Antioxidant, Antimutagenic and Cytoprotective Properties of Hydrosos Pistachio Nuts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antioxidant Tests
2.2. Antimutagenicity Test
2.3. Cytotoxic Test
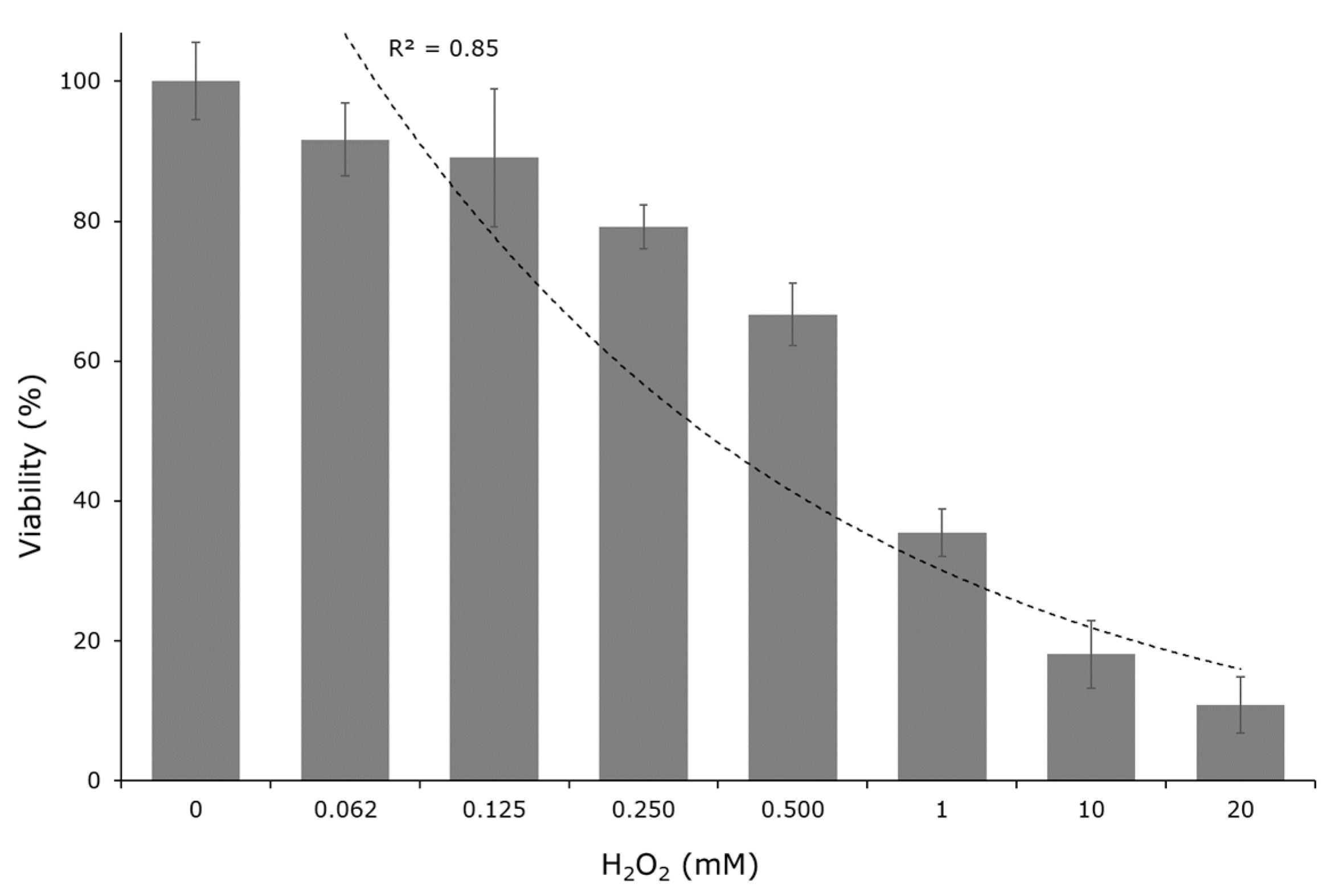
2.4. Cellular Viability and Oxidative Damage Tests
3. Materials and Methods
3.1. Plant Material and Experimental Design
3.2. Extraction of Functional Compounds
3.3. Antioxidant Tests
3.4. Antimutagenicity Test
3.5. Cell Lines
3.6. Cytotoxic Test
3.7. Cytoprotection Against H2O2-Induced Cell Damage Activity
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bozorgi, M.; Memariani, Z.; Mobli, M.; Salehi Surmaghi, M.H.; Shams-Ardekani, M.R.; Rahimi, R. Five pistacia species (P. vera, P. atlantica, P. terebinthus, P. khinjuk, and P. lentiscus): A review of their traditional uses, phytochemistry, and pharmacology. Sci. World J. 2013, 2013, 33. [Google Scholar] [CrossRef] [PubMed]
- Gijón, M.d.C.; Gimenez, C.; Perez-López, D.; Guerrero, J.; Couceiro, J.F.; Moriana, A. Rootstock influences the response of pistachio (Pistacia vera l. Cv. Kerman) to water stress and rehydration. Sci. Hortic. 2010, 125, 666–671. [Google Scholar] [CrossRef]
- Moriana, A.; Memmi, H.; Centeno, A.; Martín-Palomo, M.J.; Corell, M.; Galindo, A.; Torrecillas, A.; Pérez-López, D. Corrigendum to “influence of rootstock on pistachio (Pistacia vera L.) water relations”. Agric. Water Manag. 2019, 216, 497. [Google Scholar] [CrossRef]
- Gijón, M.d.C.; Gimenez, C.; Perez-López, D.; Guerrero, J.; Couceiro, J.F.; Moriana, A. Water relations of pistachio (Pistacia vera L.) as affected by phenological stages and water regimes. Sci. Hortic. 2011, 128, 415–422. [Google Scholar] [CrossRef]
- Noguera-Artiaga, L.; Lipan, L.; Vázquez-Araújo, L.; Barber, X.; Pérez-López, D.; Carbonell-Barrachina, Á.A. Opinion of spanish consumers on hydrosustainable pistachios. J. Food Sci. 2016, 81, S2559–S2565. [Google Scholar] [CrossRef] [PubMed]
- Corell, M.; Martín-Palomo, M.J.; Sánchez-Bravo, P.; Carrillo, T.; Collado, J.; Hernández-García, F.; Girón, I.; Andreu, L.; Galindo, A.; López-Moreno, Y.E.; et al. Evaluation of growers’ efforts to improve the sustainability of olive orchards: Development of the hydrosostainable index. Sci. Hortic. 2019, 257, 108661. [Google Scholar] [CrossRef]
- Namvar, F.; Mohamed, S.; Fard, S.G.; Behravan, J.; Mustapha, N.M.; Alitheen, N.B.M.; Othman, F. Polyphenol-rich seaweed (Eucheuma cottonii) extract suppresses breast tumour via hormone modulation and apoptosis induction. Food Chem. 2012, 130, 376–382. [Google Scholar] [CrossRef]
- World Health Organization. Cancer, Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 16 July 2019).
- Acquaviva, R.; Sorrenti, V.; Santangelo, R.; Cardile, V.; Tomasello, B.; Malfa, G.; Vanella, L.; Amodeo, A.; Genovese, C.; Mastrojeni, S.; et al. Effects of an extract of celtis aetnensis (tornab.) strobl twigs on human colon cancer cell cultures. Oncol. Rep. 2016, 36, 2298–2304. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol 2005, 100, 72–79. [Google Scholar] [CrossRef]
- Khazir, J.; Mir, B.A.; Pilcher, L.; Riley, D.L. Role of plants in anticancer drug discovery. Phytochem. Lett. 2014, 7, 173–181. [Google Scholar] [CrossRef]
- Dreher, M.L. Pistachio nuts: Composition and potential health benefits. Nutr. Rev. 2012, 70, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Noguera-Artiaga, L.; Pérez-López, D.; Burgos-Hernández, A.; Wojdyło, A.; Carbonell-Barrachina, Á.A. Phenolic and triterpenoid composition and inhibition of α-amylase of pistachio kernels (Pistacia vera L.) as affected by rootstock and irrigation treatment. Food Chem. 2018, 261, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Bisignano, C.; Filocamo, A.; Chessa, S.; Sarò, M.; Torre, G.; Faulks, R.M.; Dugo, P. Bioaccessibility of pistachio polyphenols, xanthophylls, and tocopherols during simulated human digestion. Nutrition 2013, 29, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Rakholiya, K.D.; Kaneria, M.J.; Chanda, S.V. Isolation, validation and characterization of major bioactive constituents by various techniques from mango ripe seed. In Food Technology: Applied Research and Production; Meghwal, M., Goyal, M.R., Kaneria, M., Eds.; Apple Academic Press: Waretown, NJ, USA, 2017; pp. 273–314. [Google Scholar]
- Noguera-Artiaga, L.; Salvador, M.D.; Fregapane, G.; Collado-Gonzalez, J.; Wojdylo, A.; Lopez-Lluch, D.; Carbonell-Barrachina, A.A. Functional and sensory properties of pistachio nuts as affected by cultivar. J. Sci. Food Agric. 2019, 99, 6696–6705. [Google Scholar] [CrossRef] [PubMed]
- Kaneria, M.; Rakholiya, K.; Sonagara, J.; Chanda, S. Comparative assessment of antioxidant activity and phytochemical analysis of facultative halophyte Salvadora oleoides decne. and Salvadora persica L. Am. J. Biochem. Mol. Biol. 2017, 7, 102–110. [Google Scholar] [CrossRef]
- Taghizadeh, S.F.; Rezaee, R.; Davarynejad, G.; Karimi, G.; Nemati, S.H.; Asili, J. Phenolic profile and antioxidant activity of Pistacia vera var. Sarakhs hull and kernel extracts: The influence of different solvents. J. Food Meas. Charact. 2018, 12, 2138–2144. [Google Scholar] [CrossRef]
- Horner, J.D. Nonlinear effects of water deficits on foliar tannin concentration. Biochem. Syst. Ecol. 1990, 18, 211–213. [Google Scholar] [CrossRef]
- Rajaei, A.; Barzegar, M.; Mobarez, A.M.; Sahari, M.A.; Esfahani, Z.H. Antioxidant, anti-microbial and antimutagenicity activities of pistachio (Pistachia vera) green hull extract. Food Chem. Toxicol. 2010, 48, 107–112. [Google Scholar] [CrossRef]
- Pacifico, S.; Gallicchio, M.; Fiorentino, A.; Fischer, A.; Meyer, U.; Stintzing, F.C. Antioxidant properties and cytotoxic effects on human cancer cell lines of aqueous fermented and lipophilic quince (Cydonia oblonga mill.) preparations. Food Chem. Toxicol. 2012, 50, 4130–4135. [Google Scholar] [CrossRef]
- Seifaddinipour, M.; Farghadani, R.; Namvar, F.; Mohamad, J.; Abdul Kadir, H. Cytotoxic effects and anti-angiogenesis potential of pistachio (Pistacia vera L.) hulls against mcf-7 human breast cancer cells. Molecules 2018, 23, 110. [Google Scholar] [CrossRef]
- Novotný, L.; Vachálková, A.; Biggs, D. Ursolic acid: An anti-tumorigenic and chemopreventive activity. Neoplasma 2001, 48, 241–246. [Google Scholar] [PubMed]
- Piȩt, M.; Paduch, R. Ursolic and oleanolic acids as potential anticancer agents acting in the gastrointestinal tract. Mini-Rev. Org. Chem. 2019, 16, 78–91. [Google Scholar] [CrossRef]
- Halliwell, B. Free radicals and antioxidants: Updating a personal view. Nutr. Rev. 2012, 70, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Memmi, H.; Gijón, M.C.; Couceiro, J.F.; Pérez-López, D. Water stress thresholds for regulated deficit irrigation in pistachio trees: Rootstock influence and effects on yield quality. Agric. Water Manag. 2016, 164, 58–72. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved abts radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (frap) as a measure of “antioxidant power”: The frap assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Loarca-Piña, G.; Mendoza, S.; Ramos-Gómez, M.; Reynoso, R. Antioxidant, antimutagenic, and antidiabetic activities of edible leaves from Cnidoscolus chayamansa Mc. Vaugh. J. Food Sci. 2010, 75, H68–H72. [Google Scholar] [CrossRef]
- Cardador-Martínez, A.; Loarca-Piña, G.; Oomah, B.D. Antioxidant activity in common beans (Phaseolus vulgaris l.). J. Agric. Food Chem. 2002, 50, 6975–6980. [Google Scholar] [CrossRef]
- Bolanos De La Torre, A.A.S.; Henderson, T.; Nigam, P.S.; Owusu-Apenten, R.K. A universally calibrated microplate ferric reducing antioxidant power (frap) assay for foods and applications to manuka honey. Food Chem. 2015, 174, 119–123. [Google Scholar] [CrossRef]
- Maron, D.M.; Ames, B.N. Revised methods for the salmonella mutagenicity test. Mutat. Res. Environ. Mutagenesis Relat. Subj. 1983, 113, 173–215. [Google Scholar] [CrossRef]
- Fathalizadeh, J.; Bagheri, V.; Khorramdelazad, H.; Kazemi Arababadi, M.; Jafarzadeh, A.; Mirzaei, M.R.; Shamsizadeh, A.; Hajizadeh, M.R. Induction of apoptosis by pistachio (Pistacia vera L.) hull extract and its molecular mechanisms of action in human hepatoma cell line hepg2. Cell. Mol. Biol. 2015, 61, 128–134. [Google Scholar] [PubMed]
- Hu, X.; Liang, Y.; Zhao, B.; Wang, Y. Thymoquinone protects human retinal pigment epithelial cells against hydrogen peroxide induced oxidative stress and apoptosis. J. Cell. Biochem. 2019, 120, 4514–4522. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, W.; Zhou, X.; Long, C.; Kuang, X.; Hu, J.; Tang, Y.; Liu, L.; He, J.; Huang, Z.; et al. Protective effect of lutein on arpe-19 cells upon h2o2-induced g2/m arrest. Mol. Med. Rep. 2017, 16, 2069–2074. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors until the end of 2019. The samples are stored at 4 ◦C in darkness, and it is planned to store them until the end of 2019. |


| Figure | ABTS | FRAP | DPPH |
|---|---|---|---|
| (mM TE g−1) | |||
| ANOVA 1 | |||
| Rootstock | *** | *** | *** |
| Irrigation | *** | ** | *** |
| Rootstock × Irrigation | *** | ** | ** |
| Tukey multiple range test 2 | |||
| Rootstock | |||
| P. atlantica | 2.80 c | 1.23 b | 5.44 ab |
| P. integerrima | 3.23 b | 1.37 a | 5.07 b |
| P. terebinthus | 3.67 a | 1.32 a | 5.63 a |
| Irrigation | |||
| T0 | 2.86 b | 1.29 b | 4.59 c |
| T1 | 2.96 b | 1.37 a | 5.13 b |
| T2 | 3.92 a | 1.29 b | 6.41 a |
| Rootstock × Irrigation | |||
| P. atlantica × T0 | 2.37 d | 1.24 ab | 4.83 d |
| P. atlantica × T1 | 2.61 d | 1.34 a | 6.07 b |
| P. atlantica × T2 | 3.41 b | 1.10 b | 5.42 c |
| P. integerrima × T0 | 2.95 cd | 1.36 a | 4.43 e |
| P. integerrima × T1 | 2.90 cd | 1.41 a | 4.42 e |
| P. integerrima × T2 | 3.94 ab | 1.37 a | 6.36 b |
| P. terebinthus × T0 | 3.24 bc | 1.28 a | 4.51 e |
| P. terebinthus × T1 | 3.36 bc | 1.39 a | 4.89 d |
| P. terebinthus × T2 | 4.40 a | 1.26 a | 7.47 a |
| Pooled variance | 0.32 | 0.15 | 0.22 |
| Factor | HCT-116 | A549 | HeLa | MDA-MB-231 | ARPE-19 |
|---|---|---|---|---|---|
| (% Viability) | |||||
| ANOVA 1 | |||||
| Rootstock | NS | *** | ** | NS | *** |
| Irrigation | NS | NS | NS | NS | *** |
| Rootstock × Irrigation | NS | *** | *** | NS | *** |
| Tukey multiple range test 2 | |||||
| Rootstock | |||||
| P. atlantica | 38.9 | 67.4 a | 30.1 a | 61.3 | 94.2 a |
| P. integerrima | 39.0 | 68.3 a | 30.1 a | 61.9 | 87.9 b |
| P. terebinthus | 43.4 | 68.7 a | 32.3 a | 60.3 | 86.6 b |
| CISP 3 | 40.2 | 61.1 b | 28.2 b | 57.2 | 69.2 c |
| Irrigation | |||||
| T0 | 39.7 | 65.3 | 28.3 | 58.0 | 91.7 a |
| T1 | 39.8 | 64.4 | 27.0 | 60.8 | 87.7 a |
| T2 | 40.9 | 61.1 | 28.2 | 61.0 | 89.3 a |
| CISP 3 | 40.2 | 61.1 | 28.2 | 57.2 | 69.2 b |
| Rootstock × Irrigation | |||||
| P. atlantica × T0 | 36.1 | 73.2 a | 30.1 a | 62.5 | 99.1 a |
| P. atlantica × T1 | 35.8 | 66.2 a | 29.3 a | 60.9 | 91.1 a |
| P. atlantica × T2 | 44.3 | 64.6 ab | 30.4 a | 59.6 | 90.9 a |
| P. integerrima × T0 | 36.3 | 74.5 a | 28.4 ab | 55.5 | 84.9 ab |
| P. integerrima × T1 | 41.0 | 67.2 a | 31.5 a | 64.6 | 88.3 ab |
| P. integerrima × T2 | 38.8 | 65.1 ab | 29.6 a | 64.9 | 90.6 a |
| P. terebinthus × T0 | 46.7 | 63.7 ab | 34.8 a | 58.9 | 90.1 a |
| P. terebinthus × T1 | 42.7 | 75.3 a | 28.8 ab | 59.8 | 83.8 ab |
| P. terebinthus × T2 | 39.9 | 69.1 a | 33.3 a | 61.3 | 86.2 ab |
| CISP 3 | 40.2 | 61.1 b | 28.2 b | 57.2 | 69.2 b |
| Pooled variance | 4.1 | 2.3 | 1.8 | 4.8 | 4.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noguera-Artiaga, L.; García-Romo, J.S.; Rosas-Burgos, E.C.; Cinco-Moroyoqui, F.J.; Vidal-Quintanar, R.L.; Carbonell-Barrachina, Á.A.; Burgos-Hernández, A. Antioxidant, Antimutagenic and Cytoprotective Properties of Hydrosos Pistachio Nuts. Molecules 2019, 24, 4362. https://doi.org/10.3390/molecules24234362
Noguera-Artiaga L, García-Romo JS, Rosas-Burgos EC, Cinco-Moroyoqui FJ, Vidal-Quintanar RL, Carbonell-Barrachina ÁA, Burgos-Hernández A. Antioxidant, Antimutagenic and Cytoprotective Properties of Hydrosos Pistachio Nuts. Molecules. 2019; 24(23):4362. https://doi.org/10.3390/molecules24234362
Chicago/Turabian StyleNoguera-Artiaga, Luis, Joel Said García-Romo, Ema C. Rosas-Burgos, Francisco Javier Cinco-Moroyoqui, Reyna Luz Vidal-Quintanar, Ángel Antonio Carbonell-Barrachina, and Armando Burgos-Hernández. 2019. "Antioxidant, Antimutagenic and Cytoprotective Properties of Hydrosos Pistachio Nuts" Molecules 24, no. 23: 4362. https://doi.org/10.3390/molecules24234362
APA StyleNoguera-Artiaga, L., García-Romo, J. S., Rosas-Burgos, E. C., Cinco-Moroyoqui, F. J., Vidal-Quintanar, R. L., Carbonell-Barrachina, Á. A., & Burgos-Hernández, A. (2019). Antioxidant, Antimutagenic and Cytoprotective Properties of Hydrosos Pistachio Nuts. Molecules, 24(23), 4362. https://doi.org/10.3390/molecules24234362








