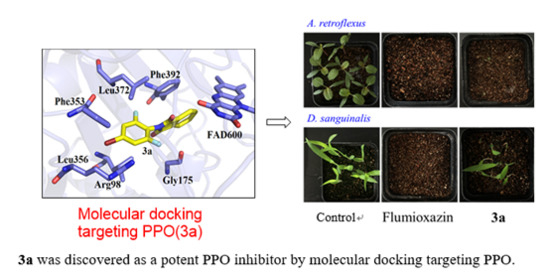
Design and Synthesis of N-phenyl Phthalimides as Potent Protoporphyrinogen Oxidase Inhibitors
Abstract
1. Introduction
2. Results and Discussion
2.1. Docking Analysis
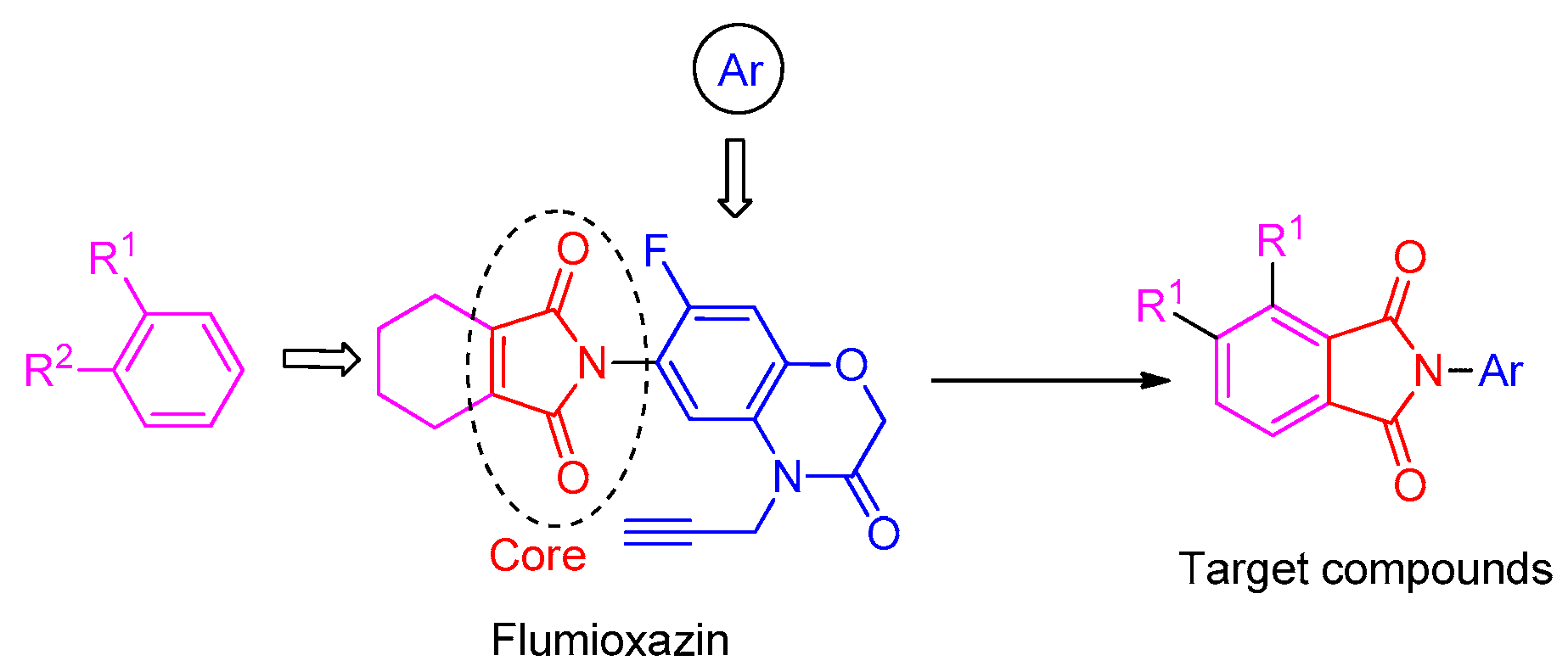
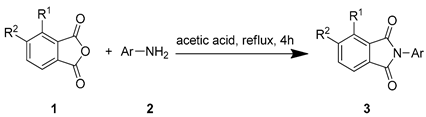
2.2. Chemistry
2.3. Herbicidal Activity
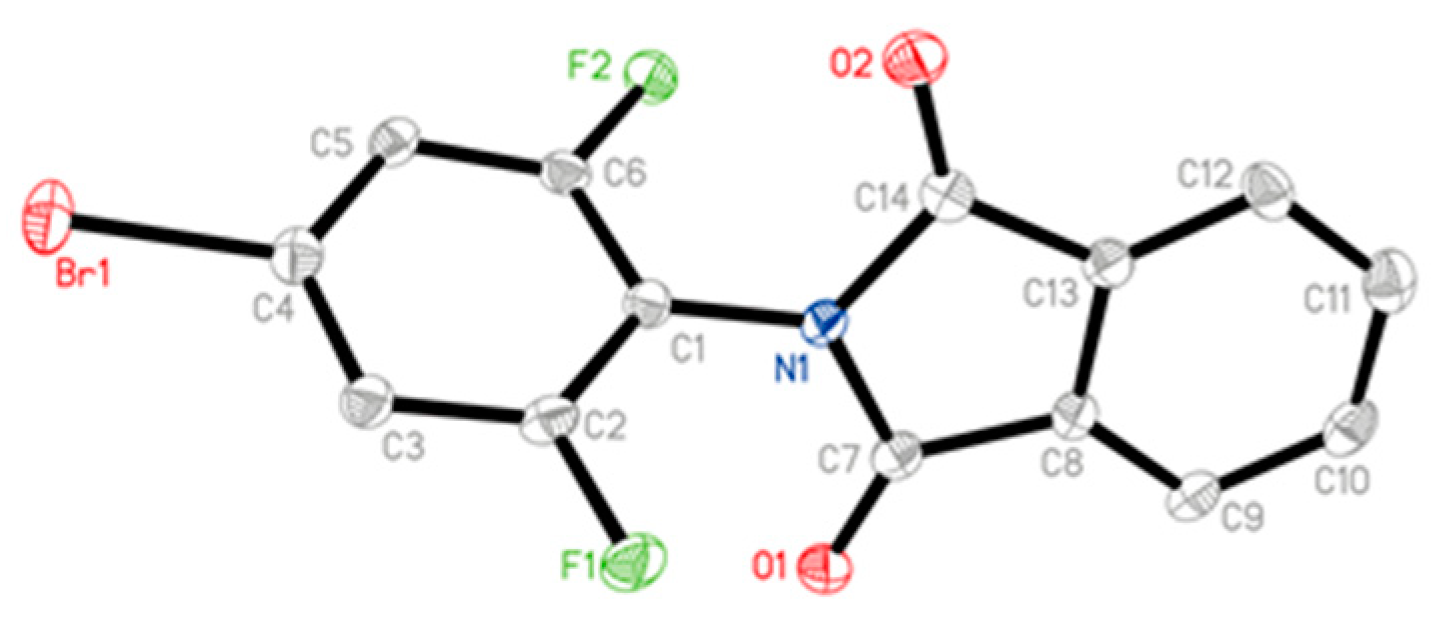
2.4. Crystal Structure Determination of Compound 3a
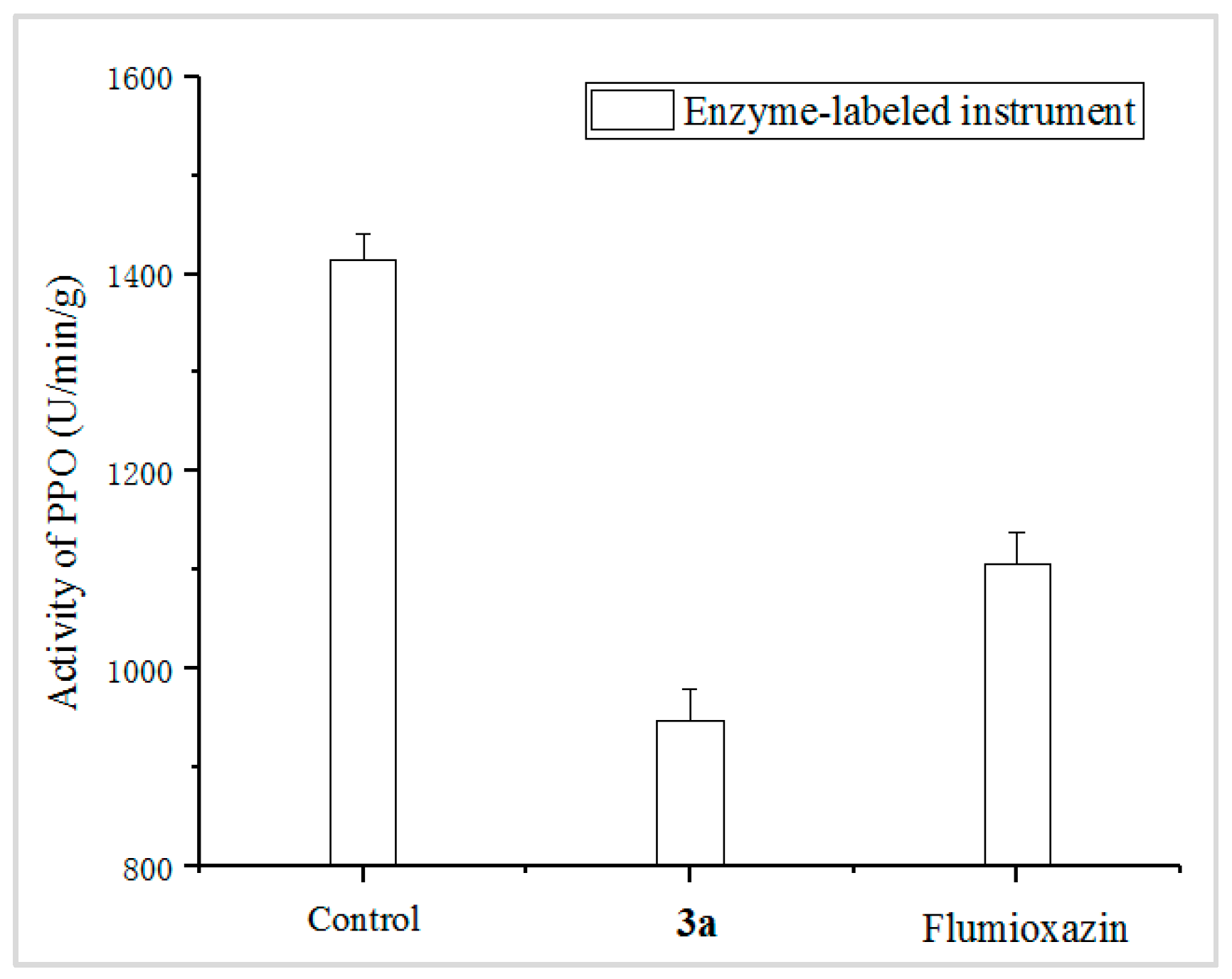
2.5. PPO Enzyme Assays
3. Materials and Methods
3.1. Molecular Docking
3.2. Equipment and Materials
3.3. General Synthetic Procedure for Compounds 3
3.4. Herbicidal Activity
3.5. PPO Enzyme Assays
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Poulson, R.; Polglase, W. The enzymic conversion of protoporphyrinogen IX to protoporphyrin IX. Protoporphyrinogen oxidase activity in mitochondrial extracts of Saccharomyces cerevisiae. J. Biol. Chem. 1975, 250, 1269–1274. [Google Scholar]
- Poulson, R. The enzymic conversion of protoporphyrinogen IX to protoporphyrin IX in mammalian mitochondria. J. Biol. Chem. 1976, 251, 3730–3733. [Google Scholar]
- Ajioka, R.S.; Phillips, J.D.; Kushner, J.P. Biosynthesis of heme in mammals. Biochim. Biophys. Acta 2006, 1763, 723–736. [Google Scholar] [CrossRef]
- Heinemann, I.U.; Jahn, M.; Jahn, D. The biochemistry of heme biosynthesis. Biochem. Biophys. 2008, 474, 238–251. [Google Scholar] [CrossRef]
- Kato, K.; Tanaka, R.; Sano, S.; Tanaka, A.; Hosaka, H. Identification of a gene essential for protoporphyrinogen IX oxidase activity in the cyanobacterium Synechocystis sp. PCC6803. Natl. Acad. Sci. 2010, 107, 16649–16654. [Google Scholar] [CrossRef]
- Wang, B.; Wen, X.; Qin, X.; Wang, Z.; Tan, Y.; Shen, Y.; Xi, Z. Quantitative structural insight into human variegate porphyria disease. J. Biol. Chem. 2013, 288, 11731–11740. [Google Scholar] [CrossRef]
- Wang, B.; Wen, X.; Xi, Z. Molecular simulations bring new insights into protoporphyrinogen IX oxidase/protoporphyrinogen IX interaction modes. Mol. Inform. 2016, 35, 476–482. [Google Scholar] [CrossRef]
- Dayan, F.E.; Barker, A.; Tranel, P.J. Origins and structure of chloroplastic and mitochondrial plant protoporphyrinogen oxidases: Implications for the evolution of herbicide resistance. Pest Manage. Sci. 2018, 74, 2226–2234. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Sun, L.; Wen, X.; Yang, X.; Tan, Y.; Jin, H.; Cao, Q.; Zhou, W.; Xi, Z.; Shen, Y. Structural insight into unique properties of protoporphyrinogen oxidase from Bacillus subtilis. J. Struct. Biol. 2010, 170, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Sherman, T.D.; Becerril, J.M.; Matsumoto, H.; Duke, M.V.; Jacobs, J.M.; Jacobs, N.J.; Duke, S.O. Physiological basis for differential sensitivities of plant species to protoporphyrinogen oxidase-inhibiting herbicides. Plant Physiol. 1991, 97, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.F.; Zuo, Y.; Yang, S.G.; Yang, G.F. Protoporphyrinogen oxidase inhibitor: An ideal target for herbicide discovery. CHIMIA Int. J. Chem. 2011, 65, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Meazza, G.; Bettarini, F.; Porta, P.L.; Piccardi, P.; Signorini, E.; Portoso, D.; Fornara, L. Synthesis and herbicidal activity of novel heterocyclic protoporphyrinogen oxidase inhibitors. Pest Manage. Sci. 2004, 60, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.L.; Tan, Y.; Zhu, X.L.; Wang, Z.F.; Zuo, Y.; Chen, Q.; Xi, Z.; Yang, G.F. Design, synthesis, and 3D-QSAR analysis of novel 1, 3, 4-oxadiazol-2 (3H)-ones as protoporphyrinogen oxidase inhibitors. J. Agric. Food Chem. 2009, 58, 2643–2651. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.L.; Zuo, Y.; Wang, Z.F.; Tan, Y.; Wu, Q.Y.; Xi, Z.; Yang, G.F. Design and syntheses of novel N-(benzothiazol-5-yl)-4,5,6,7-tetrahydro-1H-isoindole-1,3(2H)-dione and N-(benzothiazol-5-yl) isoindoline-1,3-dione as potent protoporphyrinogen oxidase inhibitors. J. Agric. Food Chem. 2011, 59, 6172–6179. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Wu, Q.; Su, S.W.; Niu, C.W.; Xi, Z.; Yang, G.F. Synthesis, herbicidal activity, and QSAR of novel N-benzothiazolyl-pyrimidine-2,4-diones as protoporphyrinogen oxidase inhibitors. J. Agric. Food Chem. 2016, 64, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.W.; Armbrust, K.L.; Grey, T.L. ydrolysis and photolysis of flumioxazin in aqueous buffer solutions. Pest Manage. Sci. 2004, 60, 939–943. [Google Scholar] [CrossRef]
- Arakawa, A.; Otani, M.; Iwashita, K.; Yamazaki, K. Molecular dynamics mechanism to generate species differences in inhibition of protoporphyrinogen oxidase by flumioxazin. Comp. Toxi. 2017, 1, 12–21. [Google Scholar] [CrossRef]
- Dayan, F.E.; Armstrong, B.M.; Weete, J.D. Inhibitory activity of sulfentrazone and its metabolic derivatives on soybean (Glycine max) protoporphyrinogen oxidase. J. Agric. Food Chem. 1998, 46, 2024–2029. [Google Scholar] [CrossRef]
- Grossmann, K.; Niggeweg, R.; Christiansen, N.; Looser, R.; Ehrhardt, T. The herbicide saflufenacil (Kixor™) is a new inhibitor of protoporphyrinogen IX oxidase activity. Weed Sci. 2010, 58, 1–9. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.; Yu, H.; Cui, D.; Li, B. Tetrahydrophthalimidobenzoates as protoporphyrinogen IX oxidase inhibiting herbicides. Pestic. Biochem. Physiol. 2017, 139, 40–45. [Google Scholar] [CrossRef]
- Chaudhari, R.; Tan, Z.; Huang, B.; Zhang, S. Computational polypharmacology: A new paradigm for drug discovery. Expert Opin. Drug Discov. 2017, 12, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.Z.; Luo, F.X.; Mo, H.B.; Ren, Y.G.; Wang, X.G.; Ou, X.M.; Lei, M.X.; Liu, A.P.; Huang, L.; Xu, M.C. Synthesis and herbicidal activity of isoindoline-1,3-dione substituted benzoxazinone derivatives containing a carboxylic ester group. J. Agric. Food Chem. 2009, 57, 9585–9592. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P. The Unique Role of Halogen Substituents in the Design of Modern Crop Protection Compounds; Wiley Online Library: Weinheim, Germany, 2012. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Tanchuk, V.Y.; Tanin, V.O.; Vovk, I.; Poda, G. A new improved hybrid scoring function for molecular docking and scoring based on autodock and autodock vina. Chem. Biol. Drug Des. 2016, 87, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, R.; Li, Z. PyMine: A PyMOL plugin to integrate and visualize data for drug discovery. BMC Res. Notes 2015, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- Giovani, S.; Penzo, M.; Butini, S.; Brindisi, M.; Gemma, S.; Novellino, E.; Campiani, G.; Blackman, M.J.; Brogi, S. Plasmodium falciparum subtilisin-like protease 1: Discovery of potent difluorostatone-based inhibitors. RSC Adv. 2015, 5, 22431–22448. [Google Scholar] [CrossRef]
- Garcia-Barrantes, P.M.; Cho, H.P.; Metts, A.M.; Blobaum, A.L.; Niswender, C.M.; Conn, P.J.; Lindsley, C.W. Lead optimization of the VU0486321 series of mGlu1 PAMs. Part 2: SAR of alternative 3-methyl heterocycles and progress towards an in vivo tool. Bioorg. Med. Chem. Lett. 2016, 26, 751–756. [Google Scholar] [CrossRef]
- Cheng, H.; Fu, Y.; Chang, Q.; Zhang, N.; Bu, M.W.; Niu, Y.; Wu, Q.Y.; Chen, C.; Verpoort, F. Synthesis, biochemical evaluation and computational simulations of new cytochrome bc1 complex inhibitors based on N-(4-aryloxyphenyl) phthalimides. Chin. Chem. Lett. 2018, 29, 1897–1900. [Google Scholar] [CrossRef]
- Yoon, H.S.; Hong, W.P.; Kang, Y.J.; Kim, J.J.; Han, S.H. Preparation of Indolocarbazole Compound for Organic Light Emitting Device. KR 2019101894, 2 September 2019. [Google Scholar]
- Barchín, B.M.; Cuadro, A.M.; Alvarez-Builla, J. Microwave-assisted parallel synthesis of a 2-aryl-1H-isoindole-1,3-dione library. Synlett 2002, 2, 343–345. [Google Scholar] [CrossRef][Green Version]
- Fujimaki, N.; Nakagomi, M.; Shudo, K. Preparation of Aromatic Amide Derivatives Affecting Gene Transcription Process. WO 2009107762, 3 September 2009. [Google Scholar]
- Wang, D.W.; Lin, H.Y.; Cao, R.J.; Chen, T.; Wu, F.X.; Hao, G.F.; Chen, Q.; Yang, W.C.; Yang, G.F. Synthesis and herbicidal activity of triketone-quinoline hybrids as novel 4-hydroxyphenylpyruvate dioxygenase inhibitors. J. Agric. Food Chem. 2015, 63, 5587–5596. [Google Scholar] [CrossRef]
- Huo, J.Q.; Zhao, B.; Zhang, Z.; Xing, J.H.; Zhang, J.L.; Dong, J.G.; Fan, Z.J. Structure-Based Discovery and Synthesis of Potential Transketolase Inhibitors. Molecules 2018, 23, 2116. [Google Scholar] [CrossRef] [PubMed]
- Sartori, S.K.; Alvarenga, E.S.; Franco, C.A.; Ramos, D.S.; Oliveira, D.F. One-pot synthesis of anilides, herbicidal activity and molecular docking study. Pest Manage. Sci. 2018, 74, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Liu., S.; Zhu., Y.; Wang., T.; Luo., J. Designed, Synthesis and Herbicidal Activity of Novel 2-Alkylthio-thieno[2,3-d] pyrimidin-4-ones. Chin. J. Chem. 2017, 37, 1877. [Google Scholar] [CrossRef][Green Version]
- Aquino-Bolaños, E.N.; Mercado-Silva, E. Effects of polyphenol oxidase and peroxidase activity, phenolics and lignin content on the browning of cut jicama. Postharvest Biol. Technol. 2004, 33, 275–283. [Google Scholar] [CrossRef]
- Queiroz, C.; da-Silva, A.J.R.; Lopes, M.L.M.; Fialho, E.; Valente-Mesquita, V.L. Polyphenol oxidase activity, phenolic acid composition and browning in cashew apple (Anacardium occidentale, L.) after processing. Food Chem. 2011, 125, 128–132. [Google Scholar] [CrossRef]
- Ciou, J.Y.; Lin, H.H.; Chiang, P.Y.; Wang, C.C.; Charles, A.L. The role of polyphenol oxidase and peroxidase in the browning of water caltrop pericarp during heat treatment. Food Chem. 2011, 127, 523–527. [Google Scholar] [CrossRef]
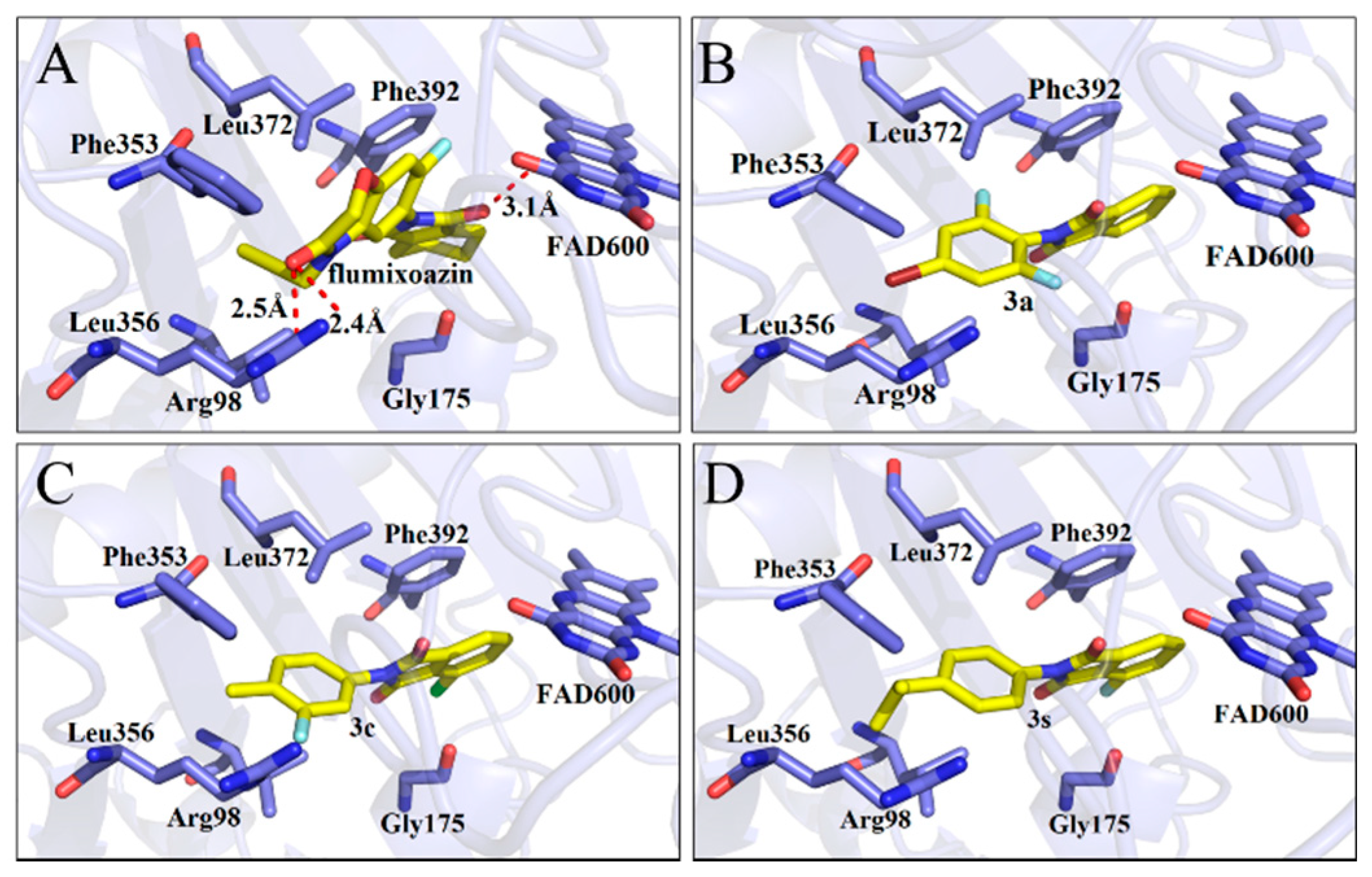
| Compd. | Affinity | Compd. | Affinity | Compd. | Affinity | Compd. | Affinity |
|---|---|---|---|---|---|---|---|
| 3a | −10.0 | 3h | −8.7 | 3o | −9.5 | 3v | −8.1 |
| 3b | −8.6 | 3i | −8.4 | 3p | −8.7 | 3w | −7.7 |
| 3c | −10.1 | 3j | −7.7 | 3q | −9.3 | 3x | −9.8 |
| 3d | −9.1 | 3k | −9.4 | 3r | −9.0 | 3y | −9.5 |
| 3e | −9.8 | 3l | −8.9 | 3s | −10.2 | Flumioxazin | −9.5 |
| 3f | −9.7 | 3m | −9.9 | 3t | −9.7 | Chlortoluron | −7.1 |
 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compd. | R1 | R2 | Ar | Yield (%) | Compd. | R1 | R2 | Ar | Yield (%) | Compd. | R1 | R2 | Ar | Yield (%) |
| 3a | H | H | 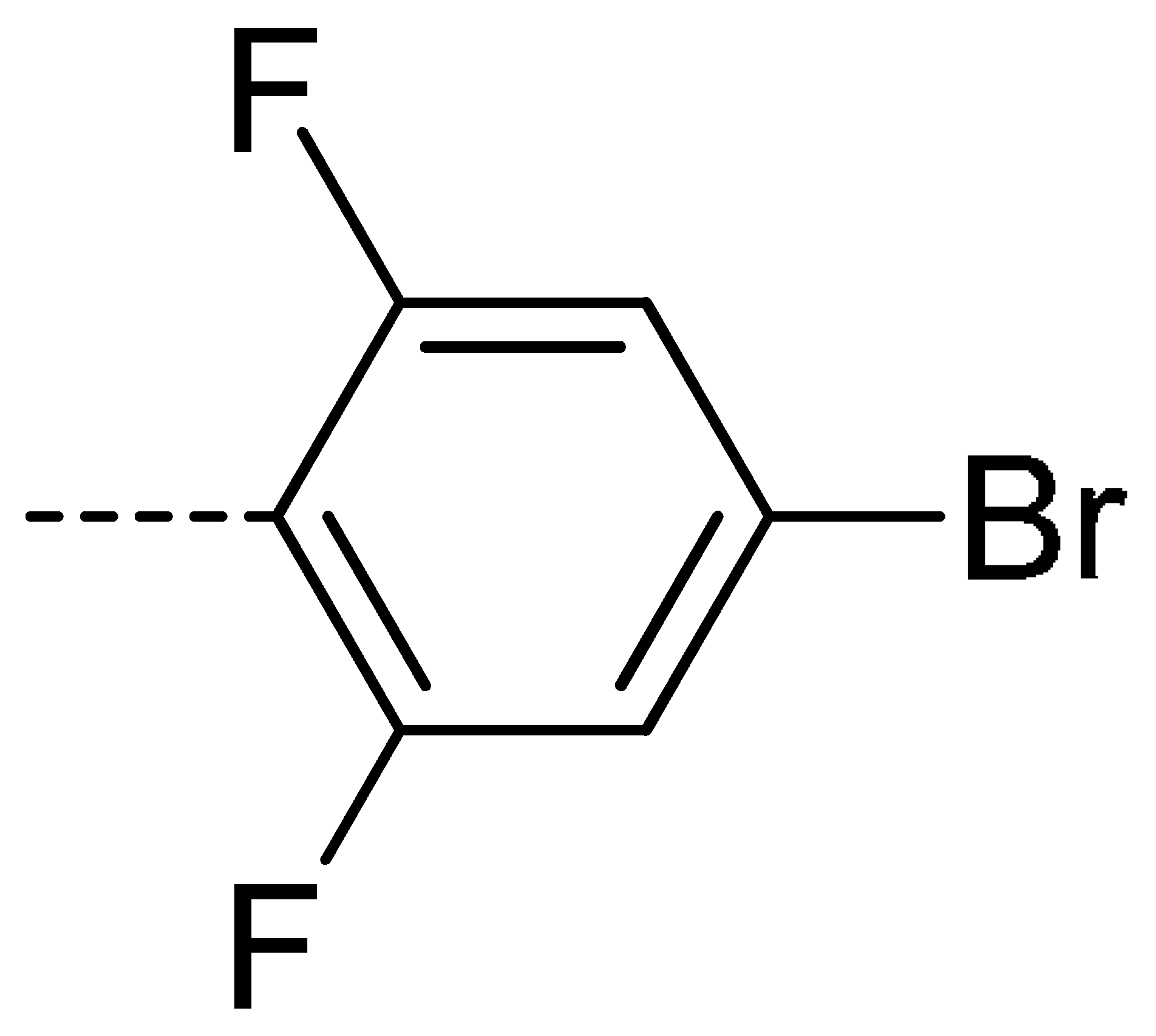 | 73 | 3j | Cl | H | 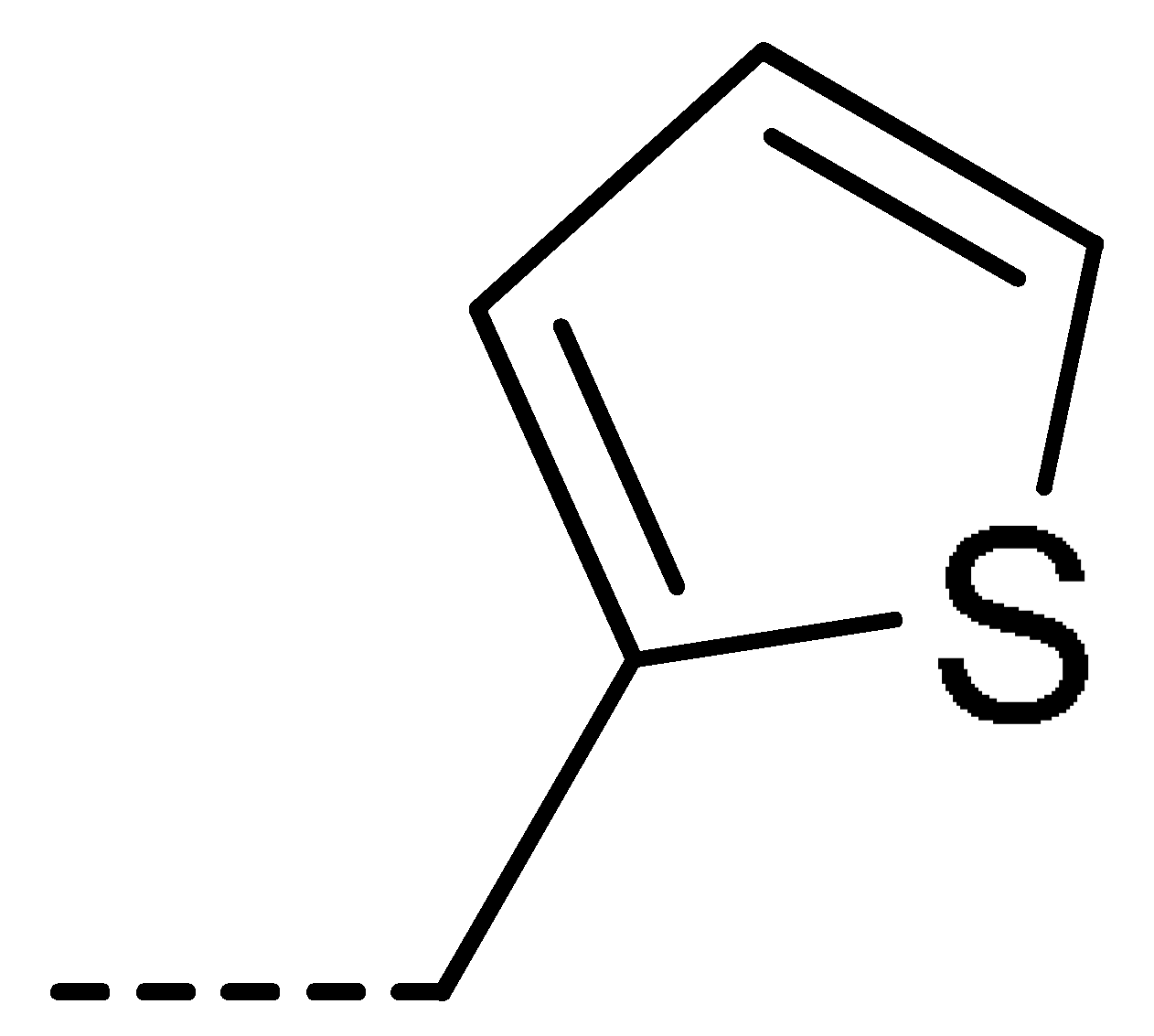 | 71 | 3s | F | H | 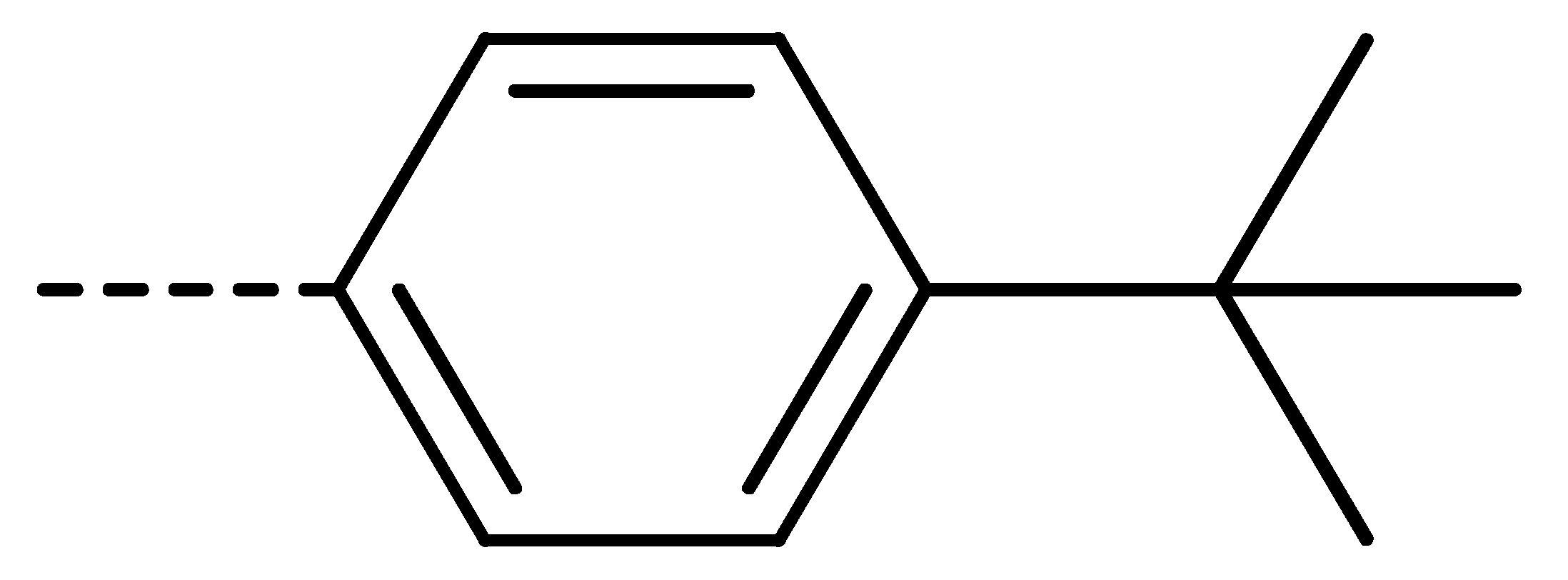 | 79 |
| 3b | H | Cl | 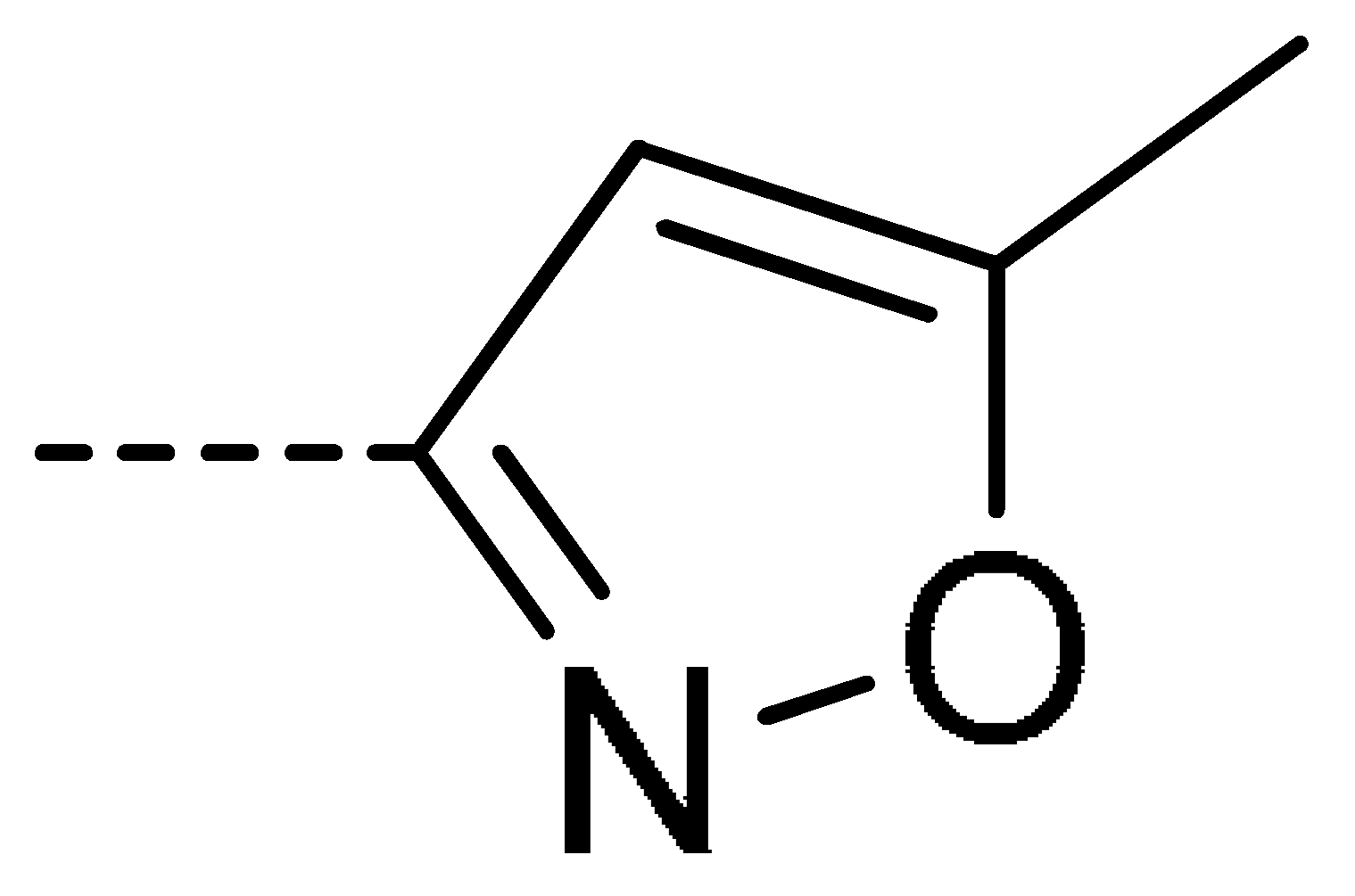 | 77 | 3k | Cl | H | 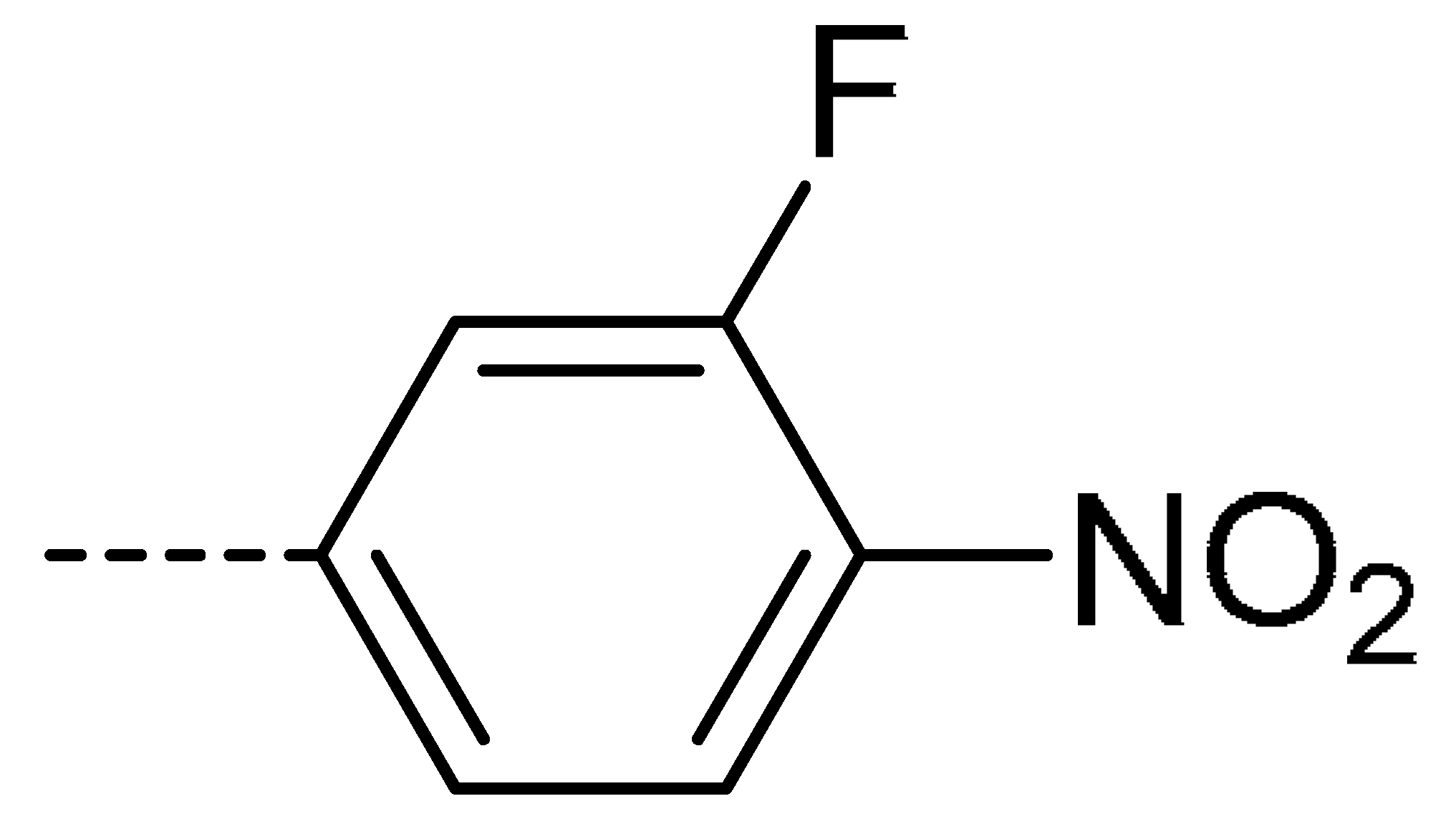 | 63 | 3t | H | Cl | 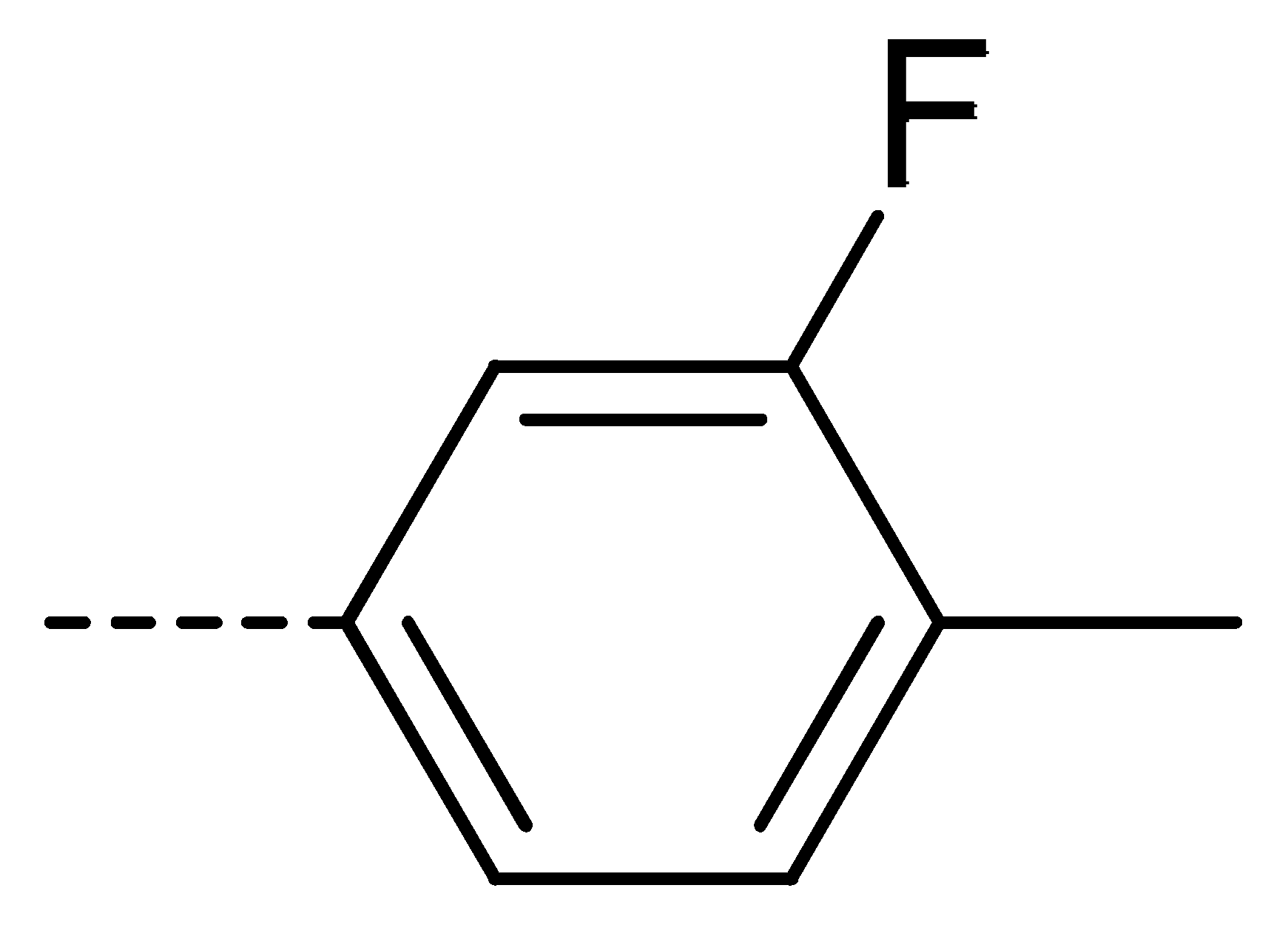 | 79 |
| 3c | Cl | H | 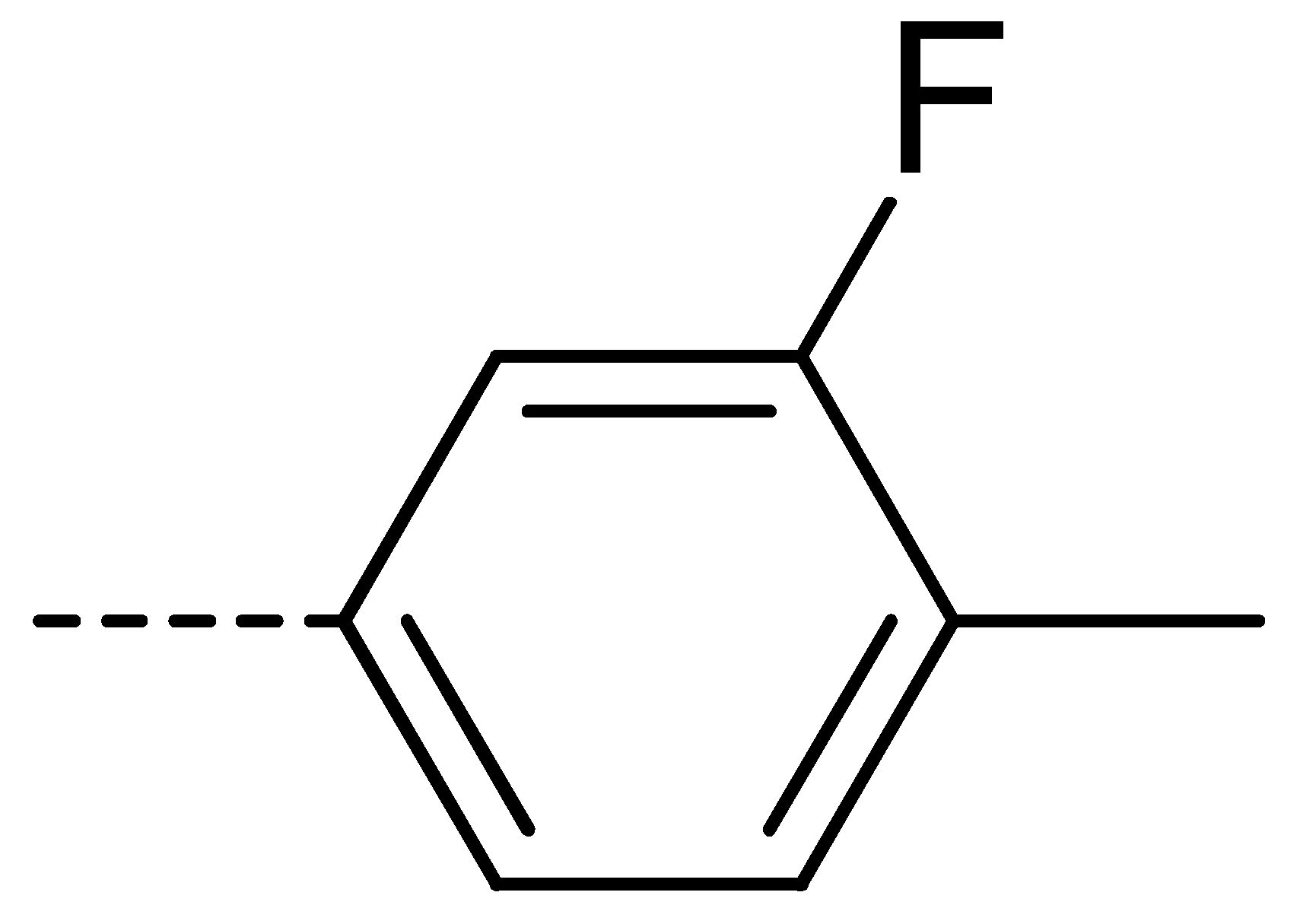 | 81 | 3l | Cl | H | 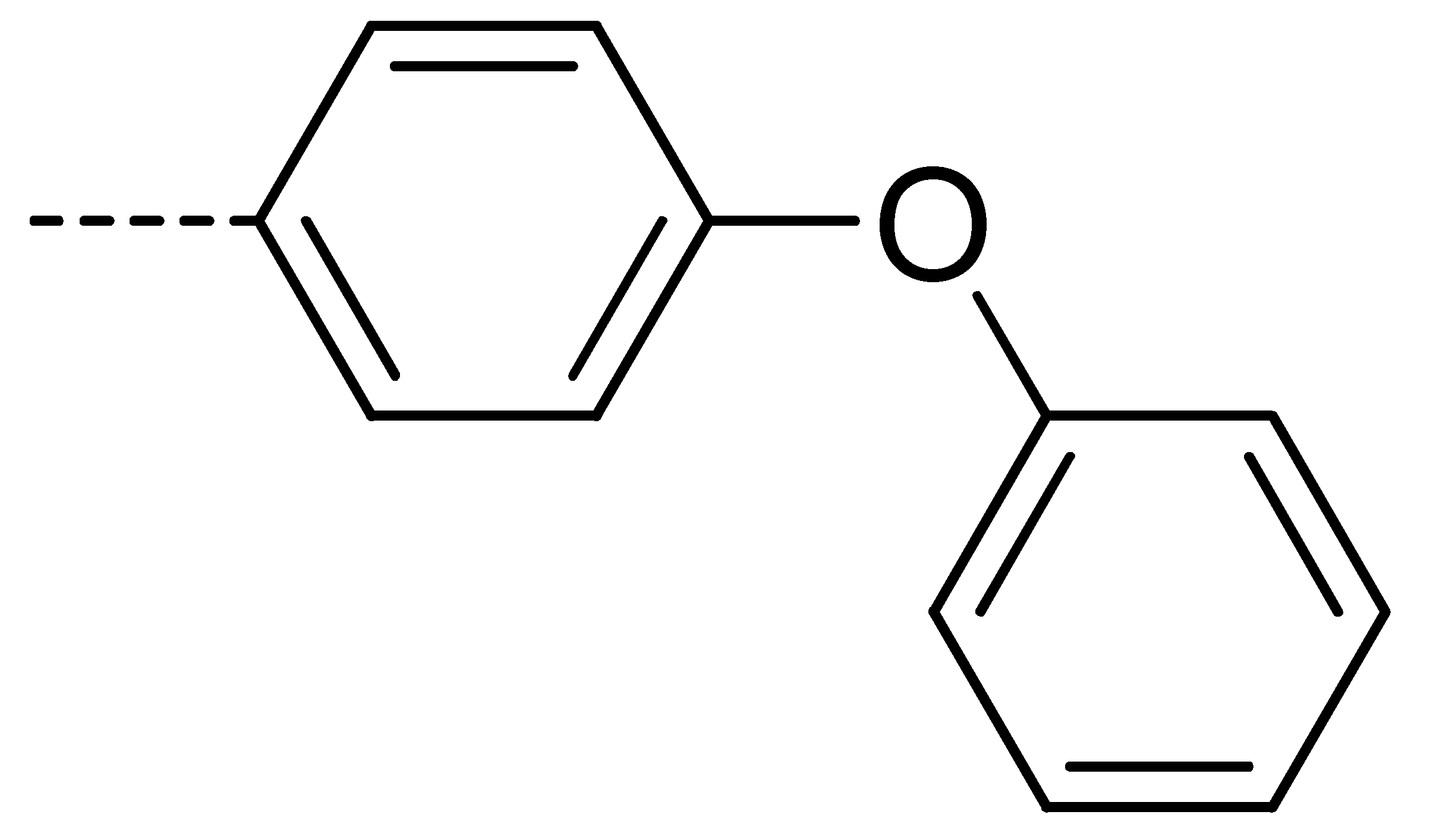 | 86 | 3u | H | Cl | 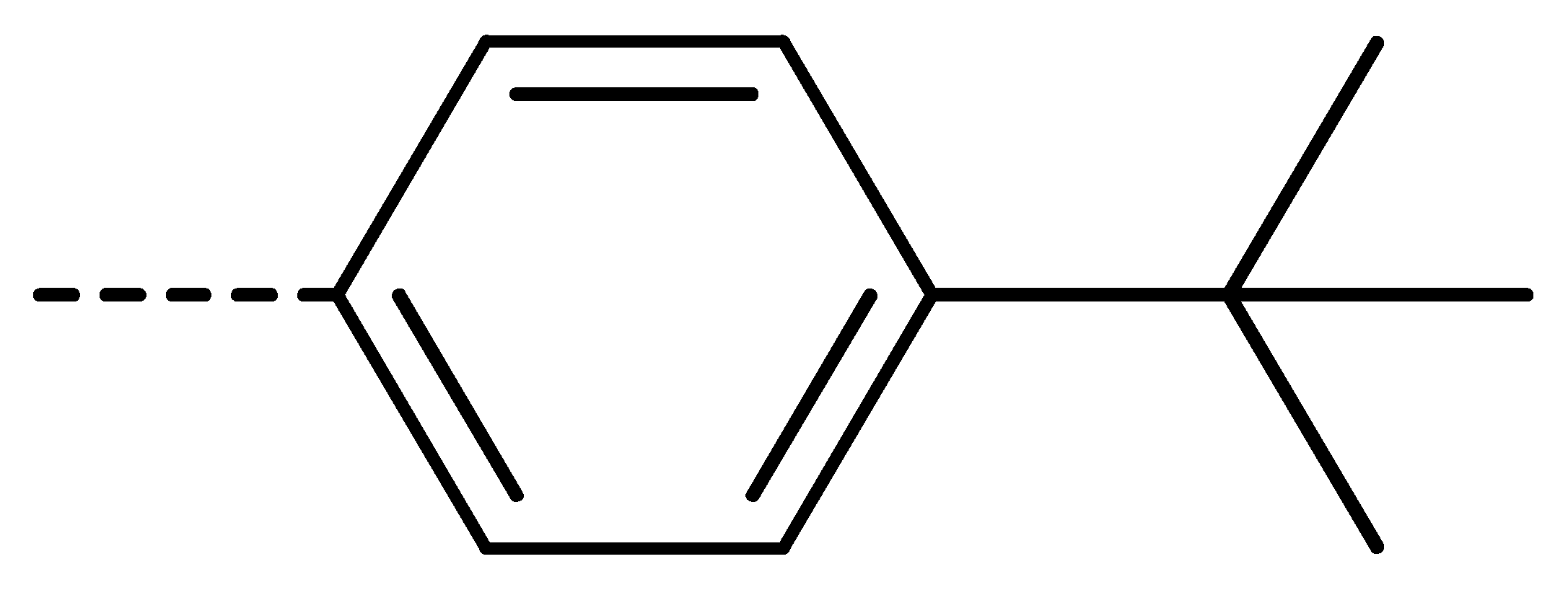 | 70 |
| 3d | Cl | H | 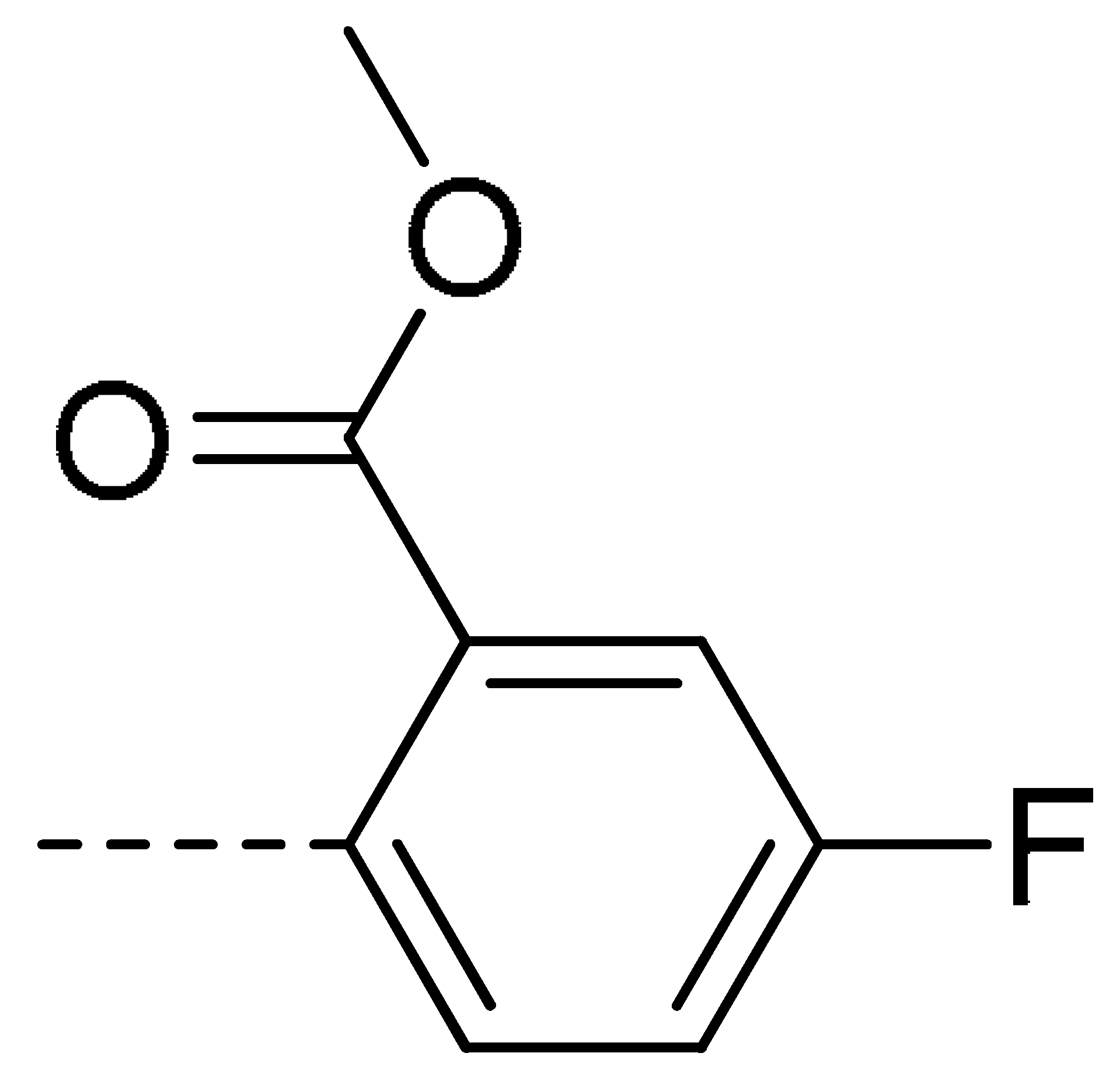 | 28 | 3m | Cl | H | 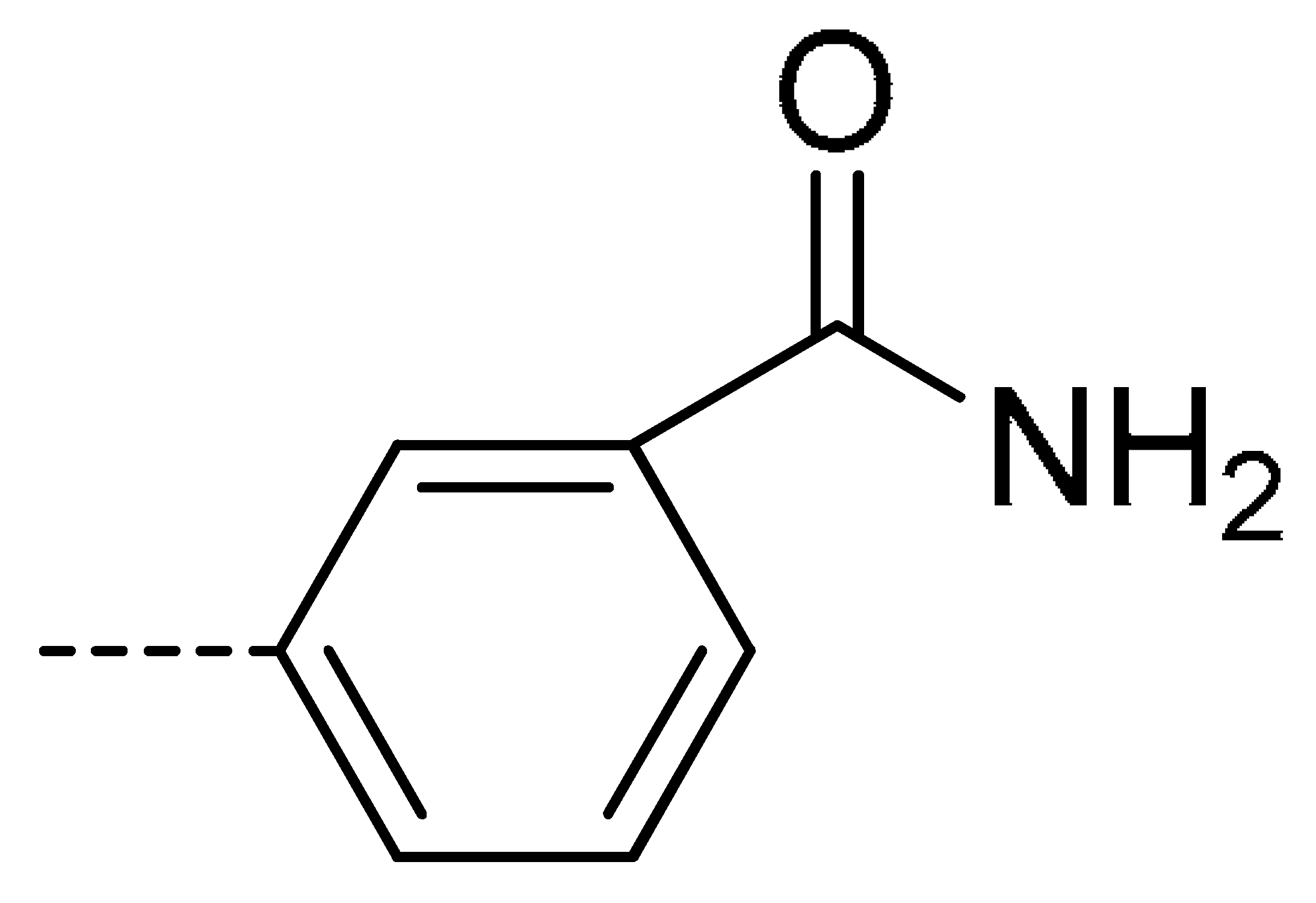 | 84 | 3v | H | Cl | 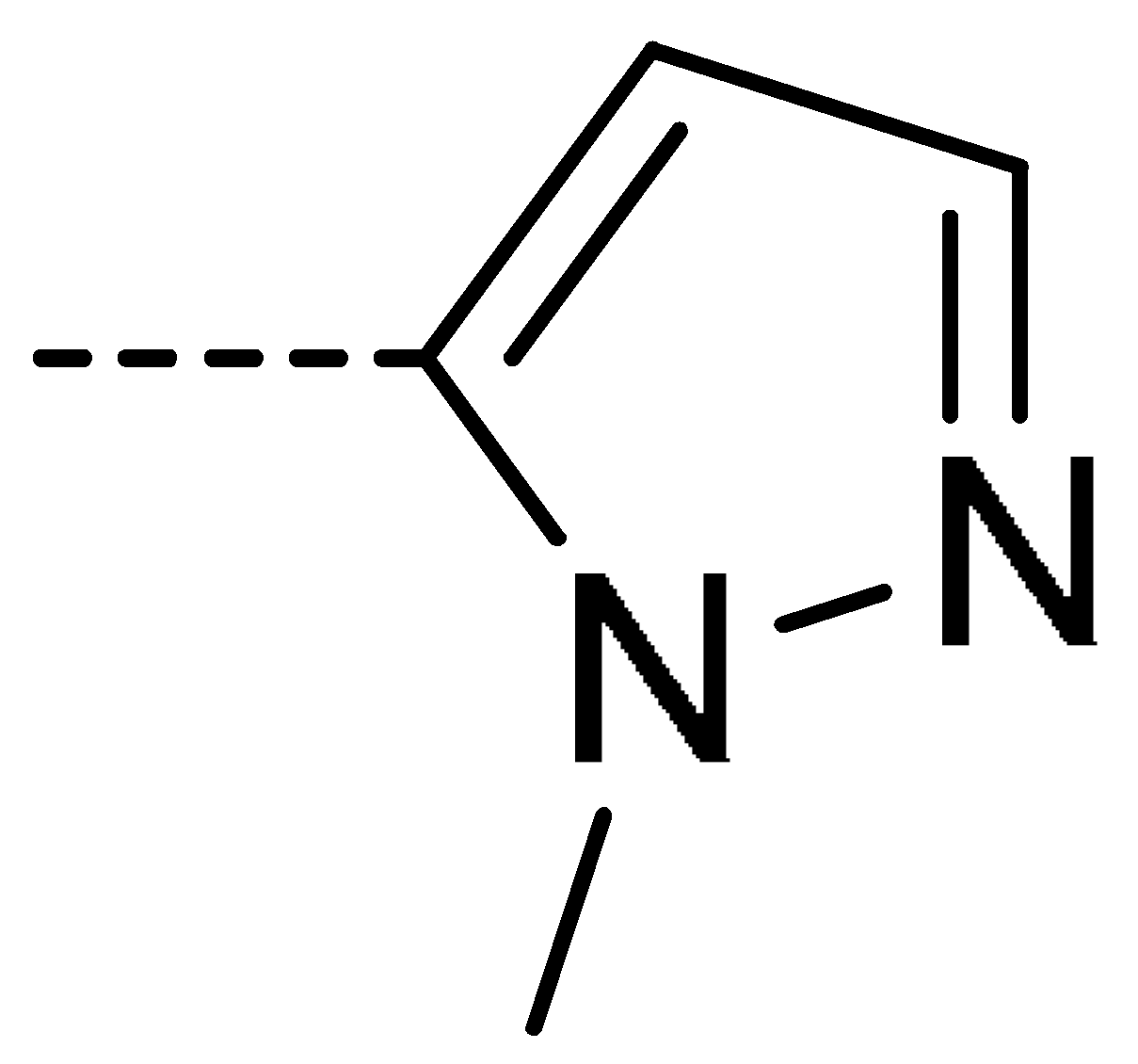 | 76 |
| 3e | Cl | H | 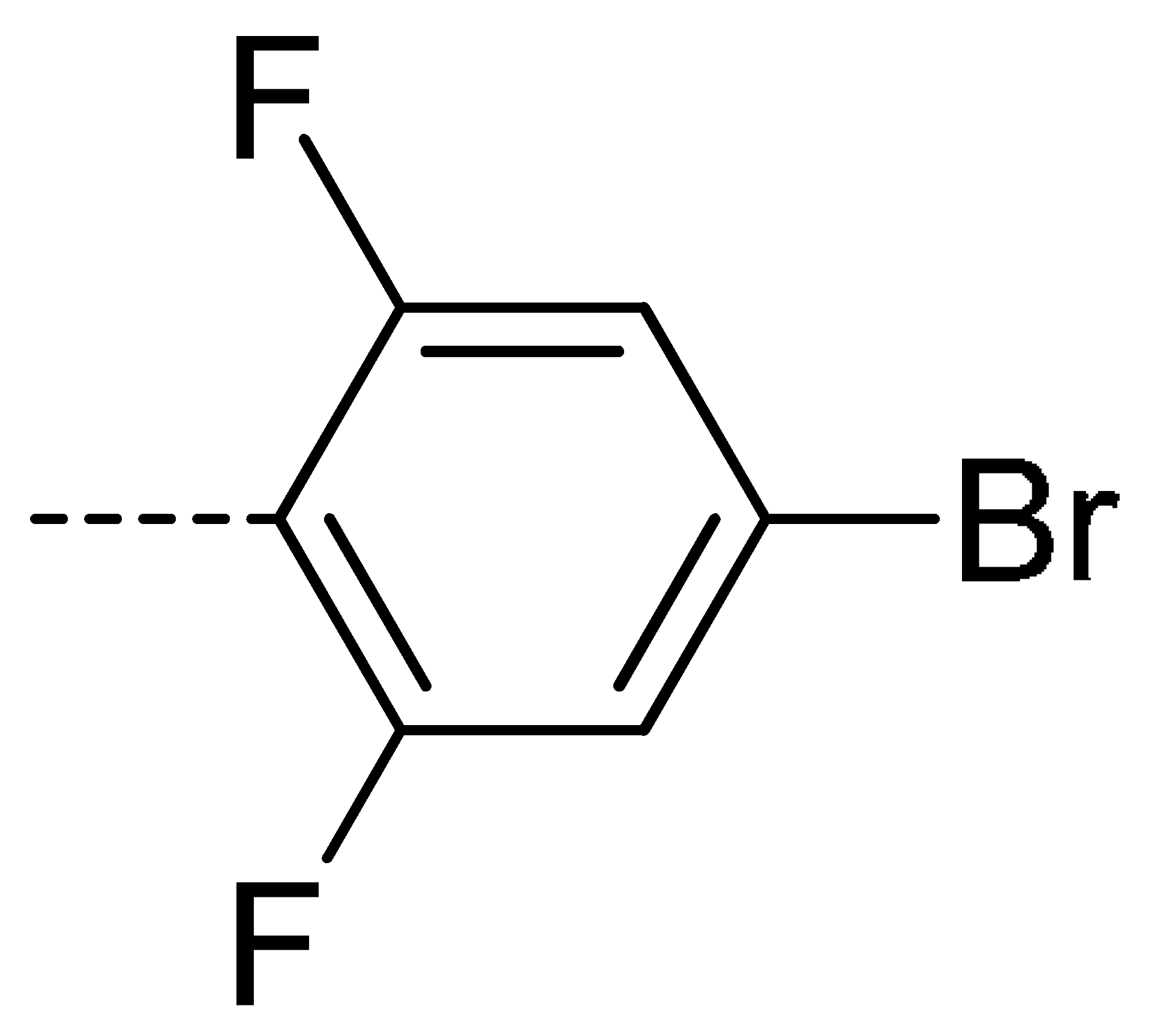 | 80 | 3n | Cl | H | 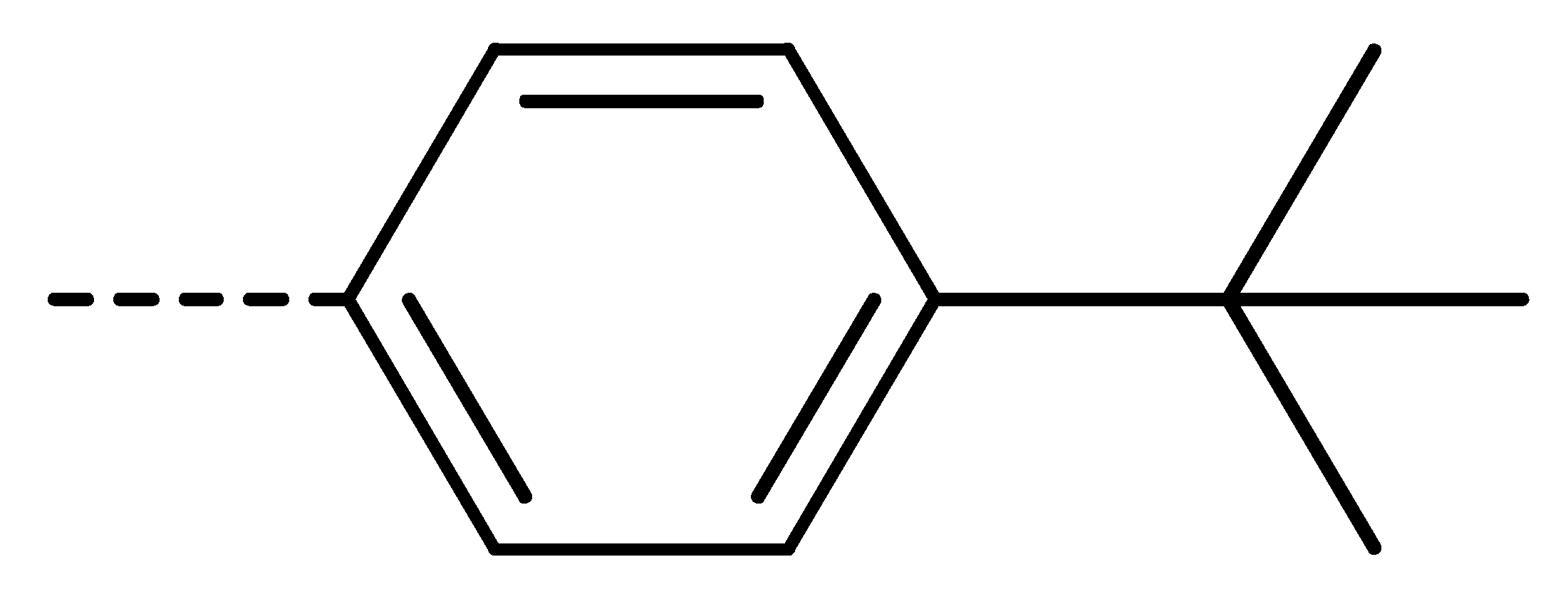 | 52 | 3w | H | Cl |  | 79 |
| 3f | Cl | H | 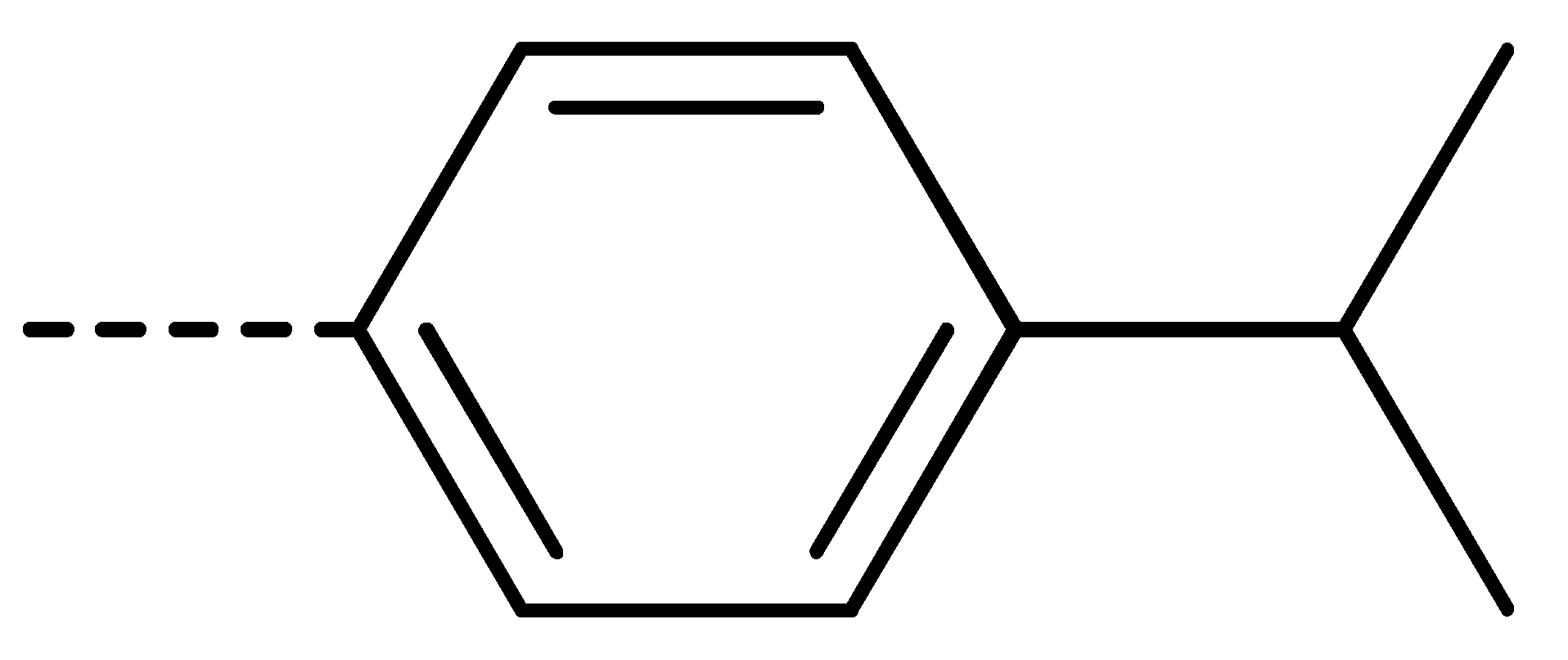 | 78 | 3o | Cl | H | 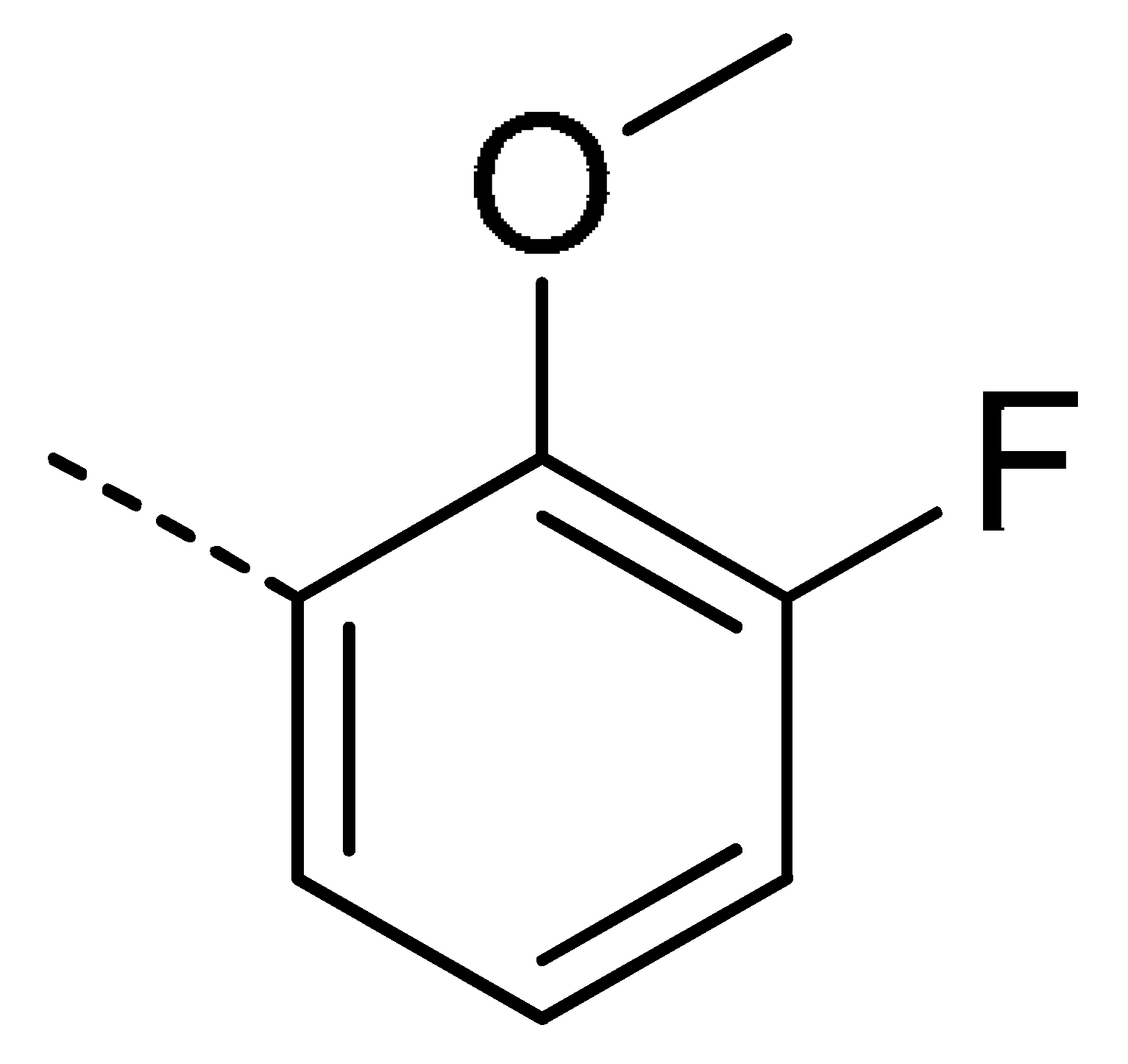 | 87 | 3x | H | CH3 |  | 47 |
| 3g | Cl | H | 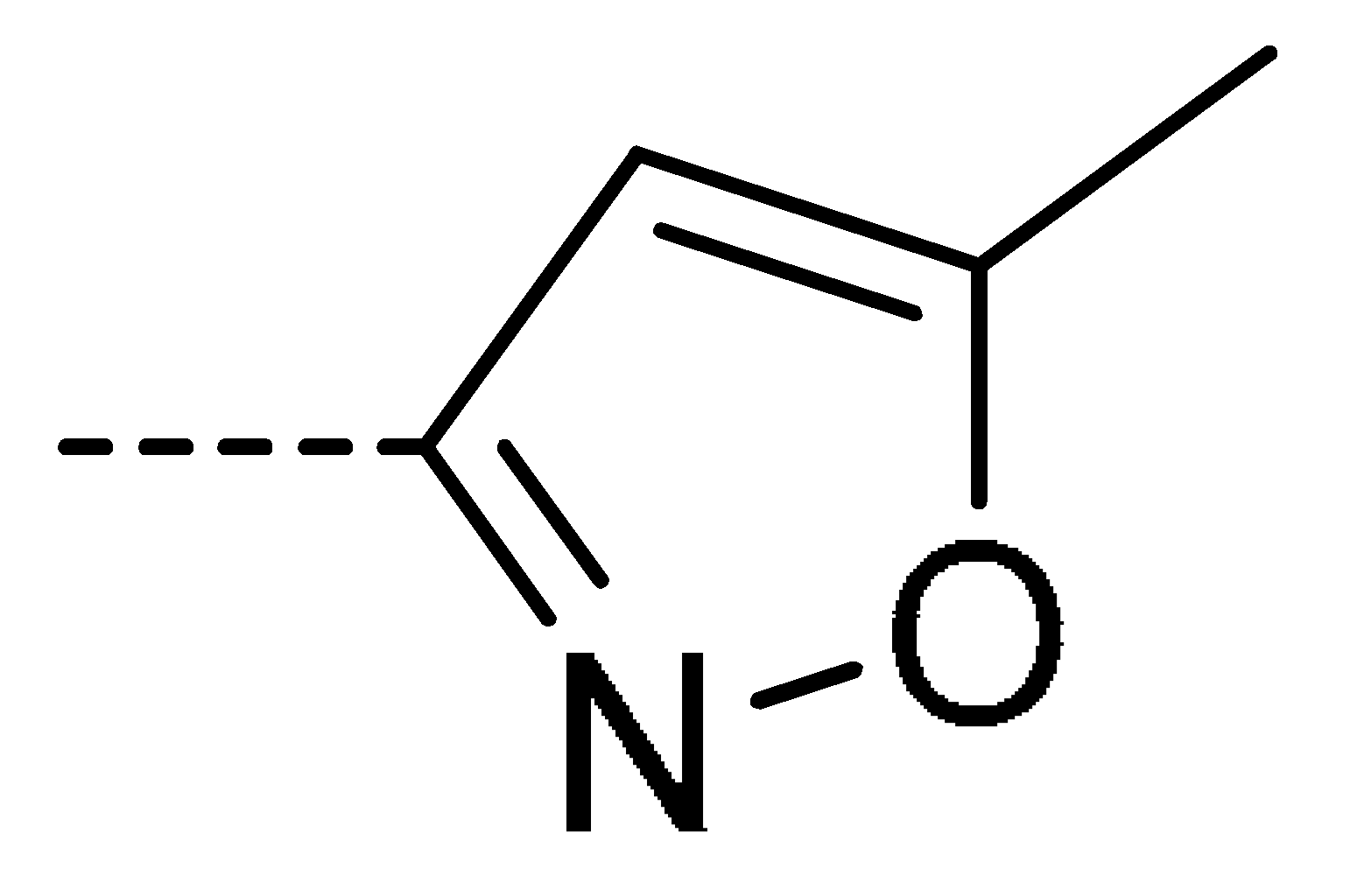 | 58 | 3p | Cl | H | 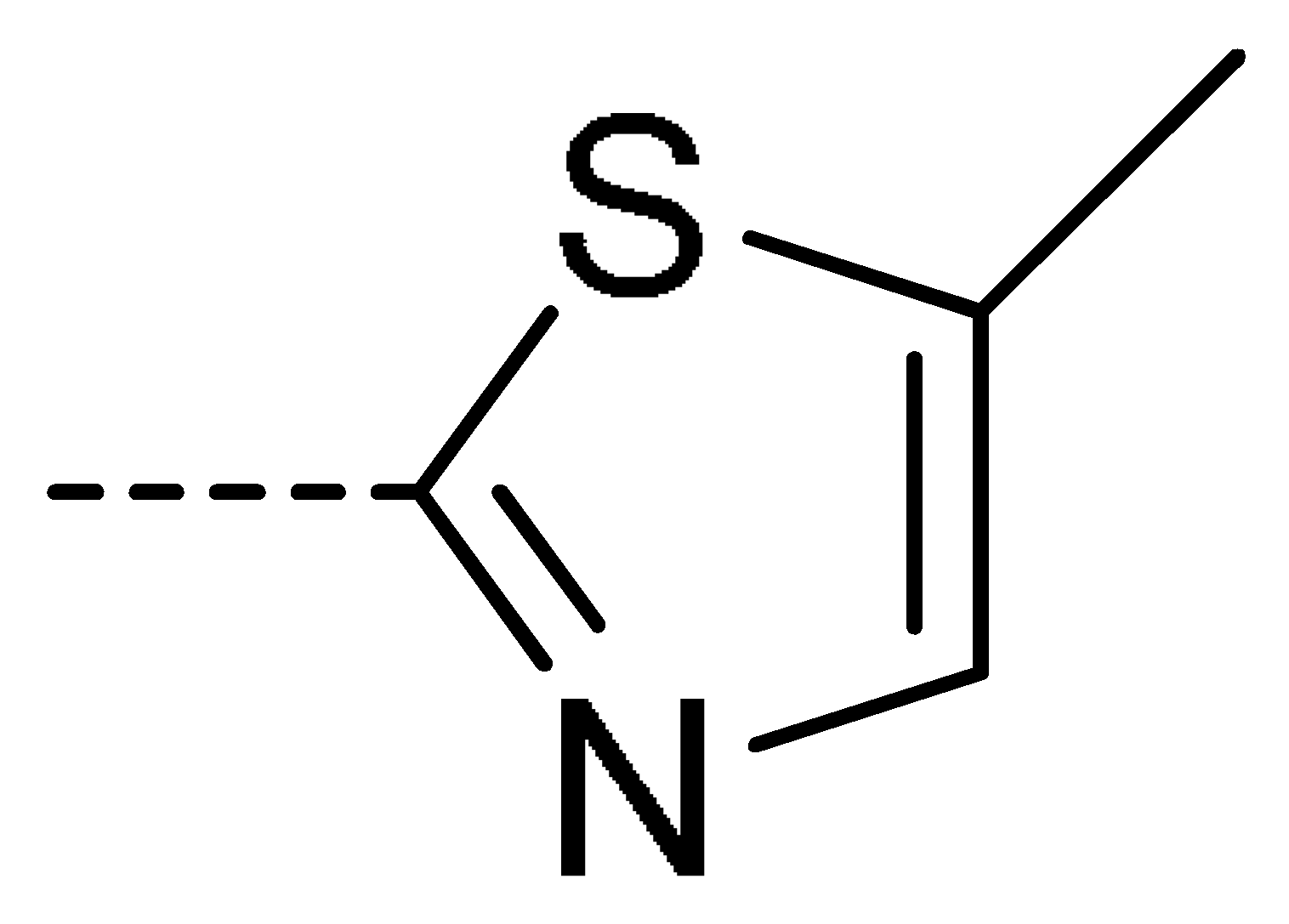 | 30 | 3y | H | NO2 | 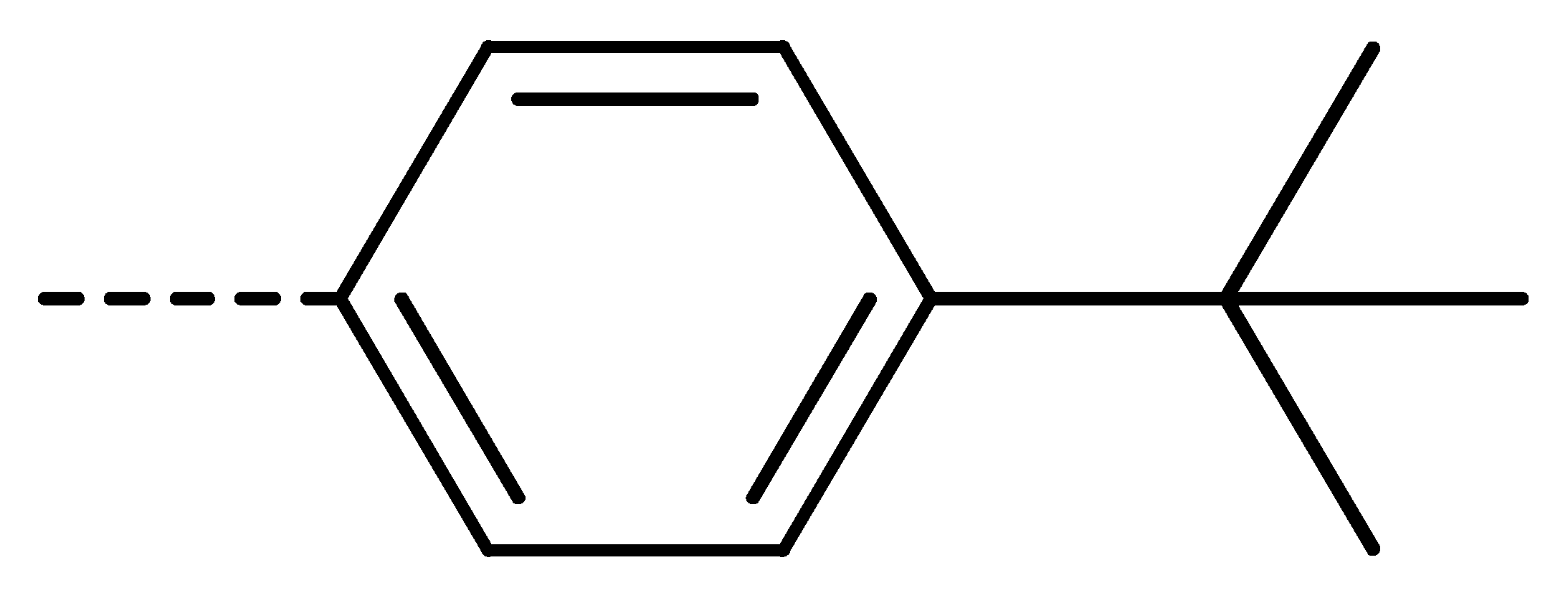 | 57 |
| 3h | Cl | H | 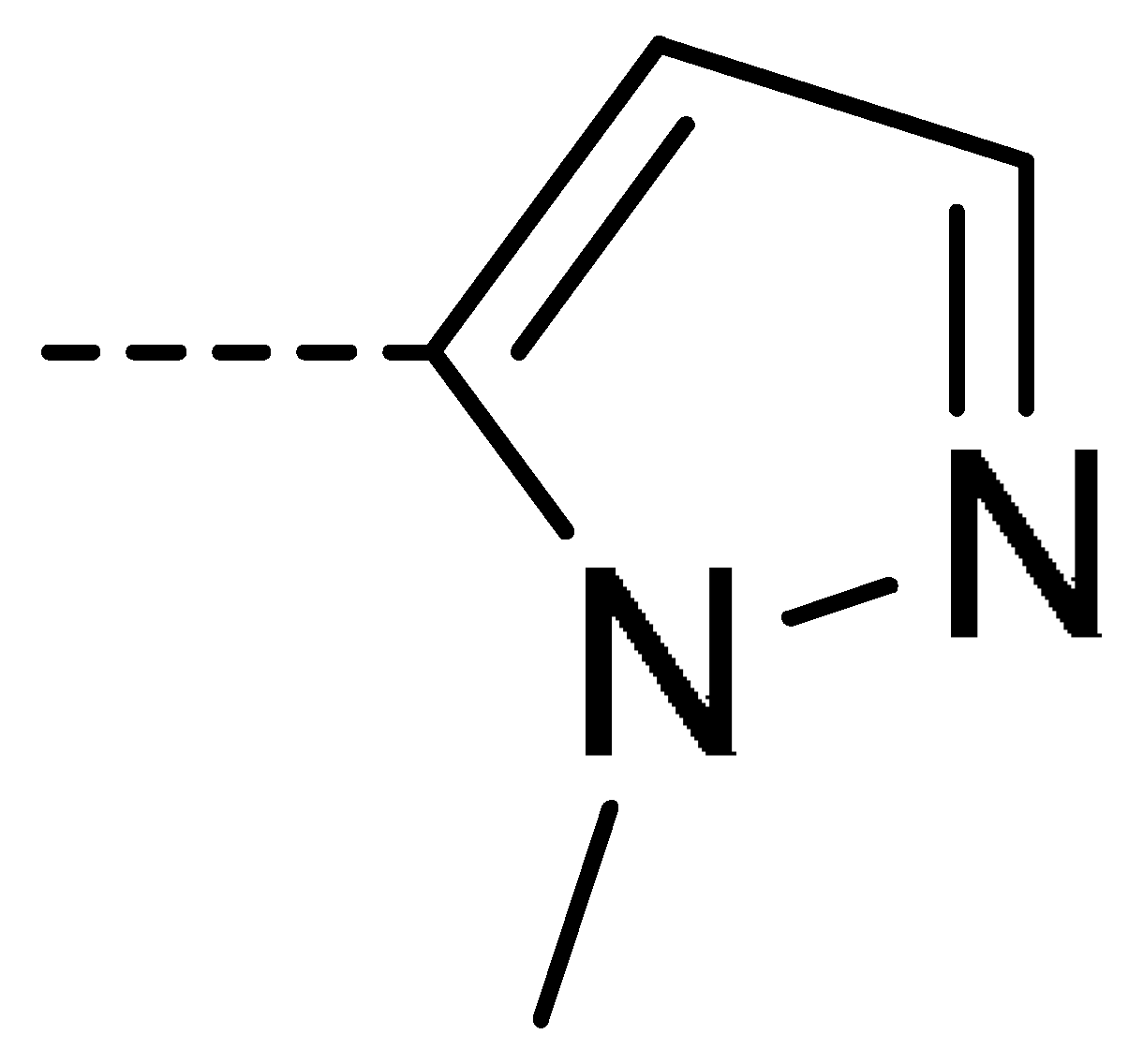 | 60 | 3q | F | H | 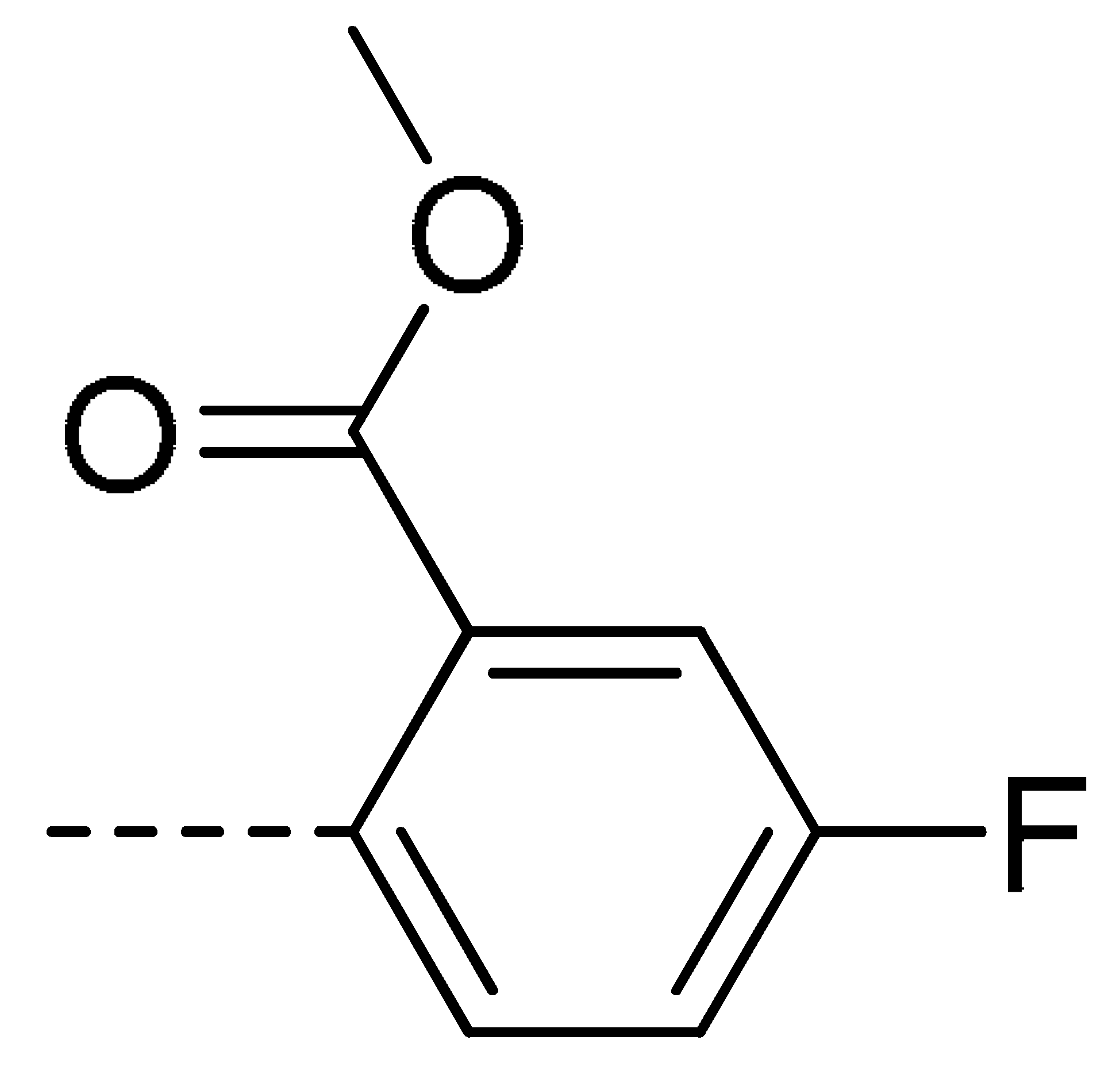 | 57 | |||||
| 3i | Cl | H | 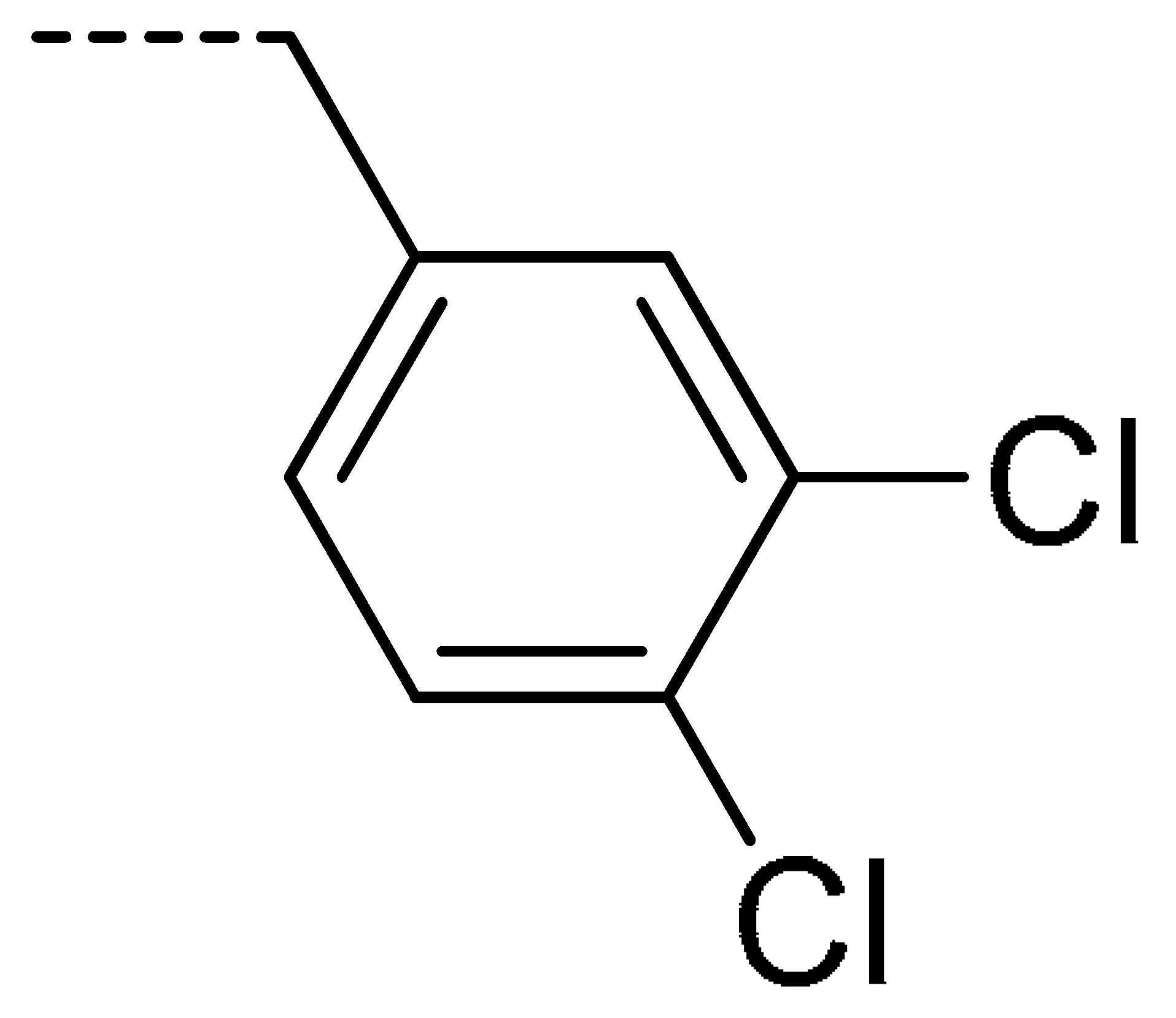 | 67 | 3r | F | H | 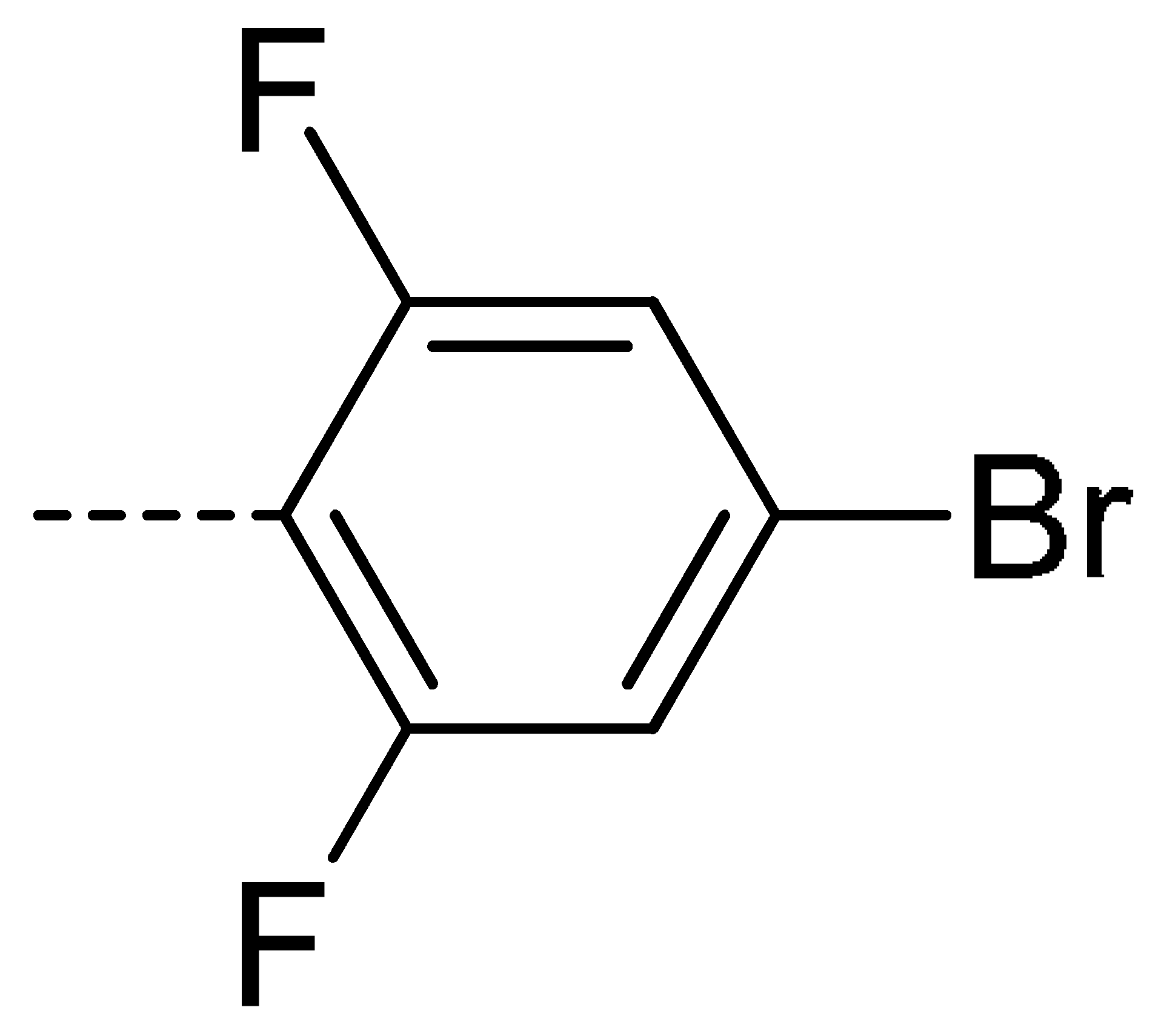 | 78 | |||||
| Compd. | BCa | ARa | DSa | |||
|---|---|---|---|---|---|---|
| Root | Stem | Root | Stem | Root | Stem | |
| 3a | 92 ± 1 | 61 ± 2 | 37 ± 1 | 87 ± 2 | 68 ± 3 | 83 ± 2 |
| 3b | 74 ± 2 | 0 | 28 ± 4 | 66 ± 4 | 77 ± 2 | 62 ± 3 |
| 3c | 78 ± 5 | 0 | 65 ± 1 | 22 ± 1 | 61 ± 1 | 0 |
| 3d | 82 ± 1 | 35 ± 1 | 81 ± 4 | 33 ± 2 | 65 ± 2 | 10 ± 1 |
| 3e | 28 ± 2 | 21 ± 2 | 51 ± 1 | 55 ± 3 | 28 ± 2 | 38 ± 3 |
| 3f | 46 ± 2 | 73 ± 3 | 68 ± 0 | 42 ± 0 | 47 ± 3 | 0 |
| 3g | 0 | 18 ± 0 | 74 ± 2 | 41 ± 2 | 91 ± 1 | 83 ± 1 |
| 3h | 77 ± 3 | 0 | 62 ± 2 | 33 ± 1 | 55 ± 1 | 34 ± 1 |
| 3i | 58 ± 4 | 0 | 55 ± 1 | 27 ± 2 | 67 ± 5 | 17 ± 3 |
| 3j | 75 ± 0 | 0 | 66 ± 1 | 34 ± 1 | 87 ± 2 | 11 ± 0 |
| 3k | 65 ± 1 | 0 | 53 ± 3 | 36 ± 2 | 57 ± 1 | 0 |
| 3l | 68 ± 2 | 6 ± 2 | 44 ± 3 | 31 ± 2 | 48 ± 4 | 51 ± 3 |
| 3m | 45 ± 3 | 0 | 55 ± 4 | 45 ± 3 | 57 ± 2 | 0 |
| 3n | 49 ± 1 | 0 | 59 ± 2 | 33 ± 2 | 59 ± 4 | 0 |
| 3o | 89 ± 1 | 77 ± 0 | 83 ± 5 | 51 ± 3 | 63 ± 4 | 0 |
| 3p | 51 ± 2 | 9 ± 2 | 37 ± 3 | 72 ± 4 | 58 ± 1 | 45 ± 2 |
| 3q | 28 ± 1 | 22 ± 1 | 22 ± 1 | 21 ± 2 | 38 ± 3 | 0 |
| 3r | 65 ± 4 | 54 ± 3 | 62 ± 2 | 75 ± 3 | 87 ± 2 | 86 ± 2 |
| 3s | 64 ± 2 | 15 ± 1 | 35 ± 4 | 61 ± 5 | 53 ± 3 | 28 ± 5 |
| 3t | 54 ± 1 | 0 | 16 ± 1 | 39 ± 4 | 46 ± 2 | 18 ± 1 |
| 3u | 77 ± 1 | 53 ± 3 | 0 | 53 ± 2 | 51 ± 2 | 32 ± 1 |
| 3v | 60 ± 5 | 0 | 10 ± 2 | 42 ± 3 | 22 ± 1 | 21 ± 2 |
| 3w | 58 ± 2 | 0 | 28 ± 1 | 61 ± 2 | 79 ± 2 | 11 ± 2 |
| 3x | 59 ± 4 | 0 | 36 ± 3 | 55 ± 3 | 31 ± 2 | 24 ± 1 |
| 3y | 68 ± 2 | 9 ± 0 | 34 ± 4 | 60 ± 4 | 44 ± 1 | 37 ± 3 |
| Chlortoluron | 85 ± 4 | 58 ± 3 | 92 ± 3 | 90 ± 5 | 98 ± 0 | 97 ± 1 |
| Atrazine | 81 ± 1 | 52 ± 1 | 32 ± 2 | 66 ± 2 | 58 ± 1 | 60 ± 2 |
| Flumioxazin | 85 ± 3 | 72 ± 5 | 82 ± 2 | 88 ± 1 | 71 ± 2 | 91 ± 0 |
| Compd. | BC | AR | DS | Compd. | BC | AR | DS |
|---|---|---|---|---|---|---|---|
| 3a | 25 ± 2 | 82 ± 3 | 37 ± 1 | 3o | 16 ± 2 | 27 ± 2 | 23 ± 2 |
| 3b | 27 ± 2 | 18 ± 2 | 12 ± 1 | 3p | 19 ± 4 | 20 ± 1 | 14 ± 0 |
| 3c | 48 ± 1 | 13 ± 1 | 19 ± 3 | 3q | 30 ± 2 | 19 ± 2 | 36 ± 1 |
| 3d | 60 ± 3 | 73 ± 2 | 20 ± 1 | 3r | 37 ± 1 | 22 ± 3 | 36 ± 5 |
| 3e | 35 ± 4 | 47 ± 3 | 33 ± 1 | 3s | 22 ± 1 | 44 ± 2 | 18 ± 1 |
| 3f | 56 ± 1 | 10 ± 2 | 11 ± 1 | 3t | 29 ± 2 | 62 ± 3 | 17 ± 1 |
| 3g | 38 ± 2 | 0 | 23 ± 2 | 3u | 60 ± 2 | 5 ± 1 | 28 ± 2 |
| 3h | 58 ± 1 | 7 ± 1 | 27 ± 2 | 3v | 33 ± 1 | 0 | 0 |
| 3i | 28 ± 2 | 34 ± 2 | 37 ± 1 | 3w | 35 ± 1 | 22 ± 4 | 36 ± 4 |
| 3j | 23 ± 2 | 20 ± 5 | 31 ± 1 | 3x | 17 ± 2 | 7 ± 2 | 7 ± 1 |
| 3k | 35 ± 5 | 24 ± 3 | 0 | 3y | 28 ± 1 | 0 | 0 |
| 3l | 33 ± 1 | 37 ± 2 | 26 ± 2 | Chlortoluron | nd a | 91 ± 1 | 65 ± 2 |
| 3m | 22 ± 4 | 35 ± 1 | 34 ± 2 | Atrazine | nd a | 91 ± 2 | 47 ± 3 |
| 3n | 25 ± 2 | 30 ± 5 | 11 ± 1 | Flumioxazin | 85 ± 6 | 92 ± 4 | 86 ± 6 |
| Compd. | AR | DS |
|---|---|---|
| 3a | 98 ± 2 | 61 ± 2 |
| 3d | 36 ± 3 | 0 |
| Flumioxazin | 100 | 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, W.; Li, X.; Ren, D.; Sun, S.; Huo, J.; Wang, Y.; Chen, L.; Zhang, J. Design and Synthesis of N-phenyl Phthalimides as Potent Protoporphyrinogen Oxidase Inhibitors. Molecules 2019, 24, 4363. https://doi.org/10.3390/molecules24234363
Gao W, Li X, Ren D, Sun S, Huo J, Wang Y, Chen L, Zhang J. Design and Synthesis of N-phenyl Phthalimides as Potent Protoporphyrinogen Oxidase Inhibitors. Molecules. 2019; 24(23):4363. https://doi.org/10.3390/molecules24234363
Chicago/Turabian StyleGao, Wei, Xiaotian Li, Da Ren, Susu Sun, Jingqian Huo, Yanen Wang, Lai Chen, and Jinlin Zhang. 2019. "Design and Synthesis of N-phenyl Phthalimides as Potent Protoporphyrinogen Oxidase Inhibitors" Molecules 24, no. 23: 4363. https://doi.org/10.3390/molecules24234363
APA StyleGao, W., Li, X., Ren, D., Sun, S., Huo, J., Wang, Y., Chen, L., & Zhang, J. (2019). Design and Synthesis of N-phenyl Phthalimides as Potent Protoporphyrinogen Oxidase Inhibitors. Molecules, 24(23), 4363. https://doi.org/10.3390/molecules24234363