Halogen-Bond Assisted Photoinduced Electron Transfer
Abstract
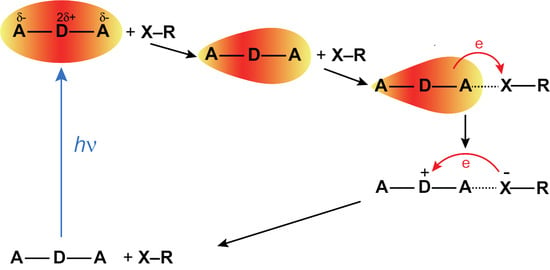
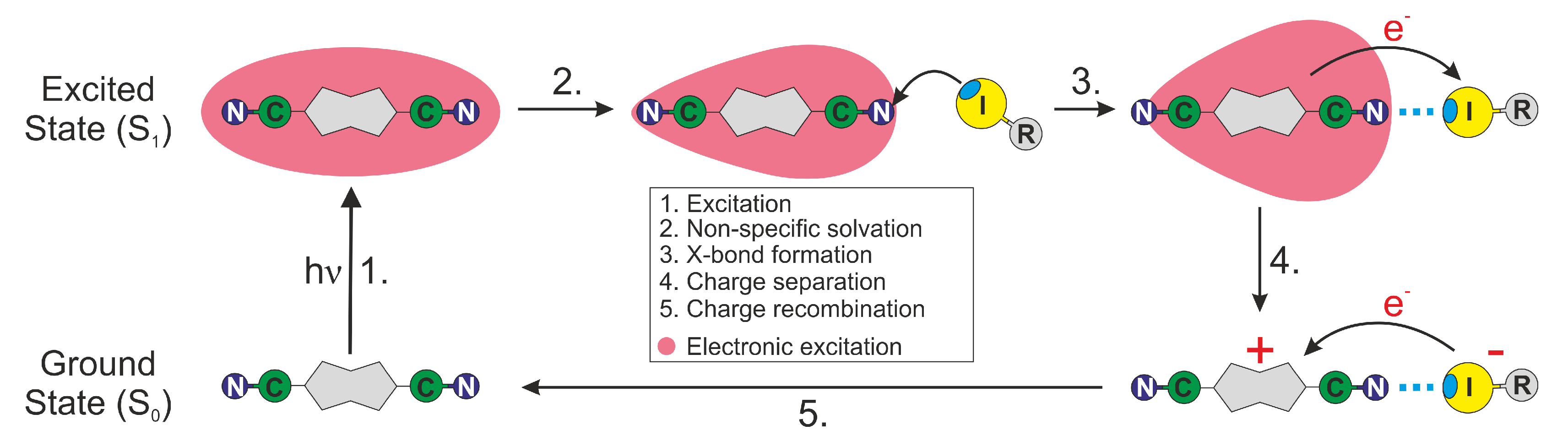
1. Introduction
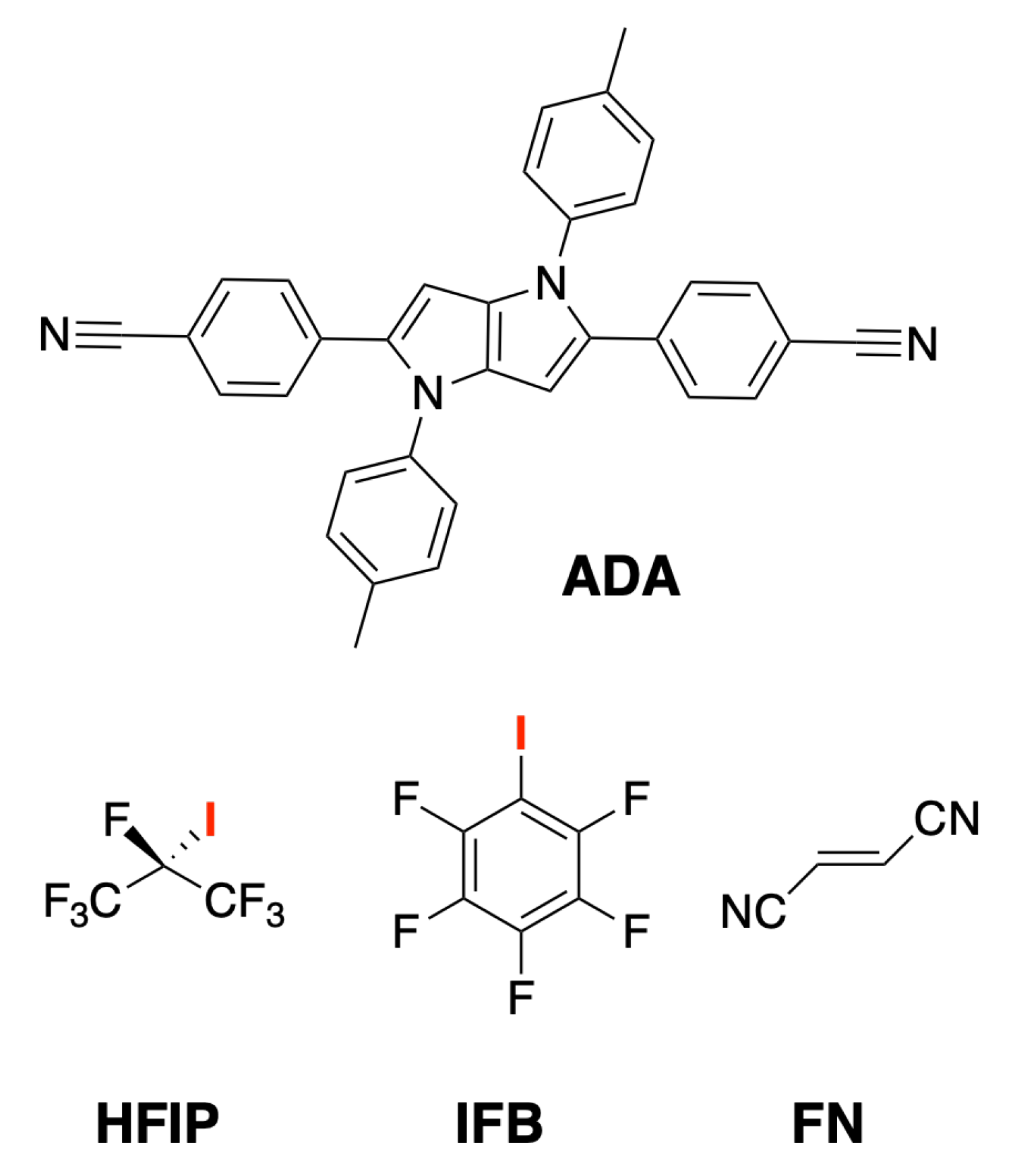
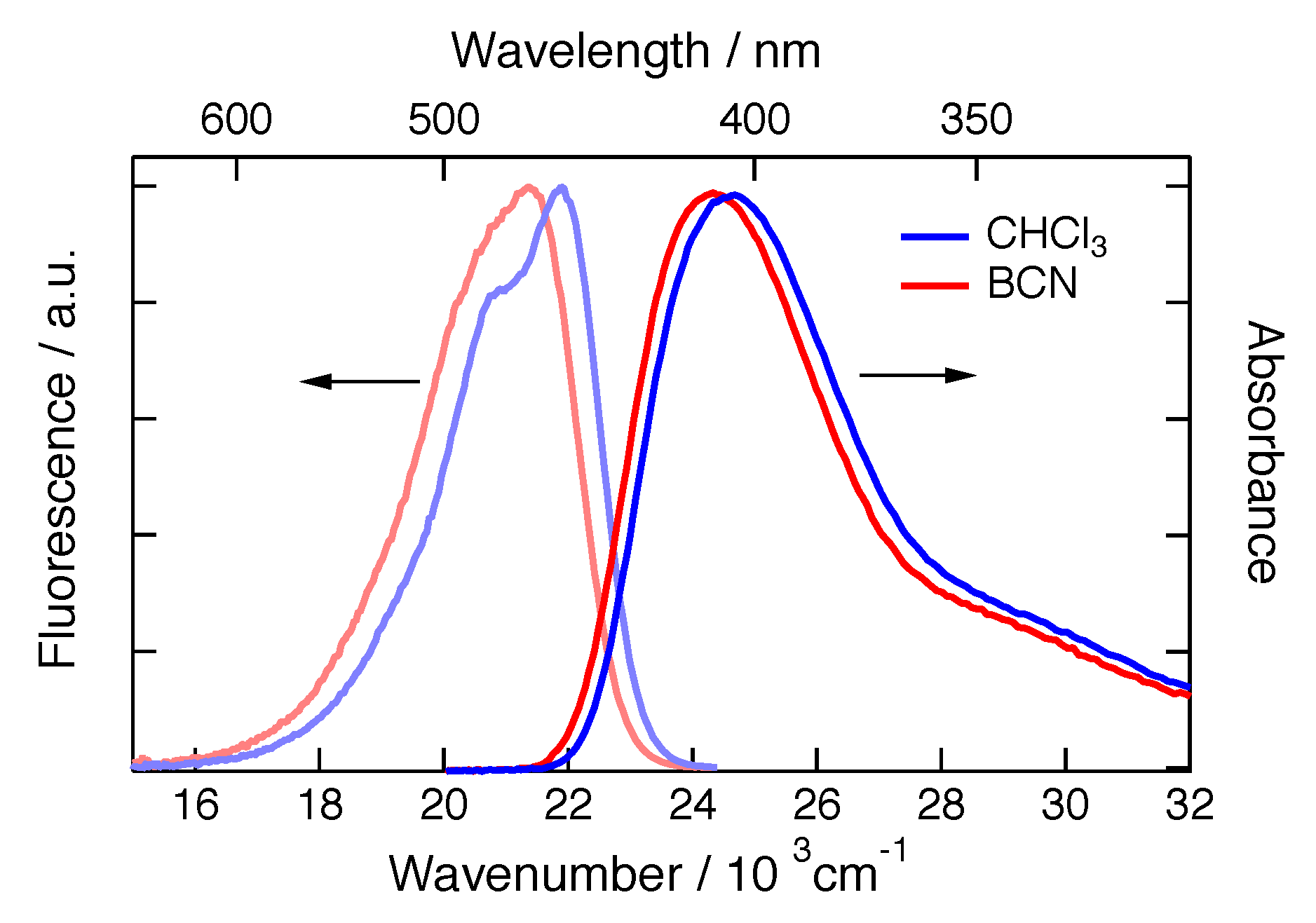
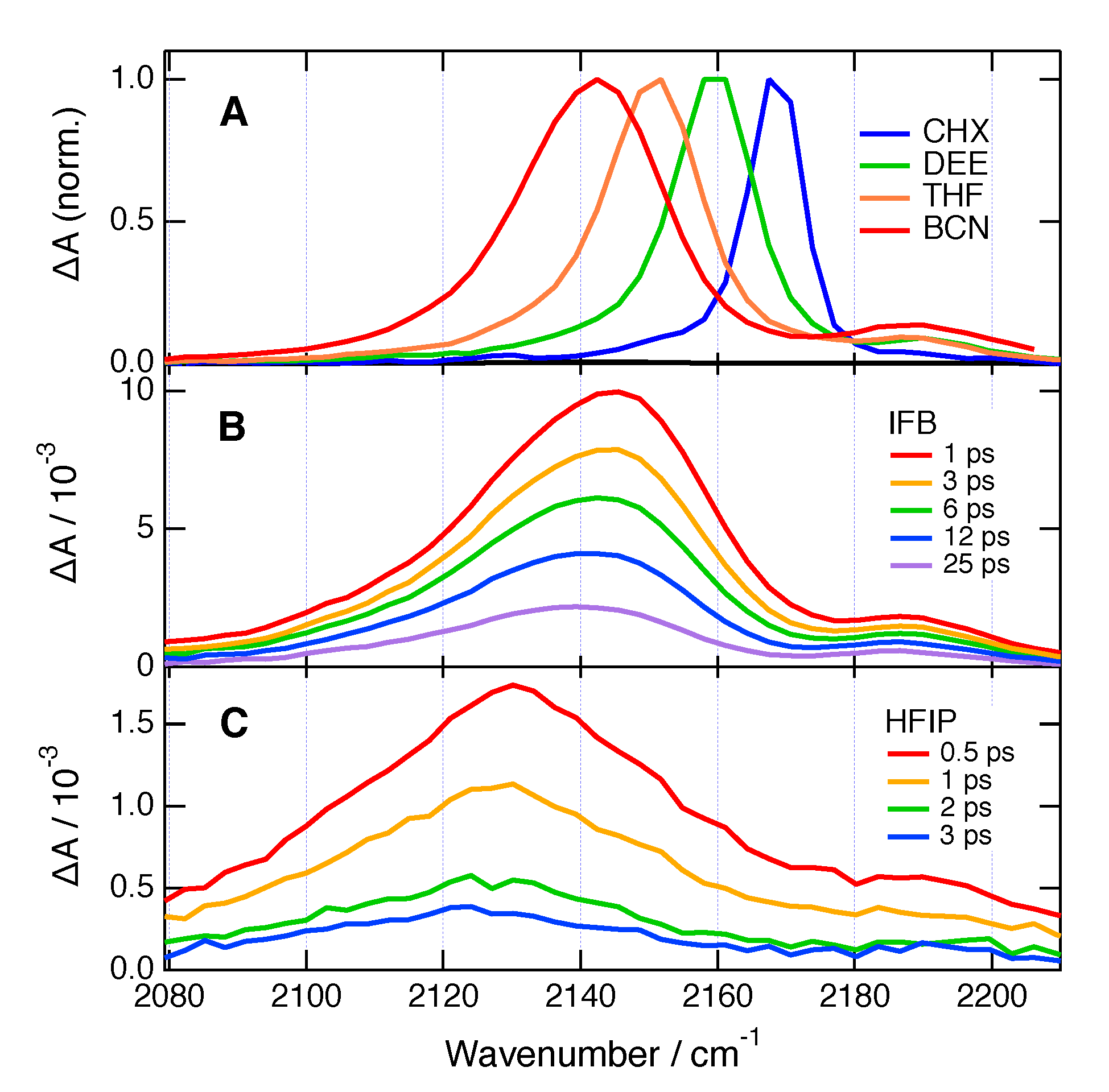
2. Results
3. Discussion
- (i)
- The intrinsic ET rate constant, , decreases in the order, FN, HFIP, IFB, in the same way as .
- (ii)
- For a given quencher, is larger or equal in BCN than in CHCl3, contrary to which is smaller in BCN.
- (iii)
- The quenching radius, R, is significantly larger for FN than for the halogenated acceptors.
- (iv)
- For a given quencher, R is larger in BCN than in CHCl3.
4. Materials and Methods
4.1. Chemicals
4.2. Stationary Spectroscopy
4.3. DOSY NMR
4.4. Time-Resolved Fluorescence
4.5. Time-Resolved Infrared Spectroscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BCN | Benzonitrile |
| DOSY | Diffusion Ordered SpectroscopY |
| ESA | Excited-State Absorption |
| ES-SB | Excited-State Symmetry Breaking |
| ET | Electron Transfer |
| FN | Fumaronitrile |
| HFIP | Perfluoroisopropyl iodide |
| IFB | Iodoperfluorobenzene (pentafluoroiodobenzene) |
| ISC | InterSystem Crossing |
| SCK | Smoluchowski–Collins–Kimball (model) |
| TA | Transient Absorption |
| TCSPC | Time-Correlated Single Photon Counting |
| TRIR | Time-Resolved IR spectroscopy |
| XB | Halogen bond |
References
- Metrangolo, P.; Resnati, G. Halogen versus Hydrogen. Science 2008, 321, 918–919. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, G.; Metrangolo, P.; Pilati, T.; Resnati, G.; Sansotera, M.; Terraneo, G. Halogen bonding: A general route in anion recognition and coordination. Chem. Soc. Rev. 2010, 39, 3772–3783. [Google Scholar] [CrossRef] [PubMed]
- Scholfield, M.R.; Vander Zanden, C.M.; Carter, M.; Ho, P.S. Halogen bonding (X-bonding): A biological perspective. Protein Sci. 2013, 22, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Gilday, L.C.; Robinson, S.W.; Barendt, T.A.; Langton, M.J.; Mullaney, B.R.; Beer, P.D. Halogen Bonding in Supramolecular Chemistry. Chem. Rev. 2015, 115, 7118–7195. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed]
- Mendez, L.; Henriquez, G.; Sirimulla, S.; Narayan, M. Looking back, looking forward at halogen bonding in drug discovery. Molecules 2017, 22, 1397. [Google Scholar] [CrossRef]
- Tepper, R.; Schubert, U.S. Halogen Bonding in Solution: Anion Recognition, Templated Self-Assembly, and Organocatalysis. Angew. Chem. Int. Ed. 2018, 57, 6004–6016. [Google Scholar] [CrossRef]
- Juanes, M.; Saragi, R.T.; Caminati, W.; Lesarri, A. The Hydrogen Bond and Beyond: Perspectives for Rotational Investigations of Non-Covalent Interactions. Chem. Eur. J. 2019, 25, 11402–11411. [Google Scholar] [CrossRef]
- Saccone, M.; Catalano, L. Halogen Bonding beyond Crystals in Materials Science. J. Phys. Chem. B 2019, 123, 9281–9290. [Google Scholar] [CrossRef]
- Priimagi, A.; Cavallo, G.; Metrangolo, P.; Resnati, G. The Halogen Bond in the Design of Functional Supramolecular Materials: Recent Advances. Acc. Chem. Res. 2013, 46, 2686–2695. [Google Scholar] [CrossRef]
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P. Halogen Bonding: The σ-Hole. J. Mol. Model. 2007, 13, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen Bonding: An Electrostatically-Driven Highly Directional Noncovalent Interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar] [CrossRef] [PubMed]
- Riley, K.E.; Murray, J.S.; Fanfrlík, J.; Řezáč, J.; Solá, R.J.; Concha, M.C.; Ramos, F.M.; Politzer, P. Halogen Bond Tunability I: The Effects of Aromatic Fluorine Substitution on the Strengths of Halogen-Bonding Interactions Involving Chlorine, Bromine, and Iodine. J. Mol. Model. 2011, 17, 3309–3318. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zheng, R.; Zhen, Y.; Yu, Z.; Dong, H.; Fu, H.; Shi, Q.; Hu, W. Rational design of charge-transfer interactions in halogen-bonded co-crystals toward versatile solid-state optoelectronics. J. Am. Chem. Soc. 2015, 137, 11038–11046. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, H.; Jin, W.J. Soft-Cavity-type Host-Guest Structure of Cocrystals with Good Luminescence Behavior Assembled by Halogen Bond and Other Weak Interactions. Cryst. Growth Des. 2017, 17, 3331–3337. [Google Scholar] [CrossRef]
- Salunke, J.K.; Durandin, N.A.; Ruoko, T.-P.; Candeias, N.R.; Vivo, P.; Vuorimaa-Laukkanen, E.; Laaksonen, T.; Priimagi, A. Halogen-Bond-Assisted Photoluminescence Modulation in Carbazole-Based Emitter. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Shi, L.; Liu, H.-Y.; Shen, H.; Hu, J.; Zhang, G.-L.; Wang, H.; Ji, L.-N.; Chang, C.-K.; Jiang, H.-F. Fluorescence properties of halogenated mono-hydroxyl corroles: the heavy-atom effects. J. Porphyr. Phthalocyanines 2009, 13, 1221–1226. [Google Scholar] [CrossRef]
- Yan, D.; Delori, A.; Lloyd, G.O.; Friščić, T.; Day, G.M.; Jones, W.; Lu, J.; Wei, M.; Evans, D.G.; Duan, X. A Cocrystal Strategy to Tune the Luminescent Properties of Stilbene-Type Organic Solid-State Materials. Angew. Chem. Int. Ed. 2011, 50, 12483–12486. [Google Scholar] [CrossRef]
- Botta, C.; Cariati, E.; Cavallo, G.; Dichiarante, V.; Forni, A.; Metrangolo, P.; Pilati, T.; Resnati, G.; Righetto, S.; Terraneo, G.; et al. Fluorine-induced J-aggregation enhances emissive properties of a new NLO push–pull chromophore. J. Mater. Chem. C 2014, 2, 5275–5279. [Google Scholar] [CrossRef]
- Sun, C.-L.; Li, J.; Geng, H.-W.; Li, H.; Ai, Y.; Wang, Q.; Pan, S.-L.; Zhang, H.-L. Understanding the Unconventional Effects of Halogenation on the Luminescent Properties of Oligo(Phenylene Vinylene) Molecules. Chem. Asian J. 2013, 8, 3091–3100. [Google Scholar] [CrossRef]
- Priimagi, A.; Cavallo, G.; Forni, A.; Gorynsztejn–Leben, M.; Kaivola, M.; Metrangolo, P.; Milani, R.; Shishido, A.; Pilati, T.; Resnati, G.; et al. Halogen Bonding versus Hydrogen Bonding in Driving Self-Assembly and Performance of Light-Responsive Supramolecular Polymers. Adv. Funct. Mater. 2012, 22, 2572–2579. [Google Scholar] [CrossRef]
- Bushuyev, O.S.; Tomberg, A.; Friščić, T.; Barrett, C.J. Shaping Crystals with Light: Crystal-to-Crystal Isomerization and Photomechanical Effect in Fluorinated Azobenzenes. J. Am. Chem. Soc. 2013, 135, 12556–12559. [Google Scholar] [CrossRef] [PubMed]
- Bushuyev, O.S.; Corkery, T.C.; Barrett, C.J.; Friščić, T. Photo-mechanical azobenzene cocrystals and in situ X-ray diffraction monitoring of their optically-induced crystal-to-crystal isomerisation. Chem. Sci. 2014, 5, 3158–3164. [Google Scholar] [CrossRef]
- Saccone, M.; Dichiarante, V.; Forni, A.; Goulet-Hanssens, A.; Cavallo, G.; Vapaavuori, J.; Terraneo, G.; Barrett, C.J.; Resnati, G.; Metrangolo, P.; et al. Supramolecular hierarchy among halogen and hydrogen bond donors in light-induced surface patterning. J. Mater. Chem. C 2015, 3, 759–768. [Google Scholar] [CrossRef]
- Virkki, M.; Tuominen, O.; Forni, A.; Saccone, M.; Metrangolo, P.; Resnati, G.; Kauranen, M.; Priimagi, A. Halogen bonding enhances nonlinear optical response in poled supramolecular polymers. J. Mater. Chem. C 2015, 3, 3003–3006. [Google Scholar] [CrossRef]
- Sun, X.; Wang, W.; Li, Y.; Ma, J.; Yu, S. Halogen-Bond-Promoted Double Radical Isocyanide Insertion under Visible-Light Irradiation: Synthesis of 2-Fluoroalkylated Quinoxalines. Org. Lett. 2016, 18, 4638–4641. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.; Jin, W.J. σ-Hole Bond vs π-Hole Bond: A Comparison Based on Halogen Bond. Chem. Rev. 2016, 116, 5072–5104. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Mao, R.; Li, Z.; Wu, J. A photoinduced reaction of perfluoroalkyl halides with 1,3-diarylprop-2-yn-1-ones catalyzed by DABSO. Org. Chem. Front. 2017, 4, 1745–1750. [Google Scholar] [CrossRef]
- Zou, W.-S.; Lin, S.; Li, J.-Y.; Wei, H.-Q.; Zhang, X.-Q.; Shen, D.-X.; Qiao, J.-Q.; Lian, H.-Z.; Xie, D.-Q.; Ge, X. Mechanism and application of halogen bond induced fluorescence enhancement and iodine molecule cleavage in solution. New J. Chem. 2015, 39, 262–272. [Google Scholar] [CrossRef]
- Tuikka, M.; Hirva, P.; Rissanen, K.; Korppi-Tommola, J.; Haukka, M. Halogen bonding—A key step in charge recombination of the dye-sensitized solar cell. Chem. Commun. 2011, 47, 4499–4501. [Google Scholar] [CrossRef]
- Simon, S.J.C.; Parlane, F.G.; Swords, W.B.; Kellett, C.W.; Du, C.; Lam, B.; Dean, R.K.; Hu, K.; Meyer, G.J.; Berlinguette, C.P. Halogen Bonding Promotes Higher Dye-Sensitized Solar Cell Photovoltages. J. Am. Chem. Soc. 2016, 138, 10406–10409. [Google Scholar] [CrossRef] [PubMed]
- Parlane, F.G.L.; Mustoe, C.; Kellett, C.W.; Simon, S.J.; Swords, W.B.; Meyer, G.J.; Kennepohl, P.; Berlinguette, C.P. Spectroscopic detection of halogen bonding resolves dye regeneration in the dye-sensitized solar cell. Nat. Commun. 2017, 8, 1761. [Google Scholar] [CrossRef] [PubMed]
- Dereka, B.; Vauthey, E. Solute–Solvent Interactions and Excited-State Symmetry Breaking: Beyond the Dipole–Dipole and the Hydrogen-Bond Interactions. J. Phys. Chem. Lett. 2017, 8, 3927–3932. [Google Scholar] [CrossRef]
- Dereka, B.; Rosspeintner, A.; Krzeszewski, M.; Gryko, D.T.; Vauthey, E. Symmetry-Breaking Charge Transfer and Hydrogen Bonding: Toward Asymmetrical Photochemistry. Angew. Chem. Int. Ed. 2016, 55, 15624–15628. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.I.; Dereka, B.; Vauthey, E. A Simple Model of Solvent-Induced Symmetry-Breaking Charge Transfer in Excited Quadrupolar Molecules. J. Chem. Phys. 2017, 146, 164306. [Google Scholar] [CrossRef]
- Mohammed, O.F.; Banerji, N.; Lang, B.; Nibbering, E.T.; Vauthey, E. Photoinduced bimolecular electron transfer investigated by femtosecond time-resolved infrared spectroscopy. J. Phys. Chem. A 2006, 110, 13676–13680. [Google Scholar] [CrossRef]
- Koch, M.; Letrun, R.; Vauthey, E. Exciplex Formation in Bimolecular Photoinduced Electron-Transfer Investigated by Ultrafast Time-Resolved Infrared Spectroscopy. J. Am. Chem. Soc. 2014, 136, 4066–4074. [Google Scholar] [CrossRef]
- Eads, D.D.; Dismer, B.G.; Fleming, G.R. A subpicosecond, subnanosecond and steady-state study of diffusion influenced fluorescence quenching. J. Chem. Phys. 1990, 93, 1136–1148. [Google Scholar] [CrossRef]
- Dorfman, R.C.; Fayer, M.D. The influence of diffusion on photoinduced electron transfer and geminate recombination. J. Chem. Phys. 1992, 96, 7410–7422. [Google Scholar] [CrossRef]
- Murata, S.; Tachiya, M. Electron transfer reactions studied through the transient effect in fluorescence quenching. J. Chem. Phys. 1996, 93, 1577–1590. [Google Scholar] [CrossRef]
- Burshtein, A.I. Non-Markovian Theories of Transfer Reactions in Luminescence and Chemiluminescence and Photo- and Electrochemistry. Adv. Chem. Phys. 2004, 129, 105–418. [Google Scholar]
- Rosspeintner, A.; Vauthey, E. Bimolecular Photoinduced Electron Transfer Reactions in Liquids under the Gaze of Ultrafast Spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 25741–25754. [Google Scholar] [CrossRef] [PubMed]
- Burshtein, A. Unified Theory of Photochemical Charge Separation. Adv. Chem. Phys. 2000, 114, 419–587. [Google Scholar]
- Von Smoluchowski, M. Versuch einer mathematischen Theorie der Koagulationskinetik kolloider Loesungen. Z. Phys. Chem. 1918, 92, 129–168. [Google Scholar]
- Rice, S.A. Diffusion Limited Reactions; Elsevier: New York, NY, USA, 1985. [Google Scholar]
- Keizer, J. Diffusion Effects on Rapid Bimolecular Chemical Reactions. Chem. Rev. 1987, 87, 167–180. [Google Scholar] [CrossRef]
- Collins, F.C.; Kimball, G.E. Diffusion-controlled reaction rates. J. Colloid Sci. 1949, 4, 425–437. [Google Scholar] [CrossRef]
- Das, R.; Periasamy, N. Diffusion-Controlled Reactions: Analysis of Quenched Fluorescence Decay Data. Chem. Phys. Lett. 1989, 136, 361–378. [Google Scholar] [CrossRef]
- Scully, A.D.; Hirayama, S. Direct determination of kinetic parameters for diffusion-influenced reactions in solution by analysis of fluorescence decay curves. J. Fluoresc. 1995, 5, 107–120. [Google Scholar] [CrossRef]
- Swallen, S.F.; Weidemaier, K.; Fayer, M.D. Excluded Volume Effects in Photoinduced Electron Transfer and Geminate Recombination: Analytical Theory and Simulations. J. Phys. Chem. 1995, 99, 1856–1866. [Google Scholar] [CrossRef][Green Version]
- Matsuda, N.; Kakitani, T.; Denda, T.; Mataga, N. Examination of the Viability of the Collins-Kimball Model and Numerical Calculation of the Time-Dependent Energy Gap Law of Photoinduced Charge Separation in Polar Solution. Chem. Phys. 1995, 190, 83–95. [Google Scholar] [CrossRef]
- Murata, S.; Matsuzaki, S.Y.; Tachiya, M. Transient Effect in Fluorescence Quenching by Electron Transfer. 2: Determination of the Rate Parameters Involved in the Marcus Equation. J. Phys. Chem. 1995, 99, 5354–5358. [Google Scholar] [CrossRef]
- Shannon, C.F.; Eads, D.D. Diffusion-controlled electron transfer reactions: subpicosecond fluorescence measurements of coumarin 1 quenched by aniline and N,N-dimethylaniline. J. Chem. Phys. 1995, 103, 5208–5223. [Google Scholar] [CrossRef]
- Gladkikh, V.; Burshtein, A.; Tavernier, H.; Fayer, M. Influence of Diffusion on the Kinetics of Donor-Acceptor Electron Transfer Monitored by the Quenching of Donor Fluorescence. J. Phys. Chem. A 2002, 106, 6982–6990. [Google Scholar] [CrossRef]
- Weller, A. Photoinduced Electron Transfer in Solutions: Exciplex and Radical Ion Pair Formation Free Enthalpies and their Solvent Dependence. Z. Phys. Chem. 1982, 133, 93–98. [Google Scholar] [CrossRef]
- Rozhkov, I.N.; Igumnov, S.M.; Bekker, G.Y.; Pletnev, S.I.; Rempel, G.D.; Deev, L.E. Oxidative properties of perfluoroalkyl halides. Russ. Chem. Bull. 1989, 38, 2041–2043. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Okamoto, T.; Ohkubo, K. Diels-Alder Reactions of Anthracenes with Dienophiles via Photoinduced Electron Transfer. J. Phys. Chem. A 2003, 107, 5412–5418. [Google Scholar] [CrossRef]
- Marcus, R.A.; Sutin, N. Electron Transfer in Chemistry and Biology. Biochim. Biophys. Acta 1985, 811, 265–322. [Google Scholar] [CrossRef]
- Vauthey, E. Direct measurements of the charge recombination dynamics of geminate ion pairs formed upon electron transfer quenching at high donor concentration. J. Phys. Chem. A 2001, 105, 340–348. [Google Scholar] [CrossRef]
- Rosspeintner, A.; Angulo, G.; Vauthey, E. Bimolecular Photoinduced Electron Transfer Beyond the Diffusion Limit: The Rehm–Weller Experiment Revisited with Femtosecond Time Resolution. J. Am. Chem. Soc. 2014, 136, 2026–2032. [Google Scholar] [CrossRef]
- Castner, E.W., Jr.; Kennedy, D.; Cave, R.J. Solvent as Electron Donor: Donor/Acceptor Electronic Coupling is a Dynamical Variable. J. Phys. Chem. A 2000, 104, 2869–2885. [Google Scholar] [CrossRef]
- Angulo, G.; Cuetos, A.; Rosspeintner, A.; Vauthey, E. Experimental Evidence of the Relevance of Orientational Correlations in Photoinduced Bimolecular Reactions in Solution. J. Phys. Chem. A 2013, 117, 8814–8825. [Google Scholar] [CrossRef] [PubMed]
- Rumble, C.A.; Vauthey, E. Structural dynamics of an excited donor–acceptor complex from ultrafast polarized infrared spectroscopy, molecular dynamics simulations, and quantum chemical calculations. Phys. Chem. Chem. Phys. 2019, 21, 11797–11809. [Google Scholar] [CrossRef] [PubMed]
- Burshtein, A.I.; Yakobson, B.I. In-cage reactions controlled by molecular rotation. Chem. Phys. 1978, 28, 415–424. [Google Scholar] [CrossRef]
- Gladkikh, V.S.; Burshtein, A.I. Photoionization affected by chemical anisotropy. J. Chem. Phys. 2007, 126, 014506. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.; Huppert, D.; Agmon, N. Non-Exponential Smoluchowski Dynamics in Fast Acid-Base Reaction. J. Am. Chem. Soc. 2000, 122, 9838–9839. [Google Scholar] [CrossRef]
- Cohen, B.; Huppert, D.; Agmon, N. Diffusion-Limited Acid-Base Nonexponential Dynamics. J. Phys. Chem. A 2001, 105, 7165–7173. [Google Scholar] [CrossRef]
- Rini, M.; Pines, D.; Magnes, B.-Z.; Pines, E.; Nibbering, E.T. Bimodal proton transfer in acid-base reactions in water. J. Chem. Phys. 2004, 121, 9593–9610. [Google Scholar] [CrossRef]
- Janiga, A.; Glodkowska-Mrowka, E.; Stoklosa, T.; Gryko, D.T. Synthesis and Optical Properties of Tetraaryl-1,4-dihydropyrrolo[3,2-b]pyrroles. Asian J. Org. Chem. 2013, 2, 411–415. [Google Scholar] [CrossRef]
- Gardecki, J.A.; Maroncelli, M. Set of Secondary Emission Standard for Calibration of the Spectral Responsivity in Emission Spectroscopy. Appl. Spectrosc. 1998, 52, 1179–1189. [Google Scholar] [CrossRef]
- Angulo, G.; Brucka, M.; Gerecke, M.; Grampp, G.; Jeannerat, D.; Milkiewicz, J.; Mitrev, Y.; Radzewicz, C.; Rosspeintner, A.; Vauthey, E.; et al. Characterization of dimethylsulfoxide/glycerol mixtures: A binary solvent system for the study of “friction-dependent” chemical reactivity. Phys. Chem. Chem. Phys. 2016, 18, 18460–18469. [Google Scholar] [CrossRef]
- Muller, P.-A.; Högemann, C.; Allonas, X.; Jacques, P.; Vauthey, E. Deuterium Isotope Effect on the Charge Recombination Dynamics of Contact Ion Pairs Formed by Electron Transfer Quenching in Acetonitrile. Chem. Phys. Lett. 2000, 326, 321–327. [Google Scholar] [CrossRef][Green Version]
Sample Availability: Sample not available. |
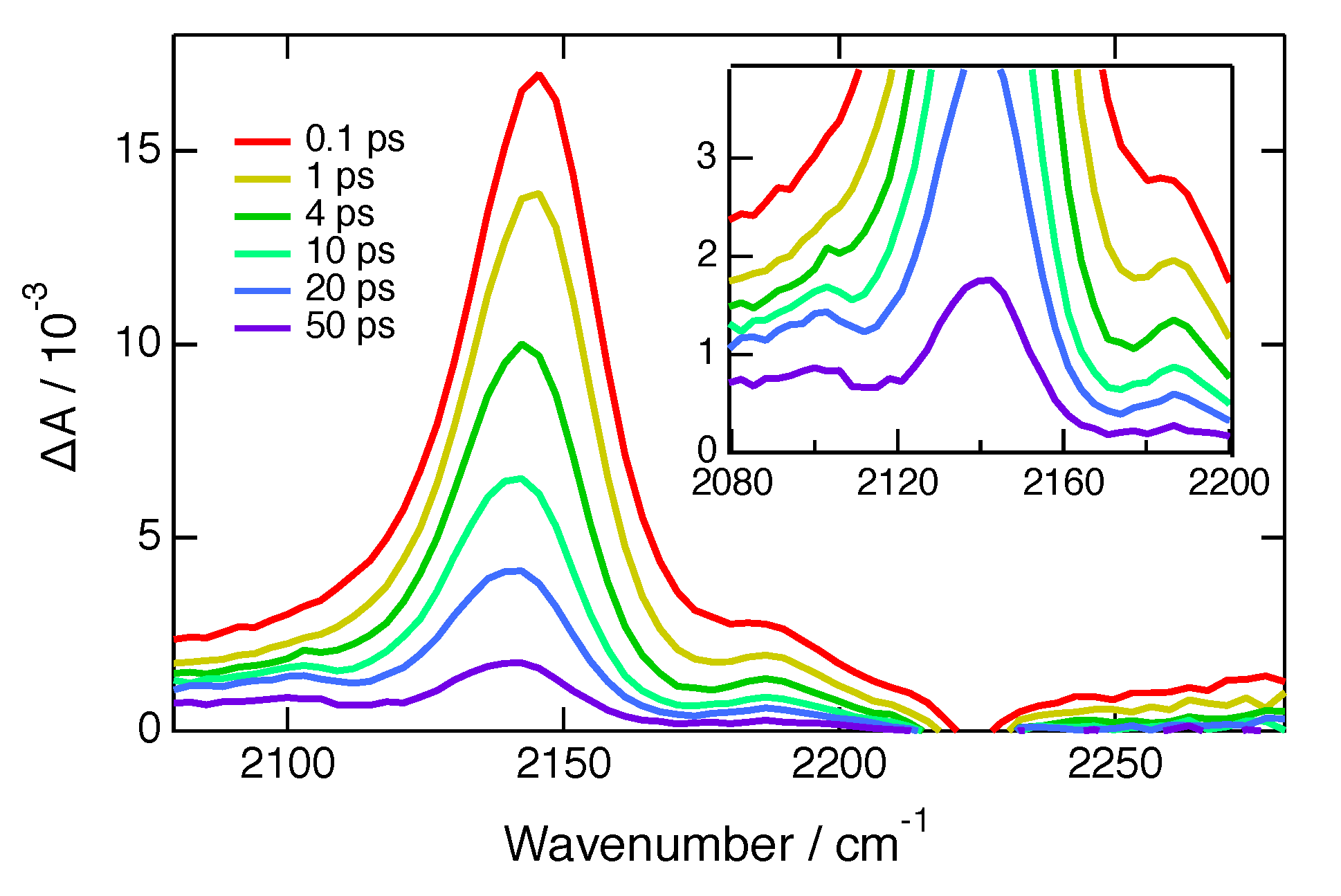
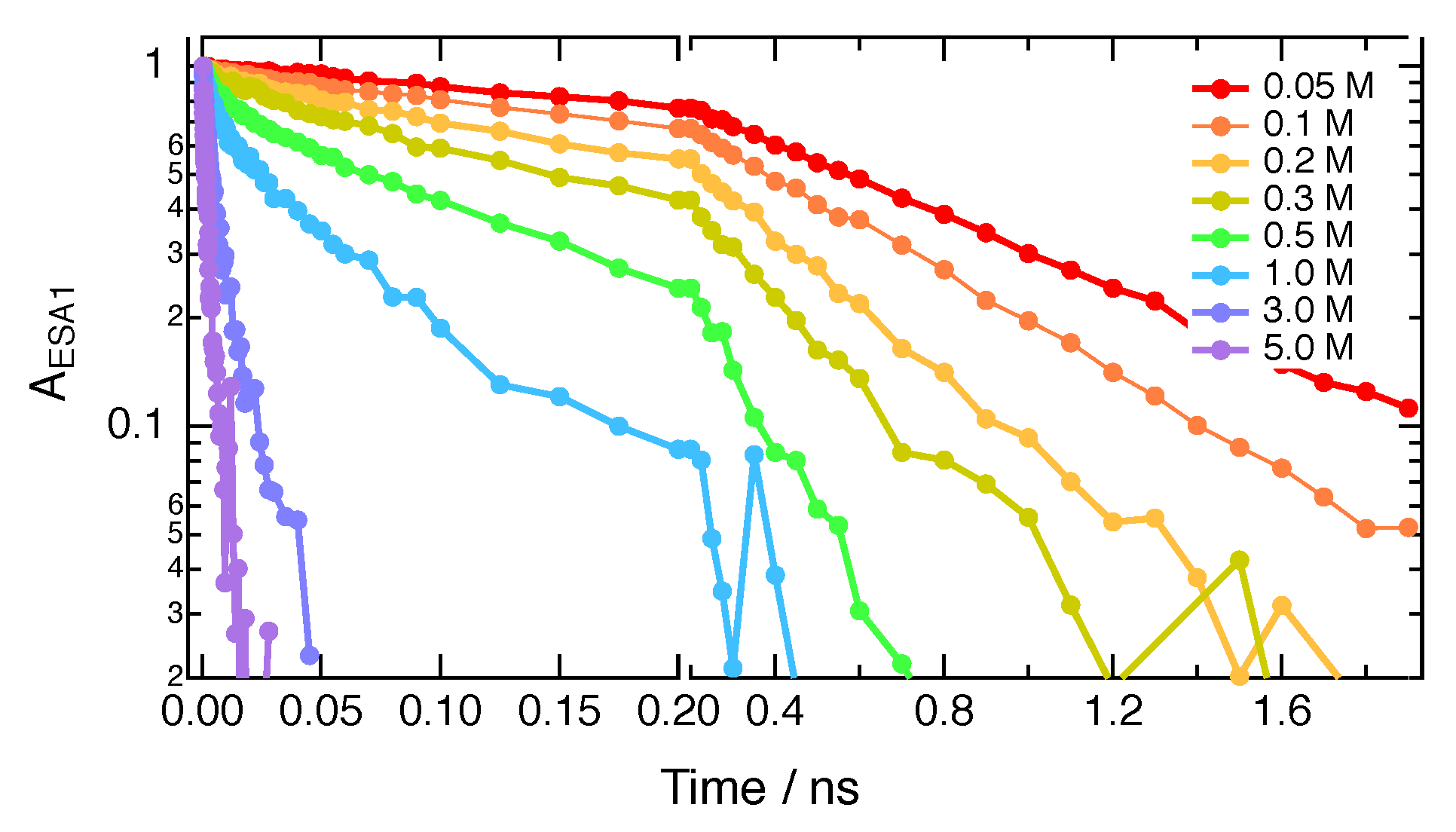
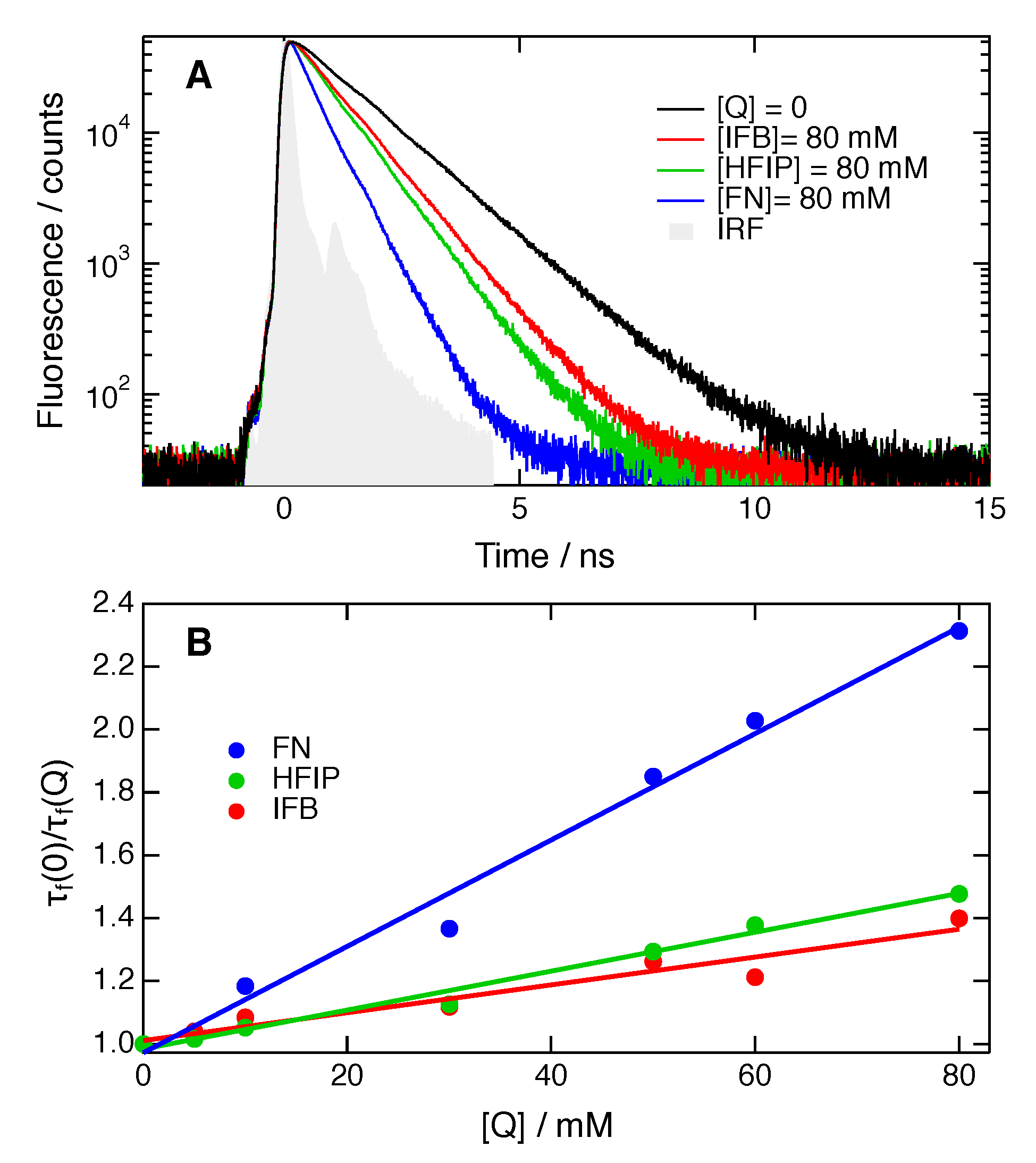
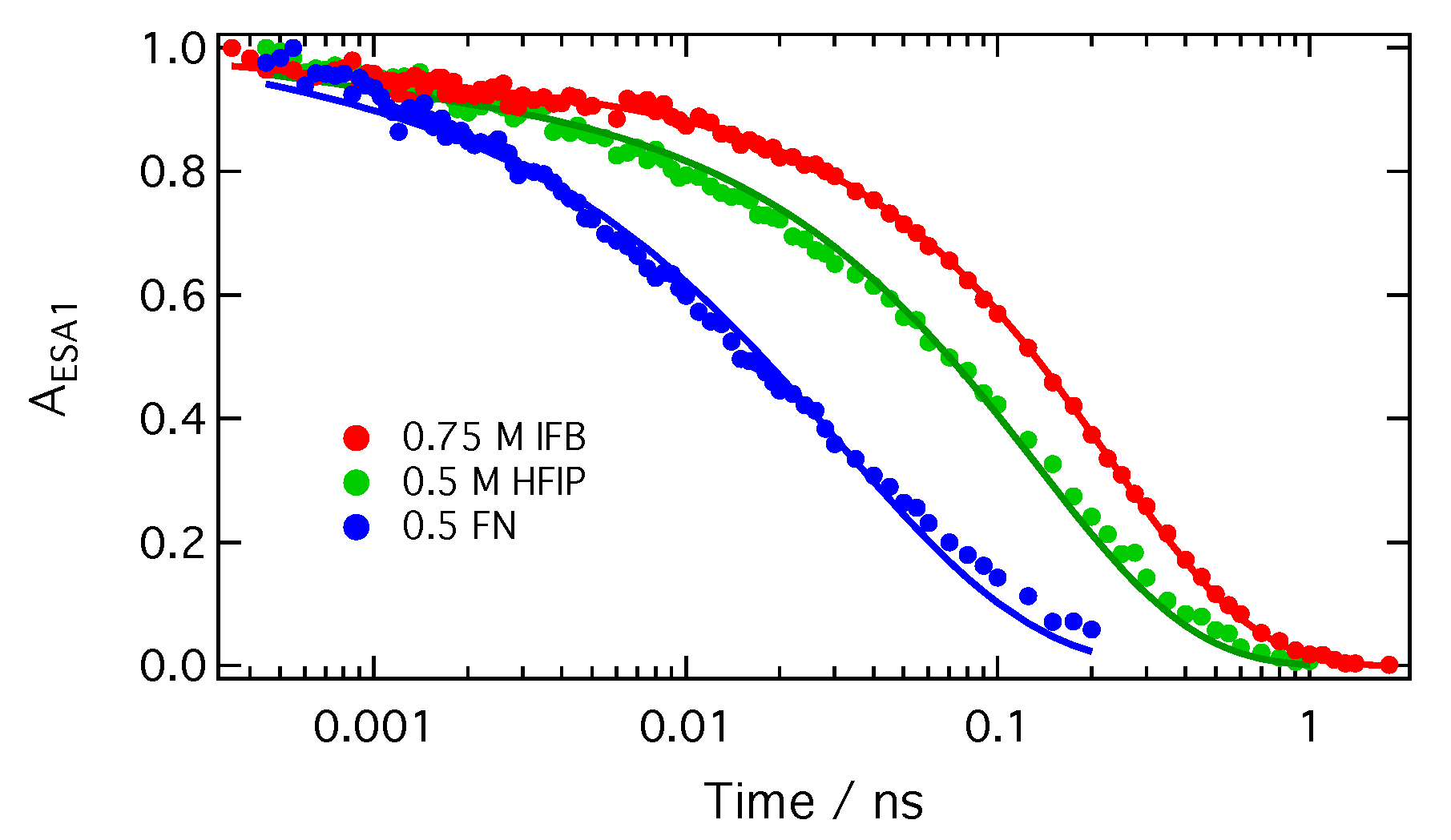
| Solvent | /M−1ns−1 | |||
|---|---|---|---|---|
| CHCl3 | 4.8 | 12 | 0.78 | 1.3 ns |
| BCN | 25.2 | 5.4 | 0.81 | 1.4 ns |
| HFIP | 8–14 | 1.5 ps | ||
| IFB | 5.6 | 12 ps |
| Quencher | Solvent | /M−1ns−1 | /M−1ns−1 | R Å | [Q] M | D Å2 ns−1 |
|---|---|---|---|---|---|---|
| HFIP | CHCl3 | 4.7 ± 0.2 | 150 ± 30 | 4.7 ± 0.2 | 0.4–1.0 | 246–242 |
| IFB | 3.4 ± 0.4 | 20 ± 13 | 3.5 ± 0.3 | 0.3–3.0 | 210–175 | |
| FN | 12.8 ± 0.6 | 220 ± 20 | 9.0 ± 1.0 | 0.1–1.0 | 260–240 | |
| HFIP | BCN | 3.3 ± 0.4 | 150 ± 25 | 6.0 ± 0.3 | 0.1–0.5 | 107–110 |
| IFB | 3.3 ± 0.2 | 25 ± 15 | 5.0 ± 0.2 | 0.3–3.0 | 93–85 | |
| FN | 6.9 ± 0.4 | – | – | – |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dereka, B.; Fureraj, I.; Rosspeintner, A.; Vauthey, E. Halogen-Bond Assisted Photoinduced Electron Transfer. Molecules 2019, 24, 4361. https://doi.org/10.3390/molecules24234361
Dereka B, Fureraj I, Rosspeintner A, Vauthey E. Halogen-Bond Assisted Photoinduced Electron Transfer. Molecules. 2019; 24(23):4361. https://doi.org/10.3390/molecules24234361
Chicago/Turabian StyleDereka, Bogdan, Ina Fureraj, Arnulf Rosspeintner, and Eric Vauthey. 2019. "Halogen-Bond Assisted Photoinduced Electron Transfer" Molecules 24, no. 23: 4361. https://doi.org/10.3390/molecules24234361
APA StyleDereka, B., Fureraj, I., Rosspeintner, A., & Vauthey, E. (2019). Halogen-Bond Assisted Photoinduced Electron Transfer. Molecules, 24(23), 4361. https://doi.org/10.3390/molecules24234361