review. Appl. Energy 2011, 88, 3389–3401. [Google Scholar] [CrossRef]
- Sforza, E.; Cipriani, R.; Morosinotto, T.; Bertucco, A.; Giacometti, G.M. Excess CO2 supply inhibits mixotrophic growth of Chlorella protothecoides and Nannochloropsis salina. Bioresour. Technol. 2012, 104, 523–529. [Google Scholar] [CrossRef]
- Ma, C.; Wen, H.; Xing, D.; Pei, X.; Zhu, J.; Ren, N.; Liu, B. Molasses wastewater treatment and lipid production at low temperature conditions by a microalgal mutant Scenedesmus sp. Z-4. Biotechnol. Biofuels 2017, 10, 111. [Google Scholar] [CrossRef]
- Liang, K.; Zhang, Q.; Gu, M.; Cong, W. Effect of phosphorus on lipid accumulation in freshwater microalga Chlorella sp. J. Appl. Phycol. 2013, 25, 311–318. [Google Scholar] [CrossRef]
- Shin, D.Y.; Cho, H.U.; Utomo, J.C.; Choi, Y.N.; Xua, X.; Park, J.M. Biodiesel production from Scenedesmus bijuga grown in anaerobically digested food wastewater effluent. Bioresour Technol. 2015, 184, 215–221. [Google Scholar] [CrossRef]
- Solovchenko, A.E. Physiological role of neutral lipid accumulation in eukaryotic microalgae under stresses. Russ. J. Plant Physiol. 2012, 59, 167–176. [Google Scholar] [CrossRef]
- Bucy, H.B.; Baumgardner, M.E.; Marchese, A.J. Chemical and physical properties of algal methyl ester biodiesel containing varying levels of methyl eicosapentaenoate and methyl docosahexaenoate. Algal Res. 2012, 1, 57–69. [Google Scholar] [CrossRef]
- Andersen, R.A. Algal Culturing Techniques; Elsevier: Amsterdam, The Netherlands, 2005; p. 578. [Google Scholar]
- Oleszek, M.; Król, A.; Tys, J.; Matyka, M.; Kulik, M. Comparison of biogas production from wild and cultivated varieties of reed canary grass. Bioresour. Technol. 2014, 156, 303–306. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 1959, 37, 911–917. [Google Scholar]
- Krzemińska, I.; Piasecka, A.; Nosalewicz, A.; Simionato, D.; Wawrzykowski, J. Alterations of the lipid content and fatty acid profile of Chlorella protothecoides under different light intensities. Bioresour. Technol. 2015, 196, 72–77. [Google Scholar] [CrossRef]
Sample Availability: Samples of the microalgae and liquid digestate are available from the authors. |
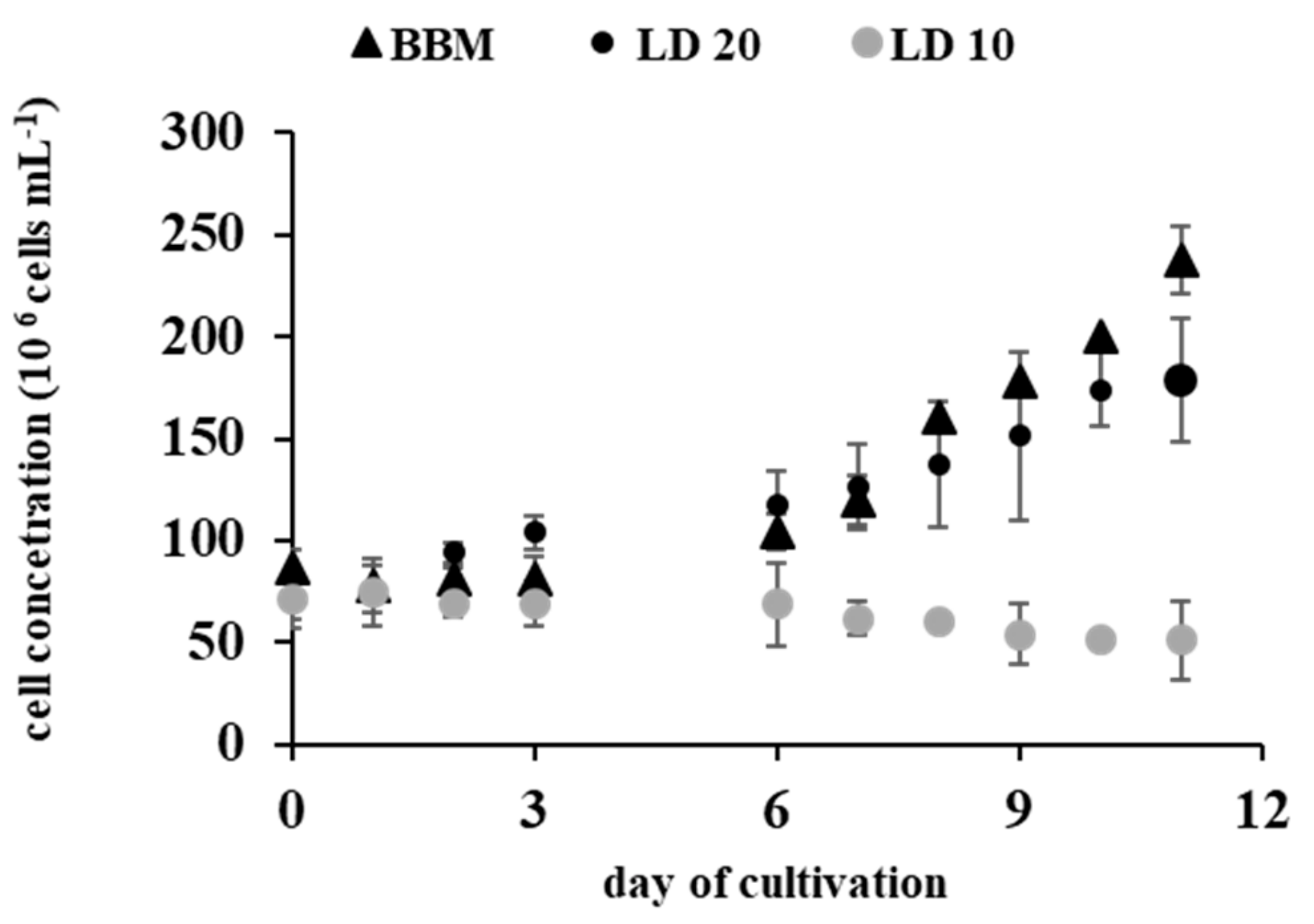
| Medium | Specific Growth Rate 0–6 (day −1) | Doubling Time 0–6 (h) | Specific Growth Rate 7–11 (day −1) | Doubling Time 7–11 (h) | Lipid Content (% of Dry Weight) * |
|---|---|---|---|---|---|
| BBM | 0.302 ± 0.056 | 51.573 ± 7.621 | 0.675 ± 0.039 | 24.71 ± 1.45 | 6.33 ± 1.4 a |
| LD 20 | 0.412 ± 0.044 | 40.767 ± 4.231 | 0.704 ± 0.053 | 23.72 ± 3.24 | 44.65 ± 2.65 b |
| Parameters | Unit | Mean ± SD |
|---|---|---|
| TS | % | 3.64 ± 0.12 |
| VS | % TS | 59.30 ± 1.55 |
| Ash | % TS | 40.70 ± 1.55 |
| BOD | mg O2 L−1 | 3985 ± 156 |
| COD | mg O2 L−1 | 9140 ± 90 |
| BOD/COD | - | 0.44 ± 0.032 |
| Removal of Element | P | K | Na | Zn | Mg | Mn | Mo | Fe | Co | Cu | N-NO2− | N-NO3− | C/N | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| unit | LD 20 | |||||||||||||
| Initial concentration | mg L−1 | 2.08 ± 0.01 a | 192.49 ± 2.67 a | 25.13 ± 0.00 a | 0.57 ± 0.02 a | 2.61 ± 0.21 a | 1.97 ± 0.01 a | 0.21 ± 0.00 a | 1.79 ± 0.04 a | 0.00 ± 0.00 a | 0.03 ± 0.00 a | 0.25 ± 0.01 a | 4.15 | 4.6 |
| Final concentration | mg L−1 | 0.45 ± 0.00 b | 174.21 ± 0.54 b | 16.97 ± 0.28 b | 0.21 ± 0.08 a | 0.61 ± 0.18 b | 1.85 ± 0.03 b | 0.08 ± 0.00 b | 1.03 ± 0.02 b | 0.00 ± 0.00 a | 0.02 ± 0.00 a | 0.15 ± 0.01 b | 1.20 | 17.8 |
| Removal | mg L−1 | 1.63 | 18.28 | 8.16 | 0.36 | 2.00 | 0.12 | 0.13 | 0.76 | 0.00 | 0.01 | 0.11 | 2.95 | |
| Removal | % | 78.4 | 9.5 | 32.5 | 63.1 | 76.7 | 5.9 | 61.2 | 42.2 | - | 32.6 | 43.1 | 71.1 | |
| BBM | ||||||||||||||
| Initial concentration | mg L−1 | 53.3 ± 0.00 c | 105.80 ± 0.00 c | 77.60 ± 0.00 c | 2.00 ± 0.00 b | 7.40 ± 0.00 c | 0.40 ± 0.00 c | 0.47 ± 0.00 c | 1.00 ± 0.01 b | 0,10 ± 0.00 b | 0.63 ± 0.01 b | nd | 45.05 | 2.8 |
| Final concentration | mg L−1 | 51.07 ± 1.04 d | 83.20 ± 0.07 d | 69.29 ± 1.33 d | 1.43 ± 0.28 c | 6.66 ± 0.43 d | 0.16 ± 0.02 d | 0.42 ± 0.01 d | 0.00 ± 0.00 c | 0.09 ± 0.02 b | 0.52 ± 0.00 c | nd | 0.00 | 12.7 |
| Removal | mg L−1 | 2.23 | 22.60 | 8.31 | 0.57 | 0.74 | 0.24 | 0.05 | 1.00 | 0.01 | 0.11 | 45.05 | ||
| Removal | % | 4.0 | 21.4 | 10.7 | 28.7 | 10.0 | 60.1 | 10.0 | 100.0 | 8.7 | 17.5 | 100 | ||
| Medium | ||
|---|---|---|
| Distribution of fatty acids (%, of total fatty acids) * | BBM | LD 1:20 |
| 16:0 (Palmitic acid) | 14.92 ± 1.88 | 9.46 ± 1.10 |
| 18:0 (Stearic acid) | 13.13 ± 2.73 | 1.76 ± 0.28 |
| 18:1 (Oleic acid) | 2.93 ± 0.34 | 35.09 ± 0.08 |
| 18:2 (Linoleic acid) | 32.85 ± 1.05 | 38.96 ± 1.05 |
| 18:3(Linolenic acid) | 34.18 ± 1.31 | 13.31 ± 0.95 |
| C16-C18 | 98.00 | 98.56 |
| Component | Stock Solution g L−1 | Quantity Used (mL L−1) | |
|---|---|---|---|
| NaNO3 | 25 | 10 | |
| CaCl2·2H2O | 2.50 | 10 | |
| MgSO4·7H2O | 7.50 | 10 | |
| K2HPO4 | 7.50 | 10 | |
| KH2PO4 | 17.50 | 10 | |
| NaCl | 2.50 | 10 | |
| EDTA solution g L−1 | |||
| EDTA | 50.00 | 1 mL | |
| KOH | 31 | ||
| Acidified Iron Solution (to 100 mL) | |||
| FeSO4·7H2O | 0.498 g | 1 mL | |
| H2SO4 (96%) | 0.1 mL | ||
| Trace metals solution (g L−1) | |||
| ZnSO4·7H2O | 8.82 | 1 mL | |
| MnCl2·4H2O | 1.44 | ||
| MoO3 | 0.71 | ||
| CuSO4·5H2O | 1.57 | ||
| Co(NO3)2·6H2O | 0.49 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzemińska, I.; Oleszek, M.; Wiącek, D. Liquid Anaerobic Digestate as a Source of Nutrients for Lipid and Fatty Acid Accumulation by Auxenochlorella Protothecoides. Molecules 2019, 24, 3582. https://doi.org/10.3390/molecules24193582
Krzemińska I, Oleszek M, Wiącek D. Liquid Anaerobic Digestate as a Source of Nutrients for Lipid and Fatty Acid Accumulation by Auxenochlorella Protothecoides. Molecules. 2019; 24(19):3582. https://doi.org/10.3390/molecules24193582
Chicago/Turabian StyleKrzemińska, Izabela, Marta Oleszek, and Dariusz Wiącek. 2019. "Liquid Anaerobic Digestate as a Source of Nutrients for Lipid and Fatty Acid Accumulation by Auxenochlorella Protothecoides" Molecules 24, no. 19: 3582. https://doi.org/10.3390/molecules24193582
APA StyleKrzemińska, I., Oleszek, M., & Wiącek, D. (2019). Liquid Anaerobic Digestate as a Source of Nutrients for Lipid and Fatty Acid Accumulation by Auxenochlorella Protothecoides. Molecules, 24(19), 3582. https://doi.org/10.3390/molecules24193582







