Improving the Yields and Reaction Rate in the Ethanolysis of Soybean Oil by Using Mixtures of Lipase CLEAs
Abstract
:1. Introduction
2. Results and Discussion
2.1. Selection of Lipases
2.2. Preparation of CLEAs of PPL and TLL
2.3. Influence of the Soybean Oil/Ethanol Molar Ratio in the Biodiesel Production
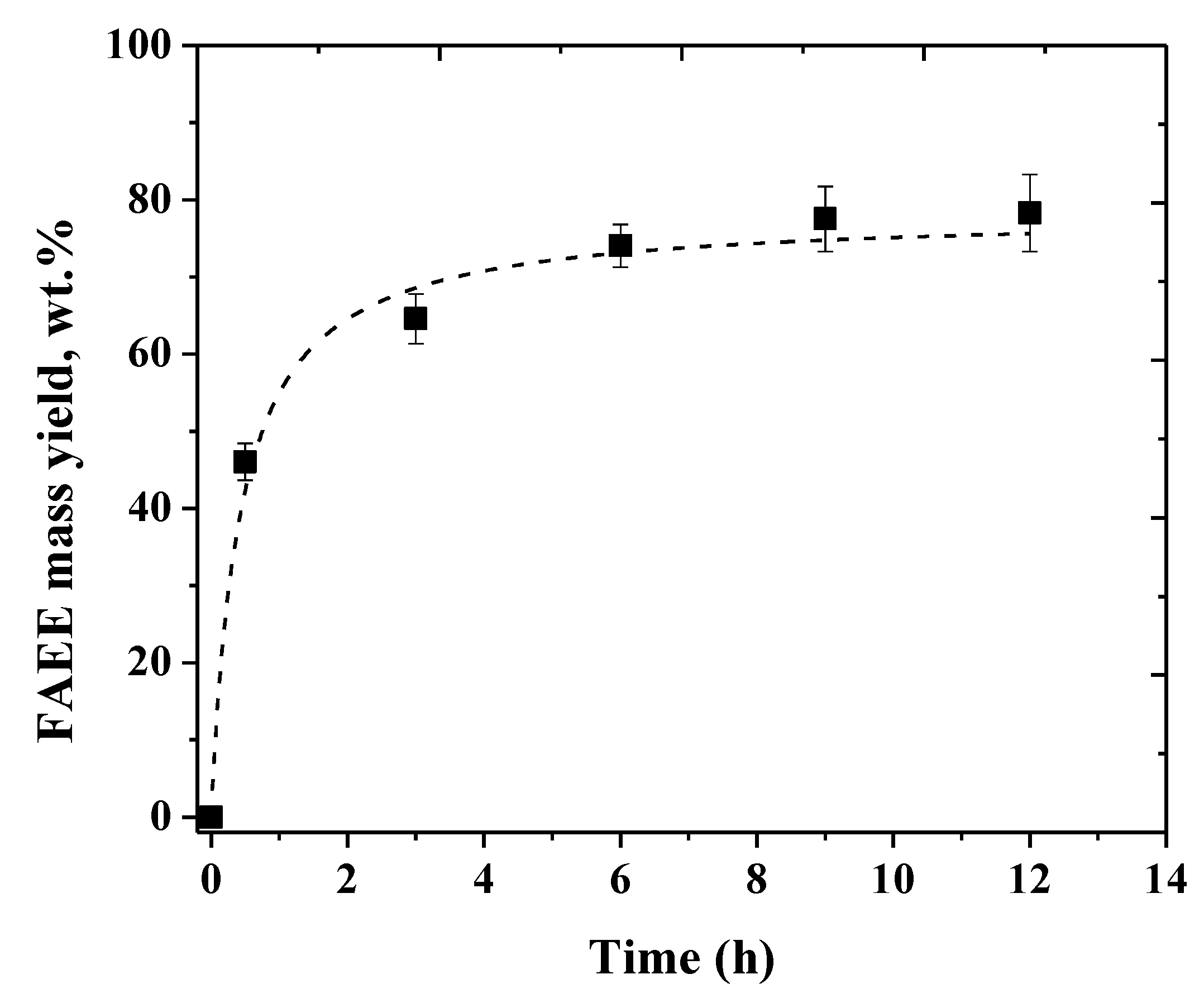
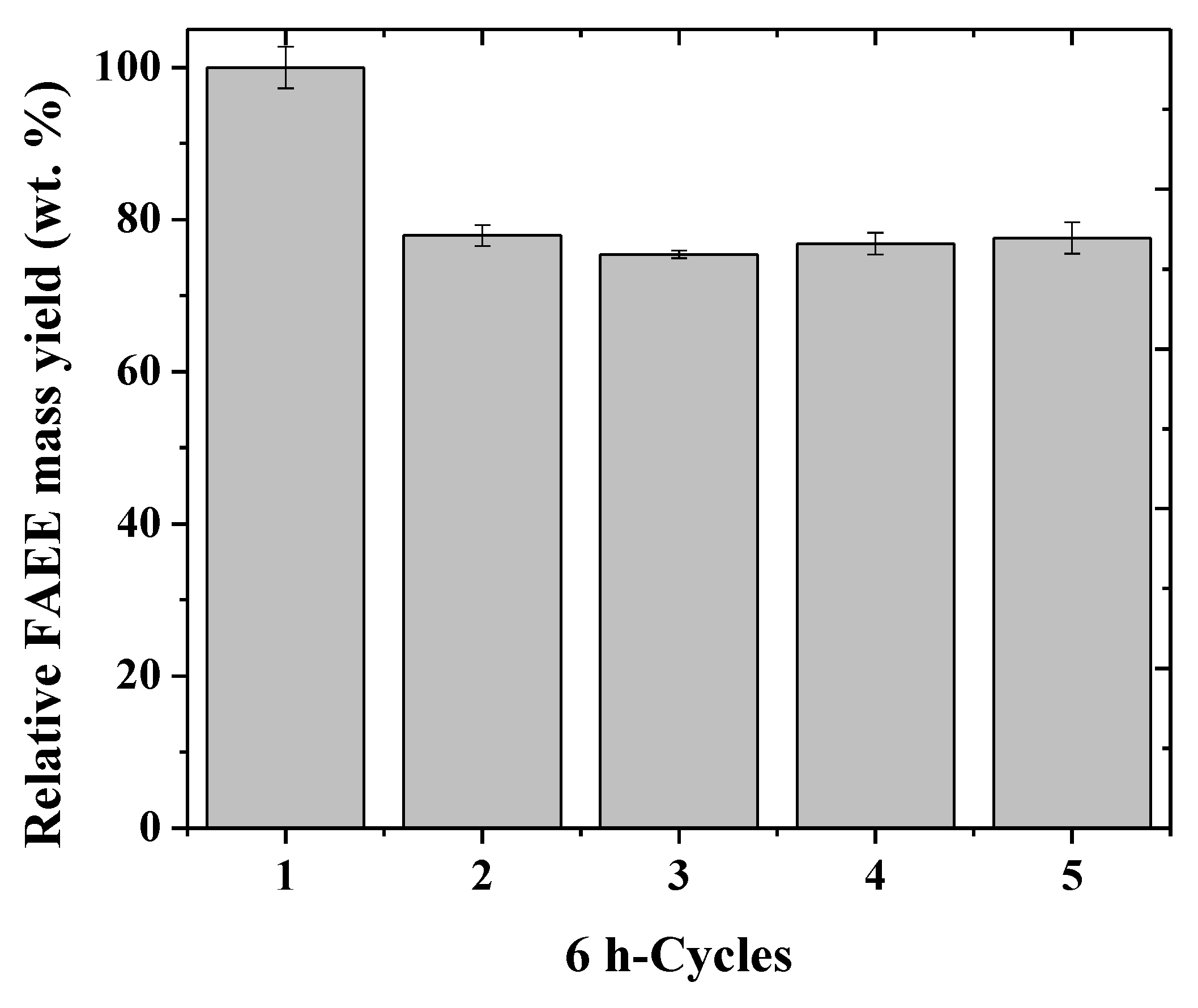
2.4. Operational Stability of The Biocatalysts
3. Material and Methods
3.1. Biodiesel Production Using Different Free Lipases
3.2. Preparation of CLEAs
3.3. Ethanolysis of Soybean Oil
3.4. Operational Stability of The Mixture of CLEAs in The Synthesis of Biodiesel
3.5. Protein Concentration Determination
3.6. Standard Activity Assay
3.7. Gas Chromatography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ma, F.; Hanna, M.A. Biodiesel production: a review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Bozbas, K. Biodiesel as an alternative motor fuel: Production and policies in the European Union. Renew. Sustain. Energy Rev. 2008, 12, 542–552. [Google Scholar] [CrossRef]
- Tan, T.; Lu, J.; Nie, K.; Deng, L.; Wang, F. Biodiesel production with immobilized lipase: A review. Biotechnol. Adv. 2010, 28, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Fjerbaek, L.; Christensen, K.V.; Norddahl, B. A review of the current state of biodiesel production using enzymatic transesterification. Biotechnol. Bioeng. 2009, 102, 1298–1315. [Google Scholar] [CrossRef] [PubMed]
- Atadashi, I.M.; Aroua, M.K.; Abdul Aziz, A.R.; Sulaiman, N.M.N. Production of biodiesel using high free fatty acid feedstocks. Renew. Sustain. Energy Rev. 2012, 16, 3275–3285. [Google Scholar] [CrossRef]
- Atadashi, I.M.M.; Aroua, M.K.; Abdul Aziz, A. Biodiesel separation and purification: A review. Renew. Energy 2011, 36, 437–443. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, F.; Yuan, C.; Du, W.; Liu, D. Lipase-catalyzed process for biodiesel production: Enzyme immobilization, process simulation and optimization. Renew. Sustain. Energy Rev. 2015, 44, 182–197. [Google Scholar] [CrossRef]
- Christopher, L.P.; Kumar, H.; Zambare, V.P. Enzymatic biodiesel: Challenges and opportunities. Appl. Energy 2014, 119, 497–520. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Torrestiana-Sánchez, B.; Dal Magro, L.; Virgen-Ortíz, J.J.; Suárez-Ruíz, F.J.; Rodrigues, R.C.; Fernandez-Lafuente, R. Comparison of acid, basic and enzymatic catalysis on the production of biodiesel after RSM optimization. Renew. Energy 2019, 135, 1–9. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Virgen-Ortíz, J.J.; Jiménez-Pérez, M.; Yates, M.; Torrestiana-Sanchez, B.; Rosales-Quintero, A.; Fernandez-Lafuente, R. Evaluation of different lipase biocatalysts in the production of biodiesel from used cooking oil: Critical role of the immobilization support. Fuel 2017, 200, 1–10. [Google Scholar] [CrossRef]
- Vargas, M.; Niehus, X.; Casas-Godoy, L.; Sandoval, G. Lipases as biocatalyst for biodiesel production. In Lipases and Phospholipases. Methods in Molecular Biology; Sandoval, G., Ed.; Humana Press: New York, NY, 2018; pp. 377–390. ISBN 978-1-4939-8672-9. [Google Scholar]
- Gog, A.; Roman, M.; Toşa, M.; Paizs, C.; Irimie, F.D. Biodiesel production using enzymatic transesterification - Current state and perspectives. Renew. Energy 2012, 39, 10–16. [Google Scholar] [CrossRef]
- Hasan, F.; Shah, A.A.; Hameed, A. Industrial applications of microbial lipases. Enzyme Microb. Technol. 2006, 39, 235–251. [Google Scholar] [CrossRef]
- Guan, F.; Peng, P.; Wang, G.; Yin, T.; Peng, Q.; Huang, J.; Guan, G.; Li, Y. Combination of two lipases more efficiently catalyzes methanolysis of soybean oil for biodiesel production in aqueous medium. Process Biochem. 2010, 45, 1677–1682. [Google Scholar] [CrossRef]
- Tongboriboon, K.; Cheirsilp, B.; H-Kittikun, A. Mixed lipases for efficient enzymatic synthesis of biodiesel from used palm oil and ethanol in a solvent-free system. J. Mol. Catal. B Enzym. 2010, 67, 52–59. [Google Scholar] [CrossRef]
- Banerjee, A.; Singh, V.; Solanki, K.; Mukherjee, J.; Gupta, M. Combi-protein coated microcrystals of lipases for production of biodiesel from oil from spent coffee grounds. Sustain. Chem. Process. 2013, 1, 14. [Google Scholar] [CrossRef]
- Sánchez-Bayo, A.; Morales, V.; Rodríguez, R.; Vicente, G.; Bautista, L. Biodiesel production (FAEEs) by heterogeneous combi-lipase biocatalysts using wet extracted lipids from microalgae. Catalysts 2019, 9, 296. [Google Scholar] [CrossRef]
- Poppe, J.K.; Matte, C.R.; Peralba, M.D.C.R.; Fernandez-Lafuente, R.; Rodrigues, R.C.; Ayub, M.A.Z. Optimization of ethyl ester production from olive and palm oils using mixtures of immobilized lipases. Appl. Catal. A Gen. 2015, 490, 50–56. [Google Scholar] [CrossRef]
- Poppe, J.K.; Matte, C.R.; Fernandez-Lafuente, R.; Rodrigues, R.C.; Ayub, M.A.Z. Transesterification of waste frying oil and soybean oil by combi-lipases under ultrasound-assisted reactions. Appl. Biochem. Biotechnol. 2018, 186, 576–589. [Google Scholar] [CrossRef]
- Poppe, J.K.; Matte, C.R.; de Freitas, V.O.; Fernandez-Lafuente, R.; Rodrigues, R.C.; Záchia Ayub, M.A. Enzymatic synthesis of ethyl esters from waste oil using mixtures of lipases in a plug-flow packed-bed continuous reactor. Biotechnol. Prog. 2018, 34, 952–959. [Google Scholar] [CrossRef]
- Alves, J.S.; Vieira, N.S.; Cunha, A.S.; Silva, A.M.; Ayub, M.A.Z.; Fernández-Lafuente, R.; Rodrigues, R.C. Combi-lipase for heterogeneous substrates: a new approach for hydrolysis of soybean oil using mixtures of biocatalysts. RSC Adv. 2014, 4, 6863–6868. [Google Scholar] [CrossRef]
- Li, L.; Du, W.; Liu, D.; Wang, L.; Li, Z. Lipase-catalyzed transesterification of rapeseed oils for biodiesel production with a novel organic solvent as the reaction medium. J. Mol. Catal. B Enzym. 2006, 43, 58–62. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, H.; Yan, Y. Optimization of lipase-catalyzed transesterification of lard for biodiesel production using response surface methodology. Appl. Biochem. Biotechnol. 2010, 160, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Kim, J.M.; Shin, H.Y.; Kang, S.W.; Kim, S.W. Biodiesel production using a mixture of immobilized Rhizopus oryzae and Candida rugosa lipases. Biotechnol. Bioprocess Eng. 2006, 11, 522–525. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, D.H.; Lim, J.S.; Um, B.-H.; Park, C.; Kang, S.W.; Kim, S.W. Optimization of the process for biodiesel production using a mixture of immobilized Rhizopus oryzae and Candida rugosa lipases. J. Microbiol. Biotechnol. 2008, 18, 1927–1931. [Google Scholar] [PubMed]
- Lee, J.H.; Kim, S.B.; Kang, S.W.; Song, Y.S.; Park, C.; Han, S.O.; Kim, S.W. Biodiesel production by a mixture of Candida rugosa and Rhizopus oryzae lipases using a supercritical carbon dioxide process. Bioresour. Technol. 2011, 102, 2105–2108. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.G.; Kim, D.K.; Park, S.C.; Lee, J.S.; Kim, S.W. Biodiesel production from crude canola oil by two-step enzymatic processes. Renew. Energy 2012, 42, 99–104. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ayub, M.A.Z. Effects of the combined use of Thermomyces lanuginosus and Rhizomucor miehei lipases for the transesterification and hydrolysis of soybean oil. Process Biochem. 2011, 46, 682–688. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Toro, E.C.; Rodríguez, D.F.; Morales, N.; García, L.M.; Godoy, C.A. Novel combi-lipase systems for fatty acid ethyl esters production. Catalysts 2019, 9, 546. [Google Scholar] [CrossRef]
- Cao, L.; van Langen, L.; Sheldon, R.A. Immobilised enzymes: Carrier-bound or carrier-free? Curr. Opin. Biotechnol. 2003, 14, 387–394. [Google Scholar] [CrossRef]
- Cao, L.; van Rantwijk, F.; Sheldon, R.A. Cross-linked enzyme aggregates: A simple and effective method for the immobilization of penicillin acylase. Org. Lett. 2000, 2, 1361–1364. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Schoevaart, R.; Van Langen, L.M. Cross-linked enzyme aggregates (CLEAs): A novel and versatile method for enzyme immobilization (a review). Biocatal. Biotransformation 2005, 23, 141–147. [Google Scholar] [CrossRef]
- Sheldon, R.A. Cross-linked enzyme aggregates (CLEAs): stable and recyclable biocatalysts. Biochem. Soc. Trans. 2007, 35, 1583–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldon, R. CLEAs, combi-CLEAs and ‘smart’ magnetic CLEAs: Biocatalysis in a bio-based economy. Catalysts 2019, 9, 261. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, R.A. Cross-linked enzyme aggregates as industrial biocatalysts. Org. Process Res. Dev. 2011, 15, 213–223. [Google Scholar] [CrossRef]
- López-Serrano, P.; Cao, L.; van Rantwijk, F.; Sheldon, R.A. Cross-linked enzyme aggregates with enhanced activity: application to lipases. Biotechnol. Lett. 2002, 24, 1379–1383. [Google Scholar] [CrossRef]
- Mafra, A.C.O.; Ulrich, L.G.; Kornecki, J.F.; Fernandez-Lafuente, R.; Tardioli, P.W.; Ribeiro, M.P. de A. Combi-CLEAs of glucose oxidase and catalase for conversion of glucose to gluconic acid eliminating the hydrogen peroxide to maintain enzyme activity in a bubble column reactor. Catalysts 2019, 9, 657. [Google Scholar] [CrossRef] [Green Version]
- Wilson, L.; Fernández-Lorente, G.; Fernández-Lafuente, R.; Illanes, A.; Guisán, J.M.; Palomo, J.M. CLEAs of lipases and poly-ionic polymers: A simple way of preparing stable biocatalysts with improved properties. Enzyme Microb. Technol. 2006, 39, 750–755. [Google Scholar] [CrossRef]
- Rojas, M.J.; Amaral-Fonseca, M.; Zanin, G.M.; Fernandez-Lafuente, R.; Giordano, R.d.L.C.; Tardioli, P.W. Preparation of crosslinked enzyme aggregates of a thermostable cyclodextrin glucosyltransferase from Thermoanaerobacter sp. Critical effect of the crosslinking agent. Catalyst 2019, 9, 120. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, J.; Giordano, R.; Fernandez-Lafuente, R.; Tardioli, P. Evaluation of strategies to produce highly porous cross-linked aggregates of porcine pancreas lipase with magnetic properties. Molecules 2018, 23, 2993. [Google Scholar] [CrossRef] [Green Version]
- Ramos, M.D.; Miranda, L.P.; Giordano, R.L.C.; Fernandez-Lafuente, R.; Kopp, W.; Tardioli, P.W. 1,3-Regiospecific ethanolysis of soybean oil catalyzed by crosslinked porcine pancreas lipase aggregates. Biotechnol. Prog. 2018, 34, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Araujo-Silva, R.; Mafra, A.C.O.; Rojas, M.J.; Kopp, W.; De Campos Giordano, R.; Fernandez-Lafuente, R.; Tardioli, P.W. Maltose production using starch from cassava bagasse catalyzed by cross-linked β-amylase aggregates. Catalysts 2018, 8, 170. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.; Sharma, A.; Gupta, M.N. Preparation of cross-linked enzyme aggregates by using bovine serum albumin as a proteic feeder. Anal. Biochem. 2006, 351, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Kumari, V.; Shah, S.; Gupta, M.N. Preparation of biodiesel by lipase-catalyzed transesterification of high free fatty acid containing oil from Madhuca indica. Energy Fuels 2007, 21, 368–372. [Google Scholar]
- Lai, J.-Q.; Hu, Z.-L.; Sheldon, R.A.; Yang, Z. Catalytic performance of cross-linked enzyme aggregates of Penicillium expansum lipase and their use as catalyst for biodiesel production. Process Biochem. 2012, 47, 2058–2063. [Google Scholar] [CrossRef]
- Schroeck, A.M.; Schober, S.; Mittelbach, M. Highly active biocatalyst for transesterification: Cross linked enzyme aggregates of Thermomyces lanuginosus and Candida antarctica B. Eur. J. Lipid Sci. Technol. 2013, 115, n. [Google Scholar]
- Cruz-Izquierdo, Á.; Picó, E.A.; López, C.; Serra, J.L.; Llama, M.J. Magnetic cross-linked enzyme aggregates (mCLEAs) of Candida antarctica lipase: An efficient and stable biocatalyst for biodiesel synthesis. PLoS ONE 2014, 9, e115202. [Google Scholar] [CrossRef]
- Mahmod, S.S.; Yusof, F.; Jami, M.S.; Khanahmadi, S.; Shah, H. Development of an immobilized biocatalyst with lipase and protease activities as a multipurpose cross-linked enzyme aggregate (multi-CLEA). Process Biochem. 2015, 50, 2144–2157. [Google Scholar] [CrossRef]
- Zhang, W.-W.; Yang, X.-L.; Jia, J.-Q.; Wang, N.; Hu, C.-L.; Yu, X.-Q. Surfactant-activated magnetic cross-linked enzyme aggregates (magnetic CLEAs) of Thermomyces lanuginosus lipase for biodiesel production. J. Mol. Catal. B Enzym. 2015, 115, 83–89. [Google Scholar] [CrossRef]
- Picó, E.A.; López, C.; Cruz-Izquierdo, Á.; Munarriz, M.; Iruretagoyena, F.J.; Serra, J.L.; Llama, M.J. Easy reuse of magnetic cross-linked enzyme aggregates of lipase B from Candida antarctica to obtain biodiesel from Chlorella vulgaris lipids. J. Biosci. Bioeng. 2018, 126, 451–457. [Google Scholar] [CrossRef]
- Badoei-dalfard, A.; Malekabadi, S.; Karami, Z.; Sargazi, G. Magnetic cross-linked enzyme aggregates of Km12 lipase: A stable nanobiocatalyst for biodiesel synthesis from waste cooking oil. Renew. Energy 2019, 141, 874–882. [Google Scholar] [CrossRef]
- Resende, M.M.; Sousa, R.; Tardioli, P.W.; Giordano, R.L.C.; Giordano, R.C. Enzymatic tailor-made proteolysis of whey in a vortex flow reactor. AIChE J. 2005, 51, 314–322. [Google Scholar] [CrossRef]
- Resende, M.M.; Tardioli, P.W.; Fernandez, V.M.; Ferreira, A.L.O.; Giordano, R.L.C.; Giordano, R.C. Distribution of suspended particles in a Taylor–Poiseuille vortex flow reactor. Chem. Eng. Sci. 2001, 56, 755–761. [Google Scholar] [CrossRef]
- Giordano, R.C.; Giordano, R.L.C.; Prazeres, D.M.F.; Cooney, C.L. Analysis of a Taylor–Poiseuille vortex flow reactor—I: Flow patterns and mass transfer characteristics. Chem. Eng. Sci. 1998, 53, 3635–3652. [Google Scholar] [CrossRef]
- Giordano, R.L.C.; Giordano, R.C.; Cooney, C.L. Performance of a continuous Taylor–Couette–Poiseuille vortex flow enzymic reactor with suspended particles. Process Biochem. 2000, 35, 1093–1101. [Google Scholar] [CrossRef]
- Resende, M.M.; Vieira, P.G.; Sousa, R.; Giordano, R.L.C.; Giordano, R.C. Estimation of mass transfer parameters in a Taylor-Couette-Poiseuille heterogeneous reactor. Brazilian J. Chem. Eng. 2004, 21, 175–184. [Google Scholar] [CrossRef]
- Mendes, A.A.; Oliveira, P.C.; de Castro, H.F. Properties and biotechnological applications of porcine pancreatic lipase. J. Mol. Catal. B Enzym. 2012, 78, 119–134. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Peirce, S.; Virgen-Ortíz, J.J.; Tacias-Pascacio, V.G.; Rueda, N.; Bartolome-Cabrero, R.; Fernandez-Lopez, L.; Russo, M.E.; Marzocchella, A.; Fernandez-Lafuente, R. Development of simple protocols to solve the problems of enzyme coimmobilization. Application to coimmobilize a lipase and a β-galactosidase. RSC Adv. 2016, 6, 61707–61715. [Google Scholar] [CrossRef]
- Zaak, H.; Kornecki, J.F.; Siar, E.-H.; Fernandez-Lopez, L.; Corberán, V.C.; Sassi, M.; Fernandez-Lafuente, R. Coimmobilization of enzymes in bilayers using pei as a glue to reuse the most stable enzyme: Preventing pei release during inactivated enzyme desorption. Process Biochem. 2017, 61, 95–101. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Mendez-Sanchez, C.; Rios, N.S.; Ortiz, C.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. New applications of glyoxyl-octyl agarose in lipases co-immobilization: Strategies to reuse the most stable lipase. Int. J. Biol. Macromol. 2019, 131, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Rios, N.S.; Arana-Peña, S.; Mendez-Sanchez, C.; Ortiz, C.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Reuse of lipase from Pseudomonas fluorescens via its step-by-step coimmobilization on glyoxyl-octyl agarose beads with least stable lipases. Catalysts 2019, 9, 487. [Google Scholar] [CrossRef] [Green Version]
- Freitas, L.; Bueno, T.; Perez, V.H.; Santos, J.C.; De Castro, H.F. Enzymatic hydrolysis of soybean oil using lipase from different sources to yield concentrated of polyunsaturated fatty acids. World J. Microbiol. Biotechnol. 2007, 23, 1725–1731. [Google Scholar] [CrossRef] [PubMed]
- Yesiloglu, Y. Immobilized lipase-catalyzed ethanolysis of sunflower oil. J. Am. Oil Chem. Soc. 2004, 81, 157–160. [Google Scholar] [CrossRef]
- Hazarika, S.; Goswami, P.; Dutta, N.N.; Hazarika, A.K. Ethyl oleate synthesis by porcine pancreatic lipase in organic solvents. Chem. Eng. J. 2002, 85, 61–68. [Google Scholar] [CrossRef]
- Bonet-Ragel, K.; Canet, A.; Benaiges, M.D.; Valero, F. Effect of acyl-acceptor stepwise addition strategy using alperujo oil as a substrate in enzymatic biodiesel synthesis. J. Chem. Technol. Biotechnol. 2018, 93, 541–547. [Google Scholar] [CrossRef] [Green Version]
- Kartal, F.; Kilinc, A. Crosslinked aggregates of Rhizopus oryzae lipase as industrial biocatalysts: Preparation, optimization, characterization, and application for enantioselective resolution reactions. Biotechnol. Prog. 2012, 28, 937–945. [Google Scholar] [CrossRef]
- Schoevaart, R.; Wolbers, M.W.; Golubovic, M.; Ottens, M.; Kieboom, A.P.G.; van Rantwijk, F.; van der Wielen, L.A.M.; Sheldon, R.A. Preparation, optimization, and structures of cross-linked enzyme aggregates (CLEAs). Biotechnol. Bioeng. 2004, 87, 754–762. [Google Scholar] [CrossRef]
- Khanahmadi, S.; Yusof, F.; Chyuan Ong, H.; Amid, A.; Shah, H. Cocoa pod husk: A new source of CLEA-lipase for preparation of low-cost biodiesel: An optimized process. J. Biotechnol. 2016, 231, 95–105. [Google Scholar] [CrossRef]
- López-Gallego, F.; Betancor, L.; Hidalgo, A.; Alonso, N.; Fernández-Lafuente, R.; Guisán, J.M. Co-aggregation of enzymes and polyethyleneimine: A simple method to prepare stable and immobilized eerivatives of glutaryl acylase. Biomacromolecules 2005, 6, 1839–1842. [Google Scholar] [CrossRef]
- Velasco-Lozano, S.; López-Gallego, F.; Vázquez-Duhalt, R.; Mateos-Díaz, J.C.; Guisán, J.M.; Favela-Torres, E. Carrier-free immobilization of lipase from Candida rugosa with polyethyleneimines by carboxyl-activated cross-linking. Biomacromolecules 2014, 15, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Soler, L.; Illanes, A.; Wilson, L. Immobilization of Alcaligenes sp. lipase as catalyst for the transesterification of vegetable oils to produce biodiesel. Catal. Today 2016, 259, 177–182. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beisson, F.F.; Tiss, A.; Riviere, C.; Verger, R.; Rivière, C.; Verger, R. Methods for lipase detection and assay: a critical review. Eur. J. Lipid Sci. Technol. 2000, 102, 133–153. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
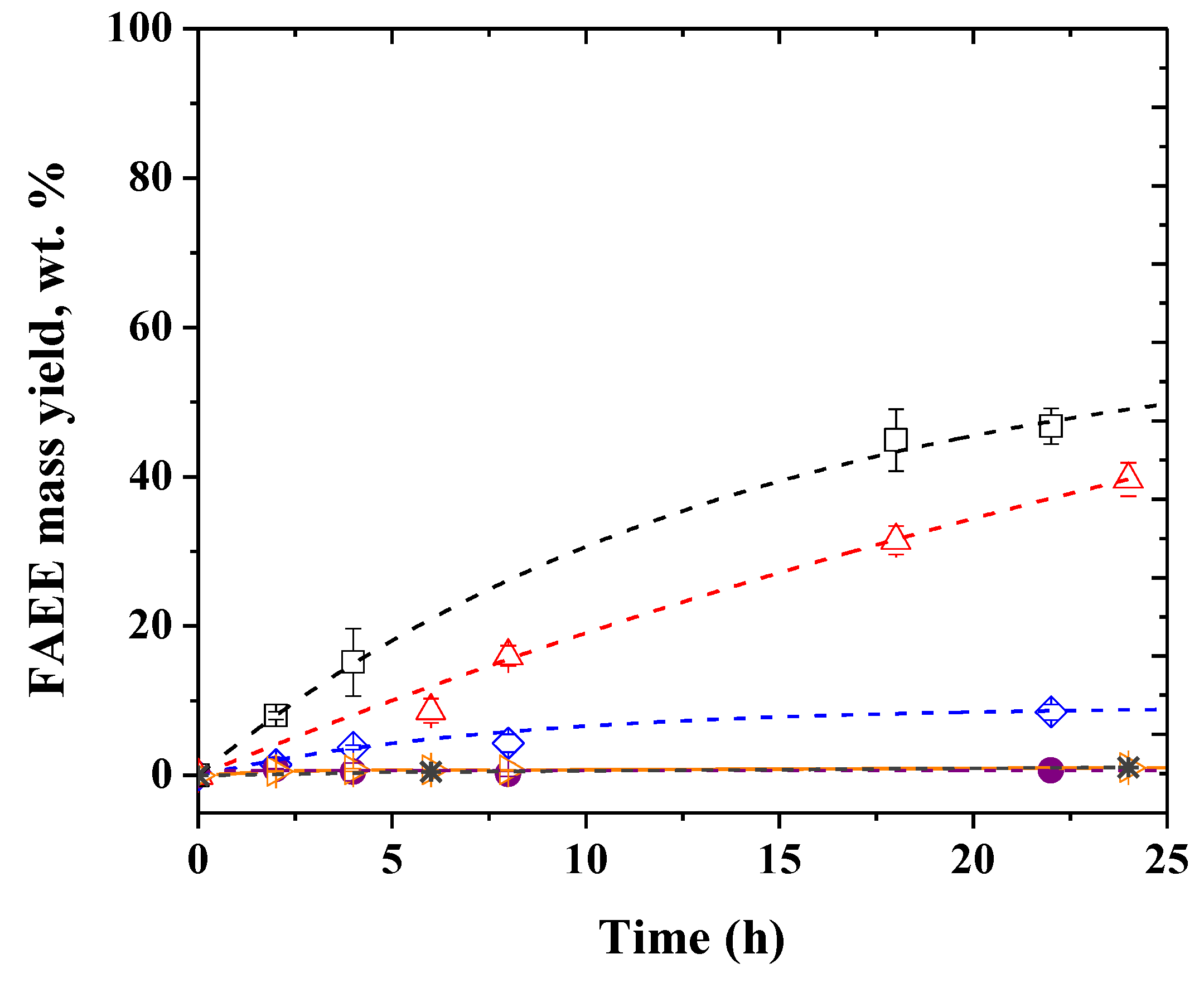
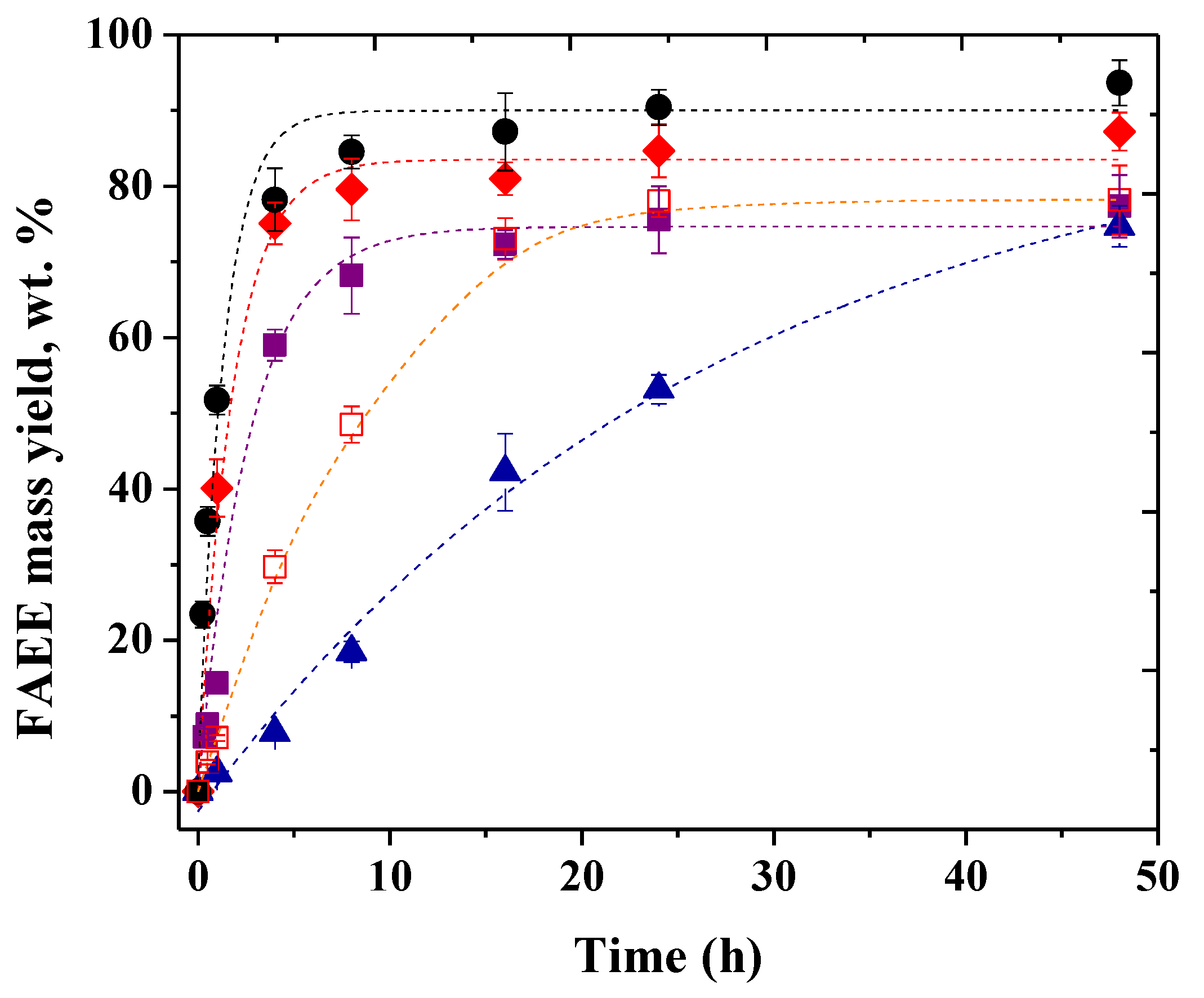
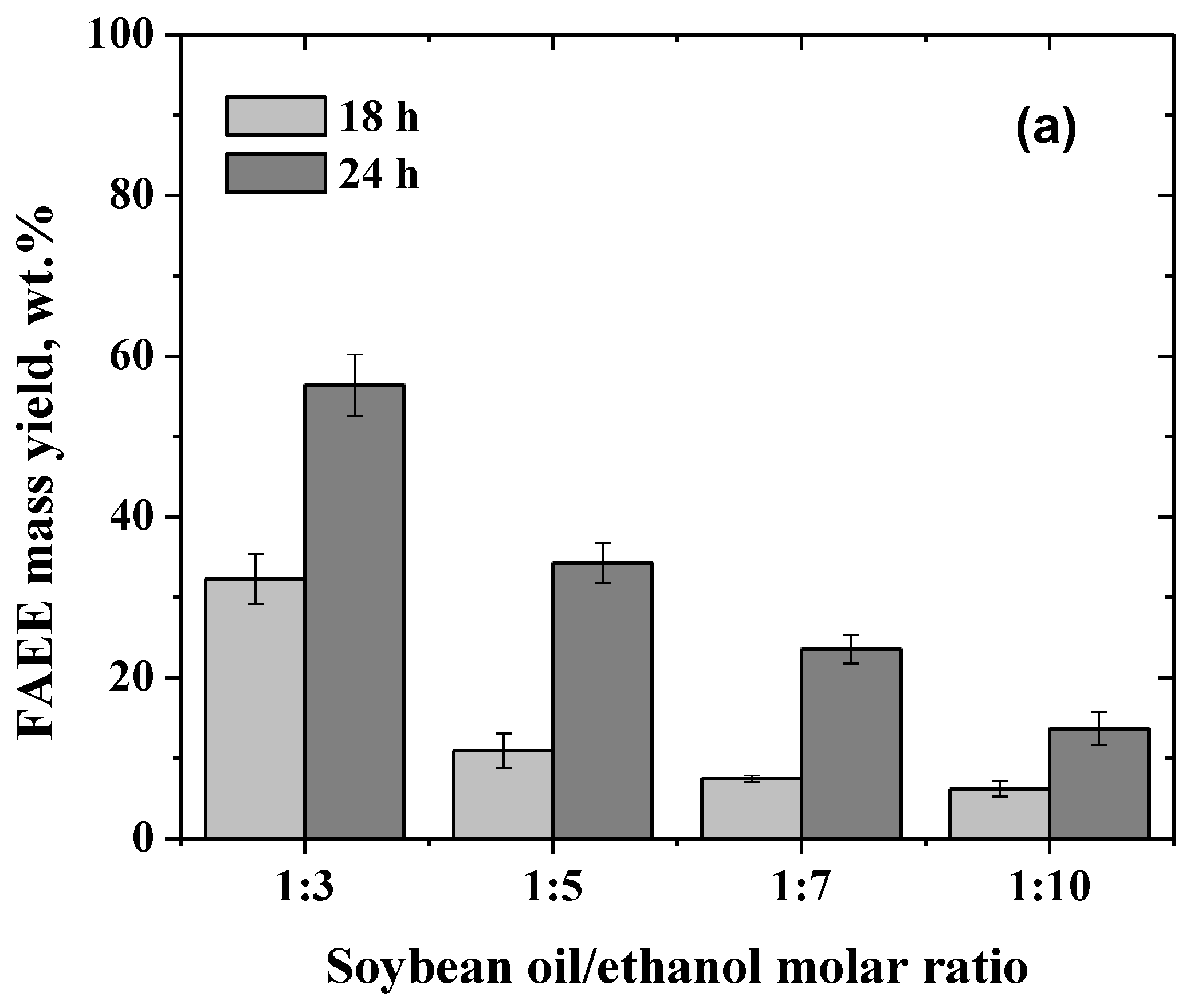
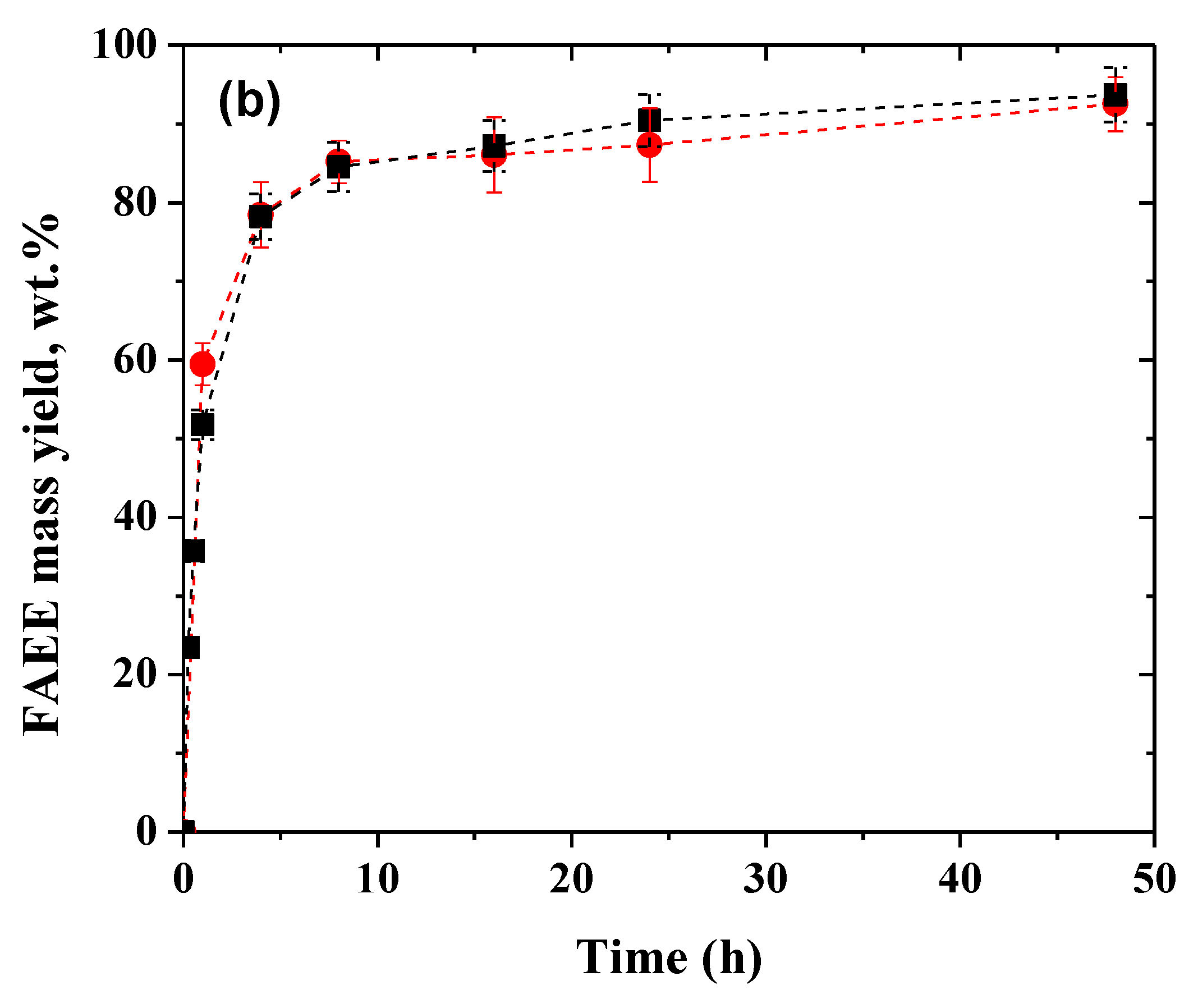
| Lipase CLEA | Immobilization Conditions | Reaction Conditions | Yield (%) | Reference |
|---|---|---|---|---|
| CLEAs of Pseudomonas cepacia lipase | Precipitant: Acetone 5 mg BSA/50 mg lipase 12-fold increase in the activity over the free enzyme powder | Jatropha seed oil/ethanol (1:4, mol/mol) CLEA (containing 6.25 mg lipase) 6 h reaction at 40 °C | 90 | [44] |
| Mahua oil/ethanol (1:4, mol/mol) CLEA (containing 50 mg enzyme) 2.5 h reaction at 40 °C | 92 | [45] | ||
| CLEA of Penicillium expansum lipase | Precipitant: Ammonium sulphate The protein content in the CLEAs: 21 wt.% | Microalgal oil in the IL [BMIm][PF6]/methanol (1:3, mol/mol) 100 mg of CLEA 48 h reaction at 50 °C | 85.7 | [46] |
| CLEA of Thermomyces lanuginosus lipase (TLL) | Precipitant: Acetone Lipase/BSA mass ratio of 1:12 8-fold increase in the activity over the non-cross-linked lipase | Rapeseed oil or fish oil/ethanol (1:4, mol/mol) 10 wt.% CLEAs (with BSA in a mass ratio of 1:4) 1.5 h reaction at 40 °C The biocatalyst remained stable within 6 cycles of reuse | 94 (Rapeseed oil) 68 (Fish oil) | [47] |
| CLEA of lipase B from Candida Antarctica (CALB) | Lipase/BSA mass ratio of 1:16 24-fold increase of the activity over the non-cross-linked lipase | Rapeseed oil or fish oil/ethanol (1:4, mol/mol) 10 wt.% CLEAs (with BSA in a mass ratio of 1:12) 24 h reaction at 40 °C | ~80 (Rapeseed oil/fish oil) | |
| CLEA of lipase B from Candida Antarctica (CALB) | Precipitant: Ammonium sulphate Insolubilized CALB was covalently cross-linked to magnetic nanoparticles | Olive oil/2-propanol (1:6, mol/mol) 1 wt.% magnetic CLEAs within oil 24 h reaction at 30 °C Reuse in 10 cycles of 24 h without apparent loss of activity. | 80 (92% after 72 h) | [48] |
| Multi-CLEA of lipase and protease from Ictalurus punctatus catfish viscera | Precipitant: Ammonium sulphate 0.113 mM of BSA Protease recovery activity: 43.82% Lipase recovery activity: 99.91% | Vegetable oil/ethanol (1:4, mol/mol) Enzyme-to-oil molar ratio: 1:10 Multi-CLEAs retained more than 34% of the initial activity after 5 batches for both enzymes | 51.7 | [49] |
| CLEA of Thermomyces lanuginosus lipase (TLL) | Tween 80 concentration: 1.0 mM 10 mg of amino- functionalized magnetite nanoparticles | Jatropha oil/methanol in isopropyl ether (1:3, mol/mol) CLEA (10 mg enzyme) 48 h reaction at 40 °C Tween 80-activated TLL- magnetic-CLEAs retained their activity during 10 cycles of 48 h | 88 | [50] |
| CLEA of pancreas porcine lipase (PPL) | Precipitant: Ethanol PPL: Soy protein mass ratio of 1:3 (The global yield was ~5-fold higher compared with standard PPL CLEAs) Immobilization yield ~60% Expressed activity ~40% | Soybean oil/ethanol (1:5, mol/mol) CLEA (50 mg enzyme) 24 h reaction at 30 °C fatty acid ethyl ester (FAEE) yield was higher than 50 wt.% within ten 24-h cycles of reuse | 60 | [42] |
| CLEA of lipase B from Candida Antarctica (CALB) | Precipitant: Ammonium sulphate Insolubilized CALB was covalently cross-linked to magnetic nanoparticles | Chlorella vulgaris lipids/methanol (1:10, mol/mol) 3 h reaction at 30 °C Magnetic CLEAs could be reused for at least ten catalytic cycles retaining 90% of the initial biodiesel conversion | 87 | [51] |
| CLEA of Km12 lipase | CLEA of Km12 was coupled with amino-coated magnetite nanoparticles Immobilization efficiency: 75% | Waste cooking oils/methanol (1:3, mol/mol) 0.3 wt.% immobilized lipase 36 h reaction at 35 °C Magnetic CLEAs retained its total activity up to 6 cycles of enzyme re-using in the standard assay condition | 71 | [52] |
| Assay | Enzymatic Activity of Each Lipase in the Mixture (%) | FAEE Mass Yield, wt.% | |||
|---|---|---|---|---|---|
| TLL | PPL | PFL | CALB | ||
| 1 | 100 | 4.32 ± 1.19 | |||
| 2 | 75 | 25 | 15.16 ± 1.72 | ||
| 3 | 50 | 50 | 22.03 ± 0.05 | ||
| 4 | 25 | 75 | 8.80 ± 0.33 | ||
| 5 | 100 | 0.22 ± 0.03 | |||
| 6 | 75 | 25 | 10.31 ± 0.19 | ||
| 7 | 50 | 50 | 11.50 ± 4.65 | ||
| 8 | 25 | 75 | 9.75 ± 2.36 | ||
| 9 | 100 | 7.42 ± 0.68 | |||
| 10 | 75 | 25 | 5.63 ± 0.39 | ||
| 11 | 50 | 50 | 4.36 ± 1.01 | ||
| 12 | 25 | 75 | 1.77 ± 0.87 | ||
| 13 | 100 | 1.90 ± 1.35 | |||
| 14 | 75 | 25 | 4.22 ± 2.23 | ||
| 15 | 50 | 50 | 9.97 ± 1.06 | ||
| 16 | 25 | 75 | 7.24 ± 1.03 | ||
| 17 | 75 | 25 | 1.22 ± 0.29 | ||
| 18 | 50 | 50 | 1.64 ± 0.80 | ||
| 19 | 25 | 75 | 2.20 ± 1.40 | ||
| 20 | 75 | 25 | 3.80 ± 1.27 | ||
| 21 | 50 | 50 | 3.53 ± 1.18 | ||
| 22 | 25 | 75 | 1.91 ± 0.16 | ||
| CLEA | Lipase/Co-feeder Mass Ratio | Immobilization Yield (%) | Expressed Activity (%) |
|---|---|---|---|
| TLL | 1:0 | 0 | 0 |
| TLL–SOY | 1:3 | 75.0 ± 0.1 | 2.9 ± 0.1 |
| TLL–BSA | 1:3 | 78.0 ± 1.0 | 15.8 ± 0.4 |
| TLL/PPL–SOY | 1:1 | 63.0 ± 1.0 | 1.9 ± 0.1 |
| TLL/PPL–SOY | 1:3 | 65.0 ± 5.0 | 10.37 ± 0.03 |
| TLL/PPL–SOY | 1:7 | 64.0 ± 0.1 | 11.2 ± 0.6 |
| TLL/PPL–BSA | 1:1 | 68.0 ± 2.0 | 1.34 ± 0.08 |
| TLL/PPL–BSA | 1:3 | 69.0 ± 1.0 | 16.3 ± 4.4 |
| TLL/PPL–BSA | 1:7 | 88.0 ± 1.0 | 15.5 ± 0.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, M.D.; Miranda, L.P.; Fernandez-Lafuente, R.; Kopp, W.; Tardioli, P.W. Improving the Yields and Reaction Rate in the Ethanolysis of Soybean Oil by Using Mixtures of Lipase CLEAs. Molecules 2019, 24, 4392. https://doi.org/10.3390/molecules24234392
Ramos MD, Miranda LP, Fernandez-Lafuente R, Kopp W, Tardioli PW. Improving the Yields and Reaction Rate in the Ethanolysis of Soybean Oil by Using Mixtures of Lipase CLEAs. Molecules. 2019; 24(23):4392. https://doi.org/10.3390/molecules24234392
Chicago/Turabian StyleRamos, Margarita Díaz, Letícia Passos Miranda, Roberto Fernandez-Lafuente, William Kopp, and Paulo Waldir Tardioli. 2019. "Improving the Yields and Reaction Rate in the Ethanolysis of Soybean Oil by Using Mixtures of Lipase CLEAs" Molecules 24, no. 23: 4392. https://doi.org/10.3390/molecules24234392
APA StyleRamos, M. D., Miranda, L. P., Fernandez-Lafuente, R., Kopp, W., & Tardioli, P. W. (2019). Improving the Yields and Reaction Rate in the Ethanolysis of Soybean Oil by Using Mixtures of Lipase CLEAs. Molecules, 24(23), 4392. https://doi.org/10.3390/molecules24234392









