Dual Insecticidal Effects of Adenanthera pavonina Kunitz-Type Inhibitor on Plodia interpunctella is Mediated by Digestive Enzymes Inhibition and Chitin-Binding Properties
Abstract
Highlights
- ApKTI increases mortality of P. interpunctella larvae.
- In vitro analyses showed that ApKTI presents chitin-binding properties.
- In silico structural studies corroborated protein-protein and protein–carbohydrate interactions.
1. Introduction
2. Results
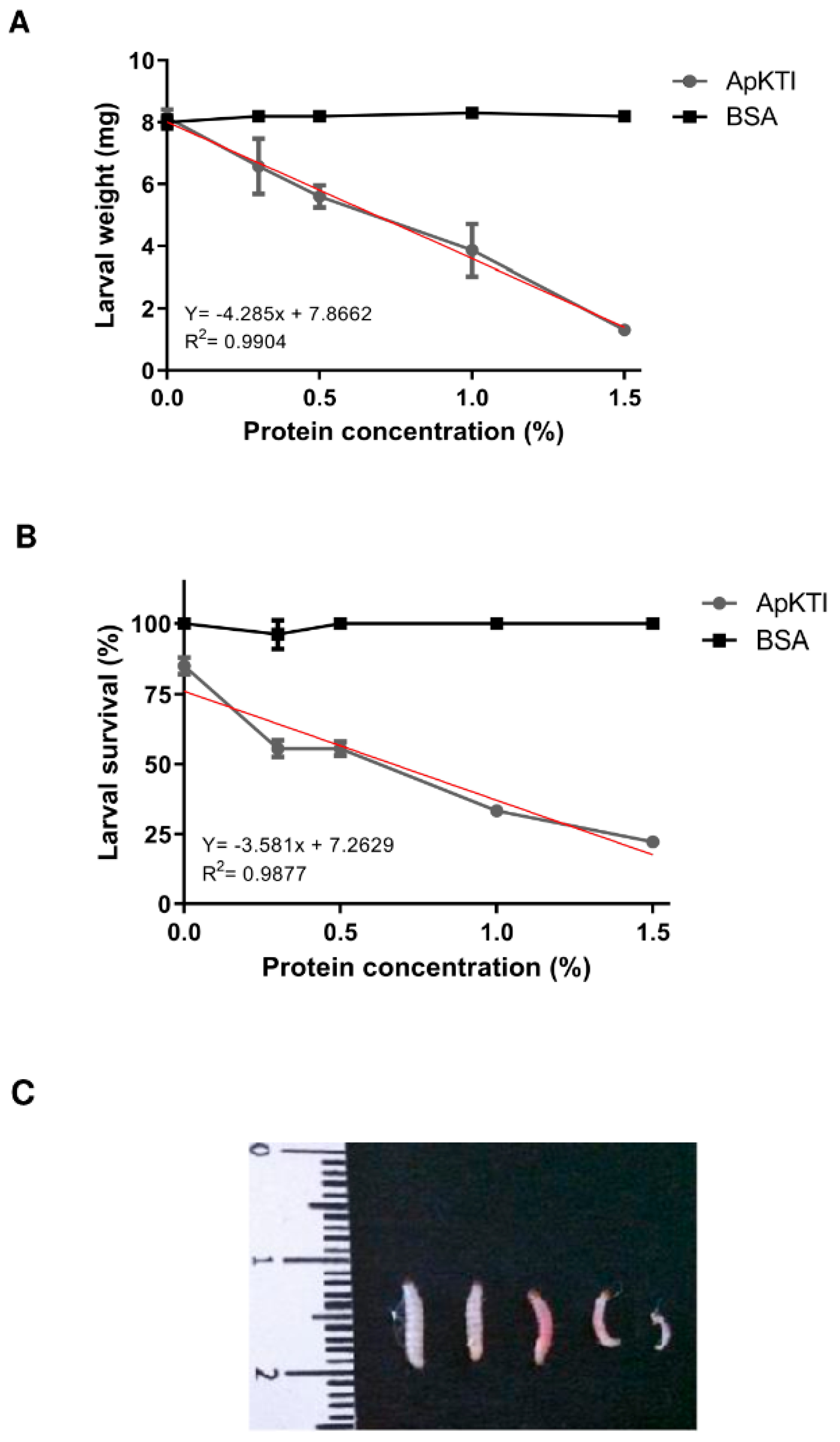
2.1. Bioassays with P. Interpunctella Larvae
2.2. Purification of Trypsin Inhibitor from Adenanthera Pavonina Seeds (ApKTI)
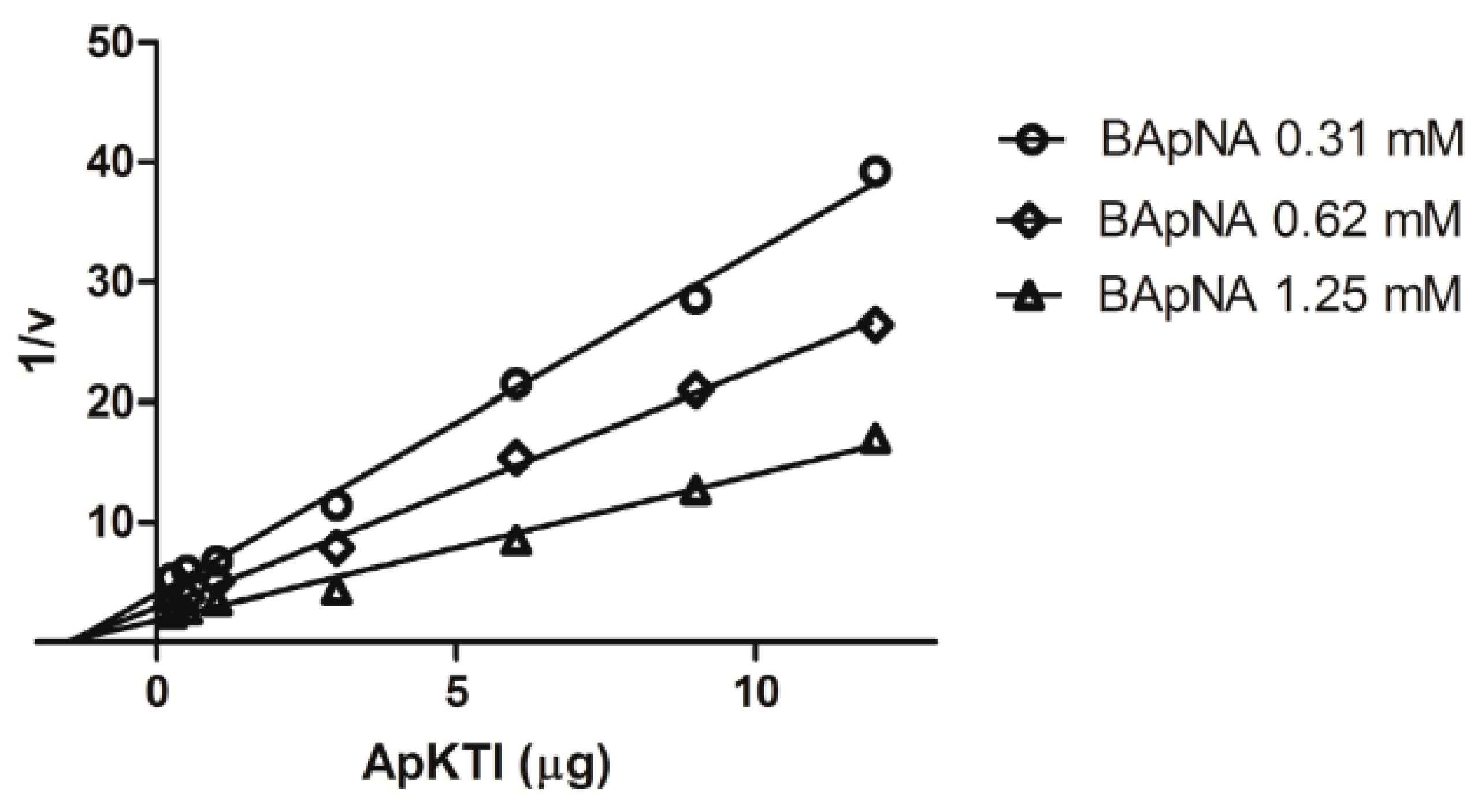
2.3. In Vitro Enzymatic Assays
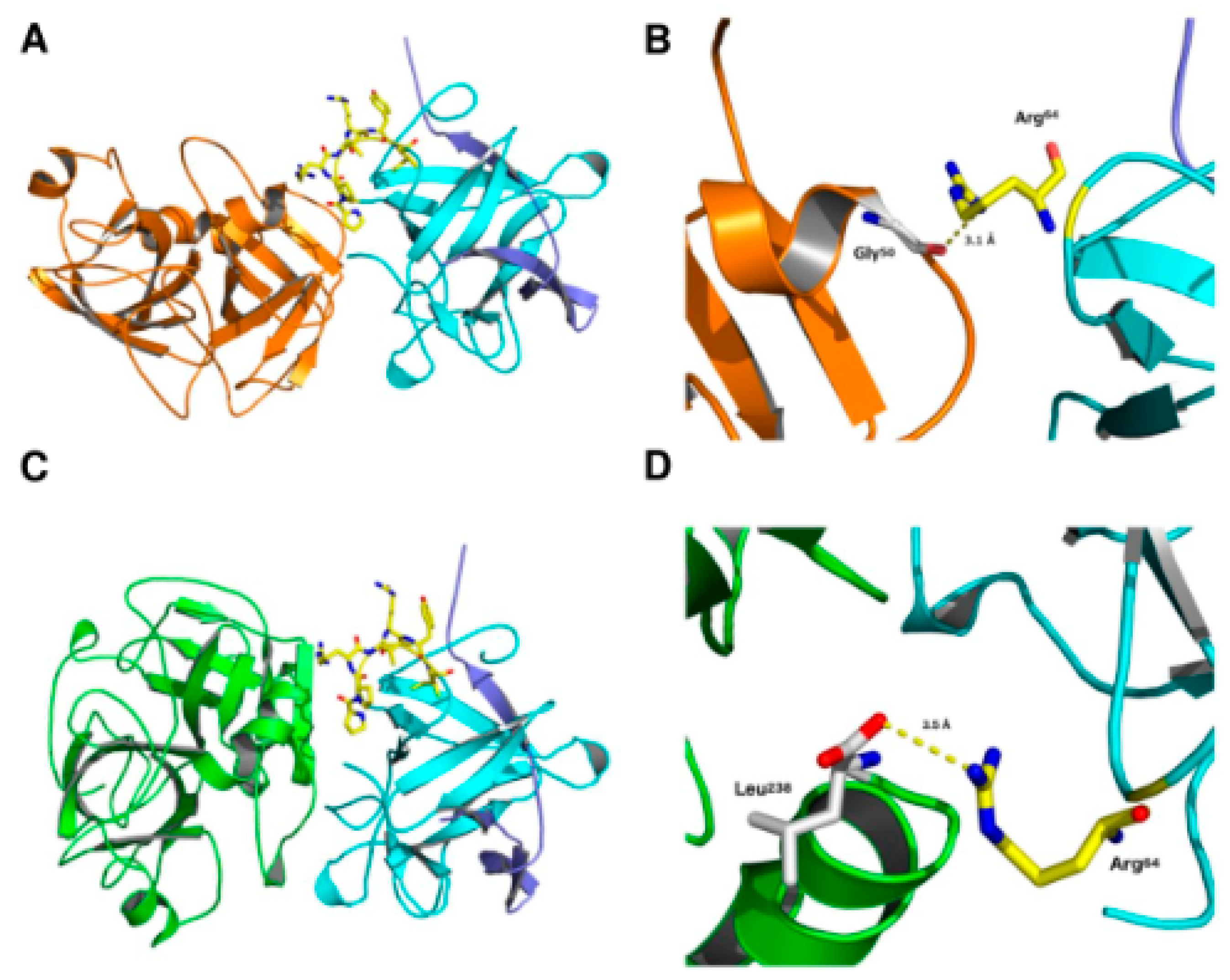
2.4. Structural Studies
2.5. Structural Studies
3. Discussion
4. Material and Methods
4.1. Chemicals
4.2. Insects
4.3. Bioassays with Plodia Interpunctella Larvae
4.4. Purification of Trypsin Inhibitor from Adenanthera Pavonina Seeds (ApKTI)
4.5. Chromatography in Chitin Column
4.6. Kinetic Studies Between ApKTI and P. Interpunctella Trypsin
4.7. In Vitro Evaluation of Inhibitory Activity
4.7.1. Inhibitory Activity for Bovine Trypsin
4.7.2. Insect Gut Peptidases
4.8. Molecular Modeling
4.9. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Santamaria, M.E.; Cambra, I.; Martinez, M.; Pozancos, C.; González-Melendi, P.; Grbic, V.; Castañera, P.; Ortego, F.; Diaz, I. Gene pyramiding of peptidase inhibitors enhances plant resistance to the spider mite Tetranychus urticae. PLoS ONE 2012, 7, e43011. [Google Scholar] [CrossRef] [PubMed]
- Le Goff, G.; Giraudo, M. Effects of Pesticides on the Environment and Insecticide Resistance. In Olfactory Concepts of Insect Control-Alternative to insecticides; Springer: Heidelberg, Germany, 2019; pp. 51–78. [Google Scholar]
- Cui, J.; Luo, J.; Van Der Werf, W.; Ma, Y.; Xia, J. Effect of pyramiding Bt and CpTI genes on resistance of cotton to Helicoverpa armigera (Lepidoptera: Noctuidae) under laboratory and field conditions. J. Econ. Entomol. 2011, 104, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, R.; Cheng, C.P.; Yeh, K.W. Genetically pyramiding protease-inhibitor genes for dual broad-spectrum resistance against insect and phytopathogens in transgenic tobacco. Plant Biotechnol. J. 2010, 8, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Amorim, T.M.; Macedo, L.L.; Uchoa, A.F.; Oliveira, A.S.; Pitanga, J.C.; Macedo, F.P.; Santos, E.A.; de Sales, M.P. Proteolytic digestive enzymes and peritrophic membranes during the development of Plodia interpunctella (Lepidoptera: Piralidae): Targets for the action of soybean trypsin inhibitor (SBTI) and chitin-binding vicilin (EvV). J. Agric. Food Chem. 2008, 56, 7738–7745. [Google Scholar] [CrossRef]
- Lopes, A.R.; Sato, P.M.; Terra, W.R. Insect chymotrypsins: Chloromethyl ketone inactivation and substrate specificity relative to possible coevolutional adaptation of insects and plants. Arch. Insect Biochem. Physiol. 2009, 70, 188–203. [Google Scholar] [CrossRef]
- Terra, W.R.; Ferreira, C. Insect digestive enzymes: Properties, compartmentalization and function. Comp. Biochem. Physiol. B Comp. Biochem. 1994, 109, 1–62. [Google Scholar] [CrossRef]
- Gatehouse, J.A. Plant resistance towards insect herbivores: A dynamic interaction. New Phytologist 2002, 156, 145–169. [Google Scholar] [CrossRef]
- Macedo, M.L.R.; Sá, C.M.; Freire, M.G.M.; Parra, J.R.P. A Kunitz-type inhibitor of coleopteran proteases, isolated from Adenanthera pavonina L. seeds and its effect on Callosobruchus maculatus. J. Agric. Food Chem. 2004, 52, 2533–2540. [Google Scholar] [CrossRef]
- Boulter, D.; Gatehouse, A.; Hilder, V. Use of cowpea trypsin inhibitor (CpTI) to protect plants against insect predation. Biotechnol. Adv. 1989, 7, 489–497. [Google Scholar] [CrossRef]
- Dunse, K.; Stevens, J.; Lay, F.; Gaspar, Y.; Heath, R.; Anderson, M. Coexpression of potato type I and II proteinase inhibitors gives cotton plants protection against insect damage in the field. Proc. Natl. Acad. Sci. USA 2010, 107, 15011–15015. [Google Scholar] [CrossRef]
- Stevens, J.; Dunse, K.; Guarino, R.; Barbeta, B.; Evans, S.; West, J.; Anderson, M. The impact of ingested potato type II inhibitors on the production of the major serine proteases in the gut of Helicoverpa armigera. Insect Biochem. Mol. Biol. 2013, 43, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Macedo, M.L.R.; Mello, G.C.; Freire, M.G.M.; Novello, J.C.; Marangoni, S.; de Matos, D.G. Effect of a trypsin inhibitor from Dimorphandra mollis seeds on the development of Callosobruchus maculatus. Plant Physiol. Biochem. 2002, 40, 891–898. [Google Scholar] [CrossRef]
- Richardson, M.; Campos, F.; Xavier-Filho, J.; Macedo, M.; Maia, G.; Yarwood, A. The amino acid sequence and reactive (inhibitory) site of the major trypsin isoinhibitor (DE5) isolated from seeds of the Brazilian Carolina tree (Adenanthera pavonina L.). Biochim. Biophys. Acta Protein Struc. Mol. Enzymol. 1986, 872, 134–140. [Google Scholar] [CrossRef]
- Migliolo, L.; de Oliveira, A.S.; Santos, E.A.; Franco, O.L.; Maurício, P. Structural and mechanistic insights into a novel non-competitive Kunitz trypsin inhibitor from Adenanthera pavonina L. seeds with double activity toward serine-and cysteine-proteinases. J. Mol. Grap. Model. 2010, 29, 148–156. [Google Scholar] [CrossRef]
- Macedo, M.L.R.; Durigan, R.A.; da Silva, D.S.; Marangoni, S.; Freire, M.d.G.M.; Parra, J.R.P. Adenanthera pavonina trypsin inhibitor retard growth of Anagasta kuehniella (Lepidoptera: Pyralidae). Arch. Insect Biochem. Physiol. 2010, 73, 213–231. [Google Scholar]
- da Silva, W.; Freire, M.d.G.M.; Parra, J.R.P.; Marangoni, S.; Macedo, M.L.R. Evaluation of the Adenanthera pavonina seed proteinase inhibitor (ApTI) as a bioinsecticidal tool with potential for the control of Diatraea saccharalis. Process Biochem. 2012, 47, 257–263. [Google Scholar] [CrossRef]
- Sasaki, D.Y.; Jacobowski, A.C.; de Souza, A.P.; Cardoso, M.H.; Franco, O.L.; Macedo, M.L.R. Effects of proteinase inhibitor from Adenanthera pavonina seeds on short-and long term larval development of Aedes aegypti. Biochimie 2015, 112, 172–186. [Google Scholar] [CrossRef]
- Prabhu, K.S.; Pattabiraman, T.N. Natural plant enzyme inhibitors. Isolation and characterisation of a trypsin/chymotrypsin inhibitor from indian red wood (Adenanthera pavonia) seeds. J. Sci. Food Agric. 1980, 31, 967–980. [Google Scholar] [CrossRef]
- Wiederstein, M.; Sippl, M.J. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007, 35, W407–W410. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Rullmann, J.A.C.; MacArthur, M.W.; Kaptein, R.; Thornton, J.M. AQUA and PROCHECK-NMR: Programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR 1996, 8, 477–486. [Google Scholar] [CrossRef]
- Chen, V.B.; Arendall, W.B.; Headd, J.J.; Keedy, D.A.; Immormino, R.M.; Kapral, G.J.; Murray, L.W.; Richardson, J.S.; Richardson, D.C. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Cryst. D 2010, 66, 12–21. [Google Scholar] [CrossRef]
- Boaventura, D.; Bolzan, A.; Padovez, F.E.; Okuma, D.M.; Omoto, C.; Nauen, R. Detection of a ryanodine receptor target-site mutation in diamide insecticide resistant fall armyworm, Spodoptera frugiperda. Pest Manag. Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Gassmann, A.J.; Crowder, D.W.; Carrière, Y. Insect resistance to Bt crops: Evidence versus theory. Nat. Biotechnol. 2008, 26, 199. [Google Scholar] [CrossRef] [PubMed]
- Hilder, V.A.; Gatehouse, A.M.; Boulter, D. Transgenic plants conferring insect tolerance: Protease inhibitor approach. In Transgenic plants. Engineering and Utilization; Academic Press: San Diego, CA, USA, 1993; pp. 317–338. [Google Scholar]
- Hilder, V.; Powell, K.; Gatehouse, A.; Gatehouse, J.; Gatehouse, L.; Shi, Y.; Hamilton, W.; Merryweather, A.; Newell, C.; Timans, J. Expression of snowdrop lectin in transgenic tobacco plants results in added protection against aphids. Transgenic Res. 1995, 4, 18–25. [Google Scholar] [CrossRef]
- Jongsma, M.A.; Beekwilder, J. Co-evolution of insect proteases and plant protease inhibitors. Curr. Protein Pep. Sci. 2011, 12, 437–447. [Google Scholar] [CrossRef]
- Habib, H.; Fazili, K.M. Plant protease inhibitors: A defense strategy in plants. Biotechnol. Mol. Biol. Rev. 2007, 2, 68–85. [Google Scholar]
- Oliva, M.L.V.; Silva, M.C.; Sallai, R.C.; Brito, M.V.; Sampaio, M.U. A novel subclassification for Kunitz proteinase inhibitors from leguminous seeds. Biochimie 2010, 92, 1667–1673. [Google Scholar] [CrossRef]
- Macedo, M.L.; de Oliveira, C.F.; Costa, P.M.; Castelhano, E.C.; Silva-Filho, M.C. Adaptive mechanisms of insect pests against plant protease inhibitors and future prospects related to crop protection: A review. Protein Pep. Lett. 2015, 22, 149–163. [Google Scholar] [CrossRef]
- Kuwar, S.S.; Pauchet, Y.; Vogel, H.; Heckel, D.G. Adaptive regulation of digestive serine proteases in the larval midgut of Helicoverpa armigera in response to a plant protease inhibitor. Insect Biochem. Mol. Biol. 2015, 59, 18–29. [Google Scholar] [CrossRef]
- Oliveira, C.F.R.; de Paula Souza, T.; Parra, J.R.P.; Marangoni, S.; de Castro Silva-Filho, M.; Macedo, M.L.R. Insensitive trypsins are differentially transcribed during Spodoptera frugiperda adaptation against plant protease inhibitors. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2013, 165, 19–25. [Google Scholar] [CrossRef]
- Bown, D.P.; Wilkinson, H.S.; Gatehouse, J.A. Regulation of expression of genes encoding digestive proteases in the gut of a polyphagous lepidopteran larva in response to dietary protease inhibitors. Physiol. Entomol. 2004, 29, 278–290. [Google Scholar] [CrossRef]
- Sales, M.P.; Pimenta, P.P.; Paes, N.S.; Grossi-de-Sá, M.F.; Xavier-Filho, J. Vicilins (7S storage globulins) of cowpea (Vigna unguiculata) seeds bind to chitinous structures of the midgut of Callosobruchus maculatus (Coleoptera: Bruchidae) larvae. Braz. J. Med. Biol. Res. 2001, 34, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Park, S.-C.; Hwang, I.; Cheong, H.; Nah, J.-W.; Hahm, K.-S.; Park, Y. Protease inhibitors from plants with antimicrobial activity. Int. J. Mol. Sci. 2009, 10, 2860–2872. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Babu, C.R. Purification and biochemical characterization of a serine proteinase inhibitor from Derris trifoliata Lour. seeds: Insight into structural and antimalarial features. Phytochemistry 2009, 70, 703–712. [Google Scholar] [CrossRef]
- Freire, M.G.M.; Franco, O.L.; Kubo, C.E.G.; Migliolo, L.; Vargas, R.H.; de Oliveira, C.F.R.; Parra, J.R.P.; Macedo, M.L.R. Structural insights regarding an insecticidal Talisia esculenta protein and its biotechnological potential for Diatraea saccharalis larval control. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2012, 161, 86–92. [Google Scholar] [CrossRef]
- Oliveira, C.F.R.; Marangoni, S.; Macedo, M.L.R. The trypsin inhibitor from Entada acaciifolia seeds affects negatively the development of Mediterranean flour moth, Anagasta kuehniella. Pestic. Biochem. Physiol. 2014, 108, 74–79. [Google Scholar] [CrossRef]
- Sumikawa, J.T.; Brito, M.V.d.; Macedo, M.L.R.; Uchoa, A.F.; Miranda, A.; Araujo, A.P.U.; Silva-Lucca, R.A.; Sampaio, M.U.; Oliva, M.L.V. The defensive functions of plant inhibitors are not restricted to insect enzyme inhibition. Phytochemistry 2010, 71, 214–220. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Leighton, S.M.; Babu, C. Bioinsecticidal activity of Archidendron ellipticum trypsin inhibitor on growth and serine digestive enzymes during larval development of Spodoptera litura. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 145, 669–677. [Google Scholar] [CrossRef]
- Zhu-Salzman, K.; Zeng, R. Insect response to plant defensive protease inhibitors. Annu. Rev. Entomol. 2015, 60, 233–252. [Google Scholar] [CrossRef]
- Souza, T.P.; Dias, R.O.; Castelhano, E.C.; Brandao, M.M.; Moura, D.S.; Silva-Filho, M.C. Comparative analysis of expression profiling of the trypsin and chymotrypsin genes from Lepidoptera species with different levels of sensitivity to soybean peptidase inhibitors. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2016, 196, 67–73. [Google Scholar] [CrossRef]
- Dias, R.O.; Via, A.; Brandão, M.M.; Tramontano, A.; Silva-Filho, M.C. Digestive peptidase evolution in holometabolous insects led to a divergent group of enzymes in Lepidoptera. Insect Biochem. Mol. Biol. 2015, 58, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.; Juliano, M.; Marana, S.; Juliano, L.; Terra, W. Substrate specificity of insect trypsins and the role of their subsites in catalysis. Insect Biochem. Mol. Biol. 2006, 36, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.R.; Juliano, M.A.; Juliano, L.; Terra, W.R. Coevolution of insect trypsins and inhibitors. Arch. Insect Biochem. Physiol. 2004, 55, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Sweet, R.; Wright, H.; Janin, J.; Chothia, C.; Blow, D. Crystal structure of the complex of porcine trypsin with soybean trypsin inhibitor (Kunitz) at 2.6 Å resolution. Biochemistry 1974, 13, 4212–4228. [Google Scholar] [CrossRef] [PubMed]
- Song, H.K.; Suh, S.W. Kunitz-type soybean trypsin inhibitor revisited: Refined structure of its complex with porcine trypsin reveals an insight into the interaction between a homologous inhibitor from Erythrina caffra and tissue-type plasminogen activator. J. Mol. Biol. 1998, 275, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Macedo, M.L.R.; Freire, M.G.M.; Franco, O.L.; Migliolo, L.; Oliveira, C.F.R. Practical and theoretical characterization of Inga laurina Kunitz inhibitor on the control of Homalinotus coriaceus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2011, 158, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Sattar, R.; Ali, S.A.; Kamal, M.; Khan, A.A.; Abbasi, A. Molecular mechanism of enzyme inhibition: Prediction of the three-dimensional structure of the dimeric trypsin inhibitor from Leucaena leucocephala by homology modelling. Biochem. Biophys. Res. Commun. 2004, 314, 755–765. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Erlanger, B.F.; Kokowsky, N.; Cohen, W. The preparation and properties of two new chromogenic substrates of trypsin. Arch. Biochem. Biophys. 1961, 95, 271–278. [Google Scholar] [CrossRef]
- Eswar, N.; Webb, B.; Marti-Renom, M.A.; Madhusudhan, M.; Eramian, D.; Shen, M.y.; Pieper, U.; Sali, A. Comparative protein structure modeling using Modeller. Curr. Protoc. Bioinformatics 2006, 15, 5.6.1–5.6.30. [Google Scholar] [CrossRef]
- Zhu, Y.; Oppert, B.; Kramer, K.; McGaughey, W.; Dowdy, A. cDNA sequence, mRNA expression and genomic DNA of trypsinogen from the indianmeal moth, Plodia interpunctella. Insect Mol. Biol. 2000, 9, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-C.; Oppert, B.; Kramer, K.J.; McGaughey, W.H.; Dowdy, A.K. cDNAs for a chymotrypsinogen-like protein from two strains of Plodia interpunctella. Insect Biochem. Mol. Biol. 1997, 27, 1027–1037. [Google Scholar] [CrossRef]
- Käll, L.; Krogh, A.; Sonnhammer, E.L. Advantages of combined transmembrane topology and signal peptide prediction—the Phobius web server. Nucleic Acids Res. 2007, 35, W429–W432. [Google Scholar] [CrossRef] [PubMed]
- Botos, I.; Meyer, E.; Nguyen, M.; Swanson, S.M.; Koomen, J.M.; Russell, D.H.; Meyer, E.F. The structure of an insect chymotrypsin. J. Mol. Biol. 2000, 298, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Aboitiz, N.; Vila-Perelló, M.; Groves, P.; Asensio, J.L.; Andreu, D.; Cañada, F.J.; Jiménez-Barbero, J. NMR and modeling studies of protein–carbohydrate interactions: Synthesis, three-dimensional structure, and recognition properties of a minimum hevein domain with binding affinity for chitooligosaccharides. ChemBioChem 2004, 5, 1245–1255. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Guex, N.; Peitsch, M.C. SWISS-MODEL and the Swiss-Pdb Viewer: An environment for comparative protein modeling. Electrophoresis 1997, 18, 2714–2723. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| Predicted Structures | Sequence Length | Fold Quality (z-Score) | Stereochemistry (G-Factors) | Ramachandran | Bad Bonds (%) | Bad Angles (%) | ||
|---|---|---|---|---|---|---|---|---|
| Most Favored (%) | Allowed (%) | Outliers (%) | ||||||
| ApKTI | 176 | −4.80 | −0.26 | 89.00 | 95.9 | 4.07 | 0 | 1.09 |
| Trypsin | 233 | −6.52 | −0.23 | 90.48 | 96.1 | 3.90 | 0 | 1.87 |
| Chymotrypsin | 238 | −6.47 | −0.24 | 91.50 | 97.9 | 2.12 | 0 | 1.70 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, C.F.R.; de Oliveira Flores, T.M.; Henrique Cardoso, M.; Garcia Nogueira Oshiro, K.; Russi, R.; de França, A.F.J.; dos Santos, E.A.; Luiz Franco, O.; de Oliveira, A.S.; Migliolo, L. Dual Insecticidal Effects of Adenanthera pavonina Kunitz-Type Inhibitor on Plodia interpunctella is Mediated by Digestive Enzymes Inhibition and Chitin-Binding Properties. Molecules 2019, 24, 4344. https://doi.org/10.3390/molecules24234344
de Oliveira CFR, de Oliveira Flores TM, Henrique Cardoso M, Garcia Nogueira Oshiro K, Russi R, de França AFJ, dos Santos EA, Luiz Franco O, de Oliveira AS, Migliolo L. Dual Insecticidal Effects of Adenanthera pavonina Kunitz-Type Inhibitor on Plodia interpunctella is Mediated by Digestive Enzymes Inhibition and Chitin-Binding Properties. Molecules. 2019; 24(23):4344. https://doi.org/10.3390/molecules24234344
Chicago/Turabian Stylede Oliveira, Caio Fernando Ramalho, Taylla Michelle de Oliveira Flores, Marlon Henrique Cardoso, Karen Garcia Nogueira Oshiro, Raphael Russi, Anderson Felipe Jácome de França, Elizeu Antunes dos Santos, Octávio Luiz Franco, Adeliana Silva de Oliveira, and Ludovico Migliolo. 2019. "Dual Insecticidal Effects of Adenanthera pavonina Kunitz-Type Inhibitor on Plodia interpunctella is Mediated by Digestive Enzymes Inhibition and Chitin-Binding Properties" Molecules 24, no. 23: 4344. https://doi.org/10.3390/molecules24234344
APA Stylede Oliveira, C. F. R., de Oliveira Flores, T. M., Henrique Cardoso, M., Garcia Nogueira Oshiro, K., Russi, R., de França, A. F. J., dos Santos, E. A., Luiz Franco, O., de Oliveira, A. S., & Migliolo, L. (2019). Dual Insecticidal Effects of Adenanthera pavonina Kunitz-Type Inhibitor on Plodia interpunctella is Mediated by Digestive Enzymes Inhibition and Chitin-Binding Properties. Molecules, 24(23), 4344. https://doi.org/10.3390/molecules24234344