Effect of the Titanium Isopropoxide:Acetylacetone Molar Ratio on the Photocatalytic Activity of TiO2 Thin Films
Abstract
1. Introduction
2. Results and Discussion
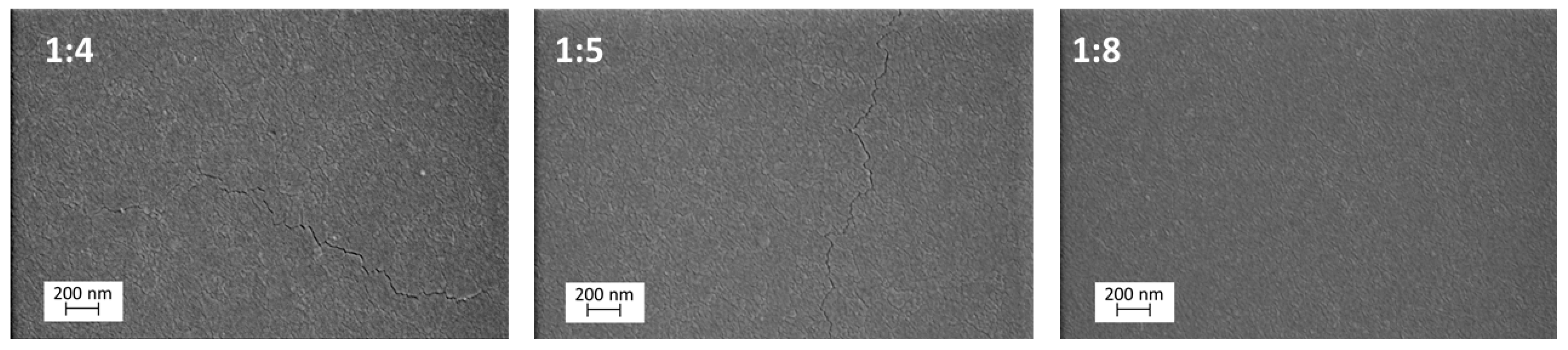
2.1. Surface Morphology
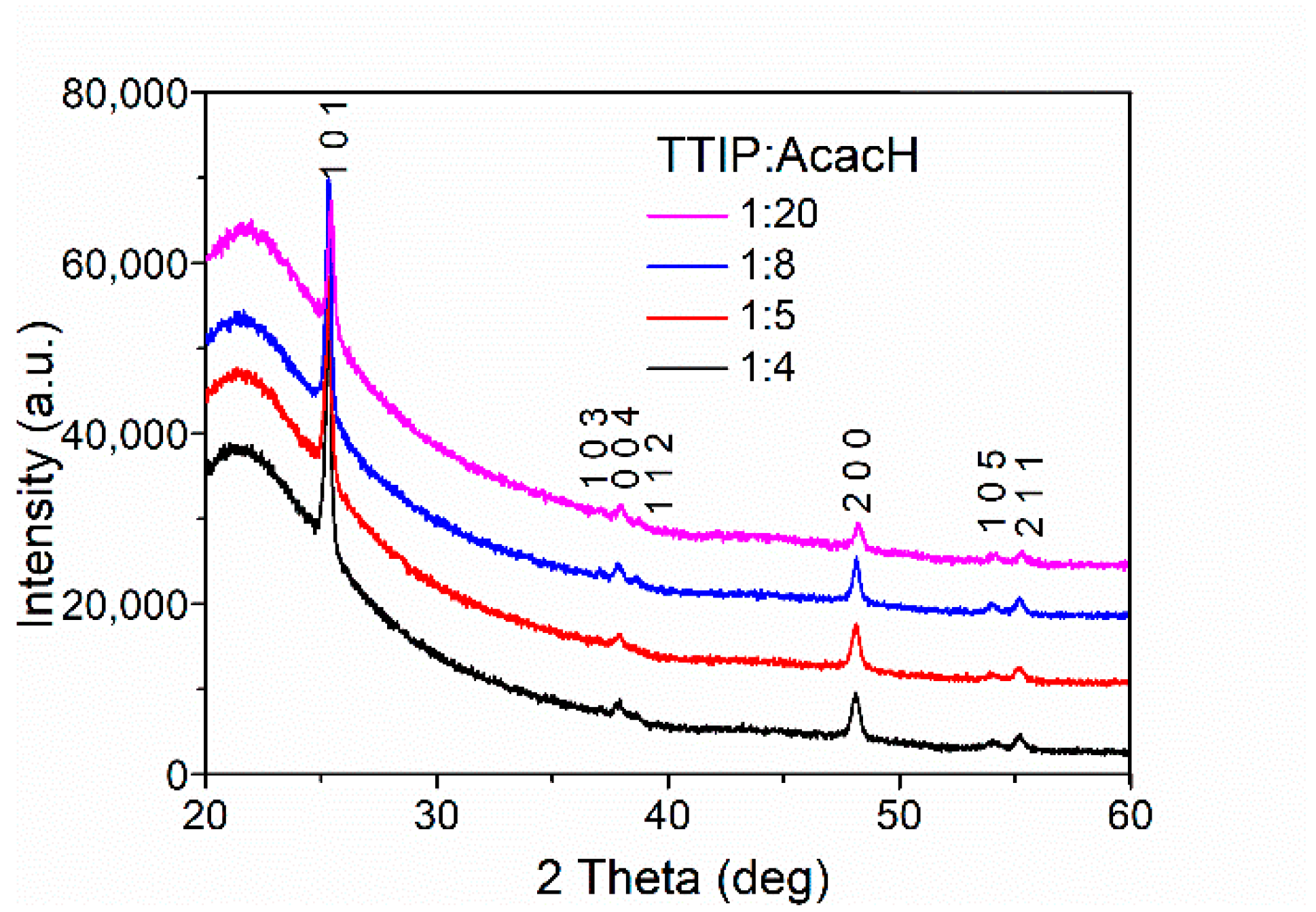
2.2. Structural Properties
2.3. Optical Properties
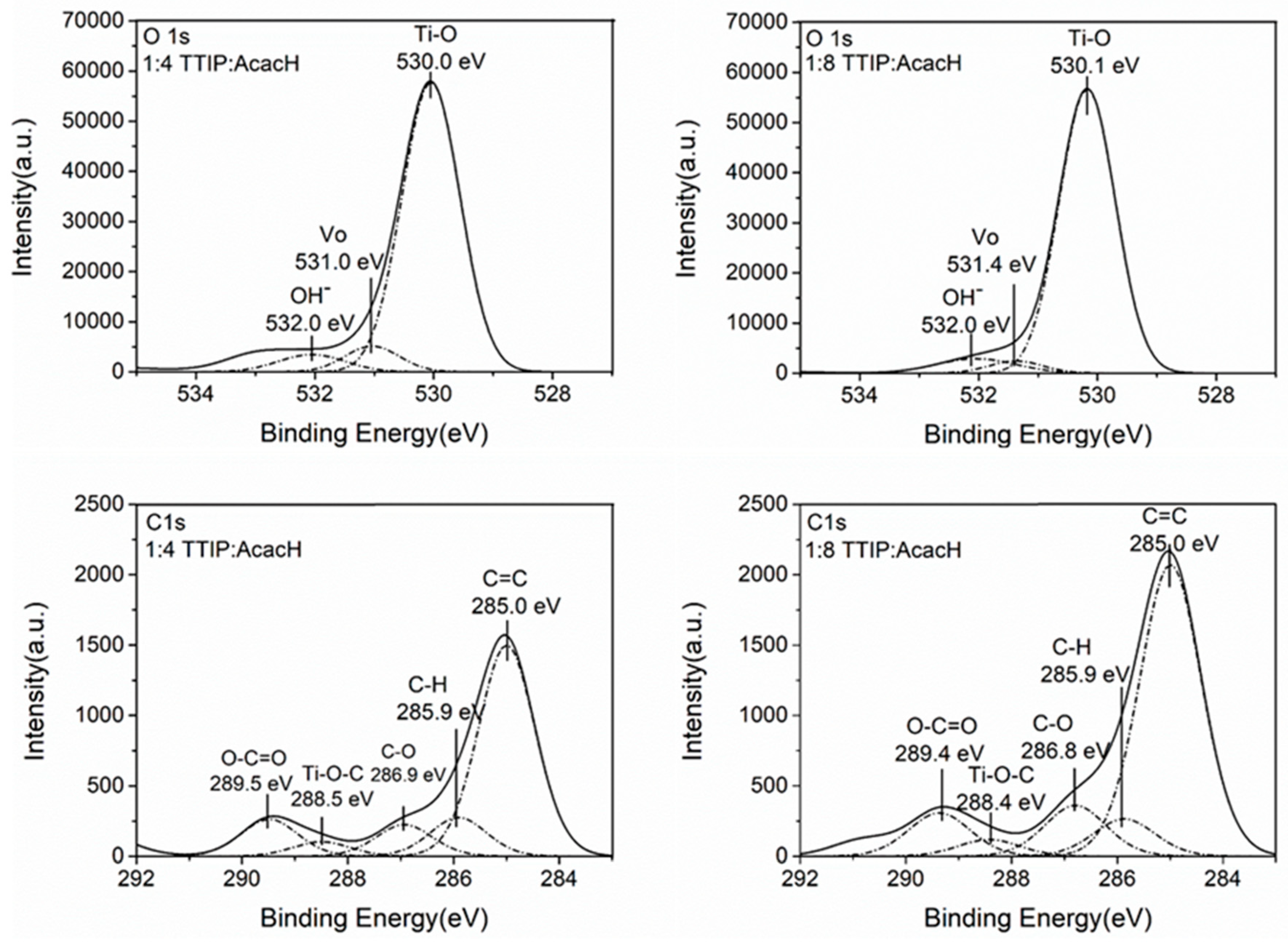
2.4. Chemical Composition and Wettability
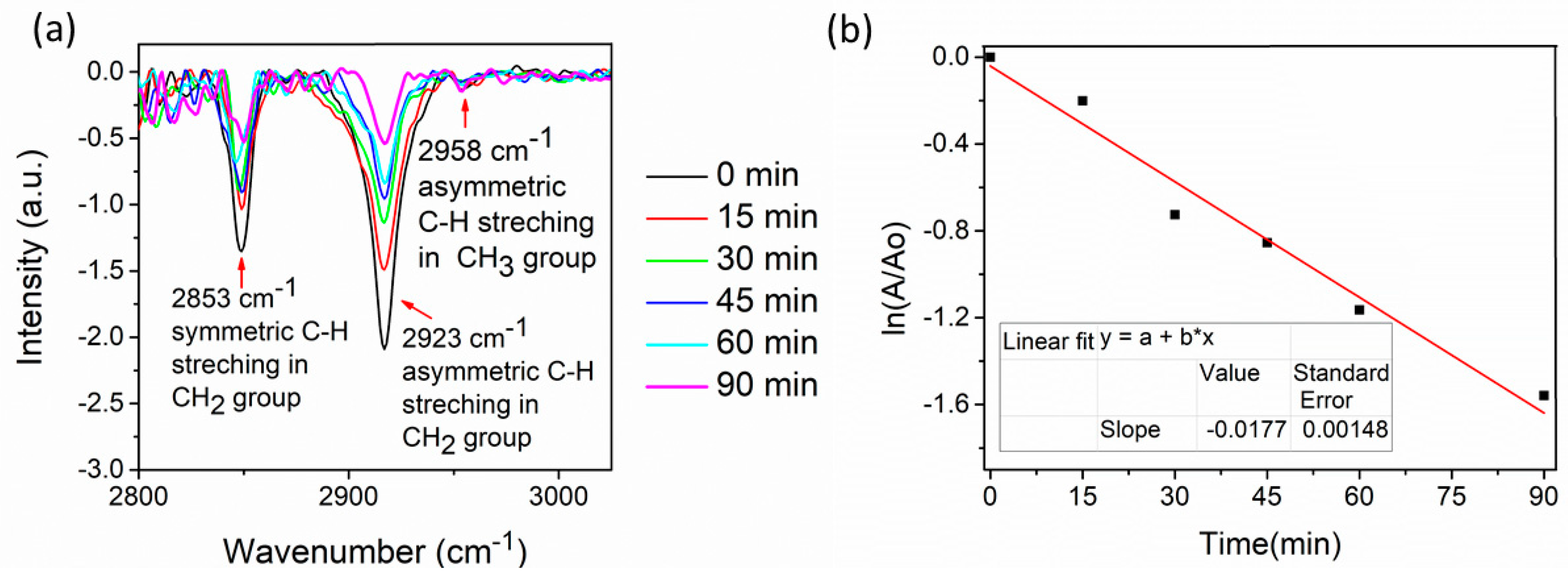
2.5. Photocatalytic Activity. Stearic Acid Test
3. Materials and Methods
3.1. TiO2 Thin Film Synthesis
3.2. Characterization of Films
3.3. Evaluation of Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adachi, T.; Latthe, S.S.; Gosavi, S.W.; Roy, N.; Suzuki, N.; Ikari, H.; Kato, K.; Katsumata, K.; Nakata, K.; Furudate, M.; et al. Photocatalytic, superhydrophilic, self-cleaning TiO2 coating on cheap, lightweight, flexible polycarbonate substrates. Appl. Surf. Sci. 2018, 458, 917–923. [Google Scholar] [CrossRef]
- Falling is Every Window Cleaner’s Nightmare. When His Scaffolding Broke Five Stories up, Jim Willingham Had to Think Fast. Available online: https://consumer.healthday.com/encyclopedia/work-and-health-41/occupational-health-news-507/window-washers-646930.html (accessed on 2 October 2019).
- Hashimiti, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Mauchauffé, R.; Kang, S.; Kim, J.; Kim, J.H.; Moon, S.Y. Spectroscopic study of an atmospheric pressure plasma generated for the deposition of titanium dioxide thin films. Curr. Appl. Phys. 2019, 19, 1296–1304. [Google Scholar] [CrossRef]
- Wei, B.; Xue, J.L.; Cao, H.T.; Li, H.G.; Wen, F.; Pei, Y.T. Effects of annealing on the composition, structure and photocatalytic properties of C-doped titania films deposited by reactive magnetron sputtering using CO2 as carbon source. Surf. Rev. Lett. 2019, 26. [Google Scholar] [CrossRef]
- Rahman, K.H.; Kumar, A.K. Effect of precursor concentration of microstructured titanium-di-oxide (TiO2) thin films and their photocatalytic activity. Mater. Res. Express 2019, 6. [Google Scholar] [CrossRef]
- Sayahi, H.; Mohsenzadeh, F.; Hamadanian, M. Cost-effective fabrication of perdurable electrodeposited TiO2 nano-layers on stainless steel electrodes applicable to photocatalytic degradation of methylene blue. Res. Chem. Intermed. 2019, 45, 4275–4286. [Google Scholar] [CrossRef]
- Tariq, M.K.; Riaz, A.; Khan, R.; Wajid, A.; Haq, H.; Javed, S.; Akram, M.A.; Islam, M. Comparative study of Ag, Sn or Zn doped TiO2 thin films for photocatalytic degradation of methylene blue and methyl orange. Mater. Res. Express 2019, 6. [Google Scholar] [CrossRef]
- Es-Souni, M.; Oja, I.; Krunks, M. Chemical solution deposition of thin TiO2-anatase films for dielectric applications. J. Mater. Sci.-Mater. El. 2014, 15, 341–344. [Google Scholar] [CrossRef]
- Acik, I.O.; Katerski, A.; Mere, A.; Aarik, J.; Aidla, A.; Dedova, T.; Krunks, M. Nanostructured solar cell by spray pyrolysis: Effect of titania barrier layer on the cell performance. Thin Solid Film. 2009, 517, 2443–2447. [Google Scholar] [CrossRef]
- Cergel, M.S.; Menir, E.; Atay, F. The effect of the structural, optical, and surface properties of anatase - TiO2 film on photocatalytic degradation of methylene blue organic contaminant. Ionic 2019, 25, 4481–4492. [Google Scholar] [CrossRef]
- Rasoulnezhad, H.; Hosseinzadeh, G.; Ghasemian, N.; Hosseinzadeh, R.; Keihan, A.H. Transparent nanostructured Fe-doped TiO2 thin films prepared by ultrasonic assisted spray pyrolysis technique. Mater. Res. Express 2018, 5. [Google Scholar] [CrossRef]
- Dündar, I.; Krichevskaya, M.; Katerski, A.; Oja-Acik, I. TiO2 thin films by ultrasonic spray pyrolysis as photocatalytic material for air purification. R. Soc. Open Sci. 2019, 6, 1–12. [Google Scholar] [CrossRef]
- Muniz-Serrato, O.; Serrato-Rodriguez, J. Formation of flow coated high catalytic activity thin films from the low temperature sol-gel titanium butoxide precursor. J. Ceram. Res. 2018, 19, 306–310. [Google Scholar]
- With, P.C.; Helmstedt, U.; Naumov, S.; Sobottka, A.; Prager, A.; Decker, U.; Heller, R.; Abel, B.; Prager, L. Low-Temperature Photochemical Conversion of Organometallic Precursor Layers to Titanium(IV) Oxide Thin Films. Chem. Mater. 2016, 28, 7715–7724. [Google Scholar] [CrossRef]
- Juma, A.O.; Oja Acik, I.; Mikli, V.; Mere, A.; Krunks, M. Effect of solution composition on anatase to rutile transformation of sprayed TiO2 thin films. Thin Solid Films 2015, 594, 287–292. [Google Scholar] [CrossRef]
- Lourduraj, S.; Williams, V. Effect of molarity on sol-gel routed nano TiO2 thin films. J. Adv. Dielectr. 2017, 7, 7. [Google Scholar] [CrossRef]
- Vernardou, D.; Vlachou, K.; Spanakis, E.; Stratakis, E.; Katsarakis, N.; Kymakis, E.; Koudoumas, E. Influence of solution chemistry on the properties of hydrothermally grown TiO2 for advanced applications. Catal. Today 2009, 144, 172–176. [Google Scholar] [CrossRef]
- Yamazaki, S.; Uchiyama, H.; Kozuka, H. Additive-free alkoxide–water–alcohol solutions as precursors for crystalline titania thin films. J. Sol.-Gel Sci. Techn. 2018, 87, 537–543. [Google Scholar] [CrossRef]
- Byrne, C.; Fagan, R.; Hinder, S.; McCormack, D.E.; Pillai, S.C. New approach of modifying the anatase to rutile transition temperature in TiO2 photocatalysts. R. Soc. Chem. 2016, 6, 95232–95238. [Google Scholar] [CrossRef]
- Kajitvichyanukul, P.; Amornchat, P. Effects of diethylene glycol on TiO2 thin film properties prepared by sol–gel process. Sci. Techn. Adv. Mater. 2005, 6, 344–347. [Google Scholar] [CrossRef][Green Version]
- Arjan, W.A.; Hector, A.L.; Levason, W. Speciation in diethanolamine-moderated TiO2 precursor sols and their use in film formation. J. Sol.-Gel Sci. Technol. 2016, 79, 22. [Google Scholar] [CrossRef]
- Wu, W.; Shan, G.; Xiang, Q.; Zhang, Y.; Yi, S.; Zhu, L. Effects of humic acids with different polarities on the photocatalytic activity of nano-TiO2 at environment relevant concentration. Water Res. 2017, 122, 78–85. [Google Scholar] [CrossRef]
- Maletic, M.; Vukcevica, M.; Kalijadis, A.; Jankovic–Castvan, I.; Dapcevica, A.; Lausevic, Z.; Lausevica, M. Hydrothermal synthesis of TiO2/carbon composites and their application for removal of organic pollutants. (in press)
- Park, Y.; Kim, W.; Park, H.; Tachikawa, T.; Majima, T.; Choi, W. Carbon-doped TiO2 photocatalyst synthesized without using an external carbon precursor and the visible light activity. Appl. Cata. B-Envoron. 2009, 91, 355–361. [Google Scholar] [CrossRef]
- Moon, J.; Li, T.; Randall, C.A.; Adair, J.H. Low temperature synthesis of lead titanate by a hydrothermal method. J. Mater. Res. 1997, 12, 189–197. [Google Scholar] [CrossRef]
- Leaustic, A.; Babonneau, F.; Livage, J. Structural Investigation of the Hydrolysis-Condensation Process of Titanium Alkoxides Ti(OR)4 (OR = OPr1, OEt) Modified by Acetylacetone. 1. Study of the Alkoxide Modification. Chem. Mater. 1989, 1, 240–247. [Google Scholar] [CrossRef]
- Kaltsum, U.; Kurniawan, A.F.; Nurhasanah, I.; Priyono, P. The Role of Concentration Ratio of TTiP:AcAc on the Photocatalytic Activity of TiO2 Thin Film in Reducing Degradation Products of Used Frying Oil. Bull. React. Eng. Cata. 2017, 12, 430–436. [Google Scholar]
- Siwinska-Stefanska, K.; Zdarta, J.; Paukszta, D.; Jesionowski, T. The influence of addition of a catalyst and chelating agent on the properties of titanium dioxide synthesized via the sol–gel method. J. Sol.-Gel Sci. Technol. 2015, 75, 264–278. [Google Scholar] [CrossRef]
- Kajitvichyanukul, P.; Pongpom, S.; Watcharenwong, A.; Ananpattarachai, J. Effect of Acetyl Acetone on Property of TiO2 Thin Film for Photocatalytic Reduction of Chromium(VI) from Aqueous Solution. J. Spec. Issue Nanotech. 2005, 4, 87–93. [Google Scholar]
- RRUFF – Database. Available online: http://rruff.info/Anatase/R060277 (accessed on 8 October 2019).
- Pimpabute, N.; Burinprakhow, T.; Somknunthot, W. Determination of optical constants and thickness of amorphous GaP thin film. Opt. Appl. 2011, 1. [Google Scholar]
- Duminica, F.D.; Maury, F.; Abisset, S. Pyrosol deposition of anatase TiO2 thin films starting from Ti(OiPr)4/acetylacetone solutions. Thin Solid Films 2007, 515, 7732–7739. [Google Scholar] [CrossRef]
- Stefanov, B.; Österlund, L. Tuning the Photocatalytic Activity of Anatase TiO2 Thin Films by Modifying the Preferred <001> Grain Orientation with Reactive DC Magnetron Sputtering. Coatings 2014, 4, 587–601. [Google Scholar] [CrossRef]
- Kumar, K.; Ntwaeaborwa, O.M.; Holsa, J.; Motaung, D.E.; Swart, H.C. The role of oxygen and titanium related defects on the emission of TiO2:Tb3+ nano-phosphor for blue lighting applications. Opt. Mater. 2015, 46, 510–516. [Google Scholar] [CrossRef]
- Naeem, M.; Hasanain, S.K.; Kobayashi, M.; Ishida, Y.; Fujimori, A.; Buzby, S.; Shah, I. Effect of reducing atmosphere on the magnetism of Zn1−xCoxO (0 ≤ x ≤ 0.10) nanoparticles. Nanotechnology 2006, 17, 2675–2680. [Google Scholar] [CrossRef] [PubMed]
- Gromyko, I.; Krunks, M.; Dedova, T.; Katerski, A.; Klauson, D.; Oja Acik, I. Surface properties of sprayed and electrodeposited ZnO rod layers. Appl. Surf. Sci. 2017, 405, 521–528. [Google Scholar] [CrossRef]
- Klaysri, R.; Ratova, M.; Praserthdam, P.; Kelly, P.J. Deposition of Visible Light-Active C-Doped Titania Films via Magnetron Sputtering Using CO2 as a Source of Carbon. Nanomaterials 2017, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, Z.; Chen, J.; Chenga, L.; Changa, J.; Shenga, W.; Huc, C.; Cao, S. C-doped hollow TiO2 spheres: In situ synthesis, controlled shell thickness, and superior visible-light photocatalytic activity. Appl. Catal. B-Environ. 2015, 165, 715–722. [Google Scholar] [CrossRef]
- Seo, H.O.; Woo, T.G.; Park, E.J.; Cha, B.J.; Kim, I.H.; Han, S.W.; Kim, Y.D. Enhanced photo-catalytic activity of TiO2 films by removal of surface carbon impurities; the role of water vapor. Appl. Surf. Sci. 2017, 420, 808–816. [Google Scholar] [CrossRef]
- Rajkumar, R.; Nisha, S. To Study the Effect of the Concentration of Carbon on Ultraviolet and Visible Light Photo Catalytic Activity and Characterization of Carbon Doped TiO2. J. Nanomed. Nanotechnol. 2015, 6, 7. [Google Scholar]
- Shi, Z.J.; Ma, M.G.; Zhu, J.F. Recent Development of Photocatalysts Containing Carbon Species: A Review. Catalysts 2019, 9, 20. [Google Scholar] [CrossRef]
- Valentin, C.D.; Pacchioni, D.; Selloni, A. Theory of Carbon Doping of Titanium Dioxide. Chem. Mater. 2005, 172, 66656–66665. [Google Scholar] [CrossRef]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B-Environ. 2015, 176–177, 396–428. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Bao, S.H.; Gou, Y.; Jin, P. Natural Hydrophobicity and Reversible Wettability Conversion of Flat Anatase TiO2 Thin Film. Appl. Mater. Inter. 2014, 6, 1351–1355. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Puzenat, E.; Guillard, G.; Geantet, C.; Matsuzawa, S.; Negishi, N. Improvement of Photocatalytic Degradation Activity of Visible-Light-Responsive TiO2 by Aid of Ultraviolet-Light Pretreatment. J. Phys. Chem. C 2009, 113, 5535–5540. [Google Scholar] [CrossRef]
- Higashimotoa, S.; Katsuura, K.; Yamamoto, M.; Takahashi, M. Photocatalytic activity for decomposition of volatile organic compound on Pt-WO3 enhanced by simple physical mixing with TiO2. Catal. Commun. 2020, 133, 1566–7367. [Google Scholar] [CrossRef]
- Mills, A.; Lepre, A.; Elliott, N.; Bhopal, S.; Parkin, I.P.; O’Neill, S.A. Characterisation of the photocatalyst Pilkington ActivTM: A reference film photocatalyst? J. Photoch. Photobio. A 2003, 160, 213–224. [Google Scholar] [CrossRef]
- Mills, A.; Wang, J. Simultaneous monitoring of the destruction of stearic acid and generation of carbon dioxide by self-cleaning semiconductor photocatalytic films. J. Photoch. Photobio. A 2006, 182, 181–186. [Google Scholar] [CrossRef]
- Pore, V.; Ritala, M.; Lekelä, M.; Areva, S.; Järn, M.; Järnström, J. H2S modified atomic layer deposition process for photocatalytic TiO2 thin films. J. Mater. Chem. 2007, 17, 1361–1371. [Google Scholar] [CrossRef]
- Smirnova, N.; Fesenko, T.; Zhukovsky, M.; Goworek, J.; Eremenko, A. Photodegradation of Stearic Acid Adsorbed on Superhydrophilic TiO2 Surface: In Situ FTIR and LDI Study. Nanoscale Res. Lett. 2015, 10, 7. [Google Scholar] [CrossRef][Green Version]
- Montero, J.; Österlund, L. Photodegradation of Stearic Acid Adsorbed on Copper Oxide Heterojunction Thin Films Prepared by Magnetron Sputtering. ChemEngineering 2018, 2, 40. [Google Scholar] [CrossRef]
- Wang, S.-H.; Chen, T.-K.; Rao, K.K.; Wong, M.-S. Nanocolumnar titania thin films uniquely incorporated with carbon for visible light photocatalysis. Appl. Catal. B-Environ. 2007, 76, 328–334. [Google Scholar] [CrossRef]
- Ratova, M.; Klaysri, R.; Praserthdam, P.; Kelly, P.J. Visible light active photocatalytic C-doped titanium dioxide films deposited via reactive pulsed DC magnetron co-sputtering: Properties and photocatalytic activity. Vacuum 2018, 149, 214–224. [Google Scholar] [CrossRef]
- Varnagiris, S.; Medvids, A.; Lelis, M.; Milcius, D.; Antuzevics, A. Black carbon-doped TiO2 films: Synthesis, characterization and photocatalysis. J. Photochem. Photobio. A-Chem. 2019, 382, 9. [Google Scholar] [CrossRef]
- Aiying, B.; Wei, L.; Gengle, Z.; Jinbo, X. Preparation and Enhanced Daylight-Induced Photo-Catalytic. Activity of Transparent C-Doped TiO2 Thin Films. J. Wuhan Univ. Tehnol.-Mater. Sci. Ed. 2010, 25, 738–742. [Google Scholar]
- Klauson, D.; Poljakova, A.; Pronina, N.; Krichevskaya, M.; Moiseev, A.; Dedova, T.; Preis, S. Aqueous Photocatalytic Oxidation of Doxycycline. Adv. Oxid. Technol. 2013, 16, 234–243. [Google Scholar] [CrossRef]
- Klauson, D.; Budarnaja, O.; Stepanova, K.; Krichevskaya, M.; Dedova, T.; Käkinen, A.; Preis, S. Selective Performance of Sol–Gel Synthesised Titanium Dioxide Photocatalysts in Aqueous Oxidation of Various-Type Organic Pollutants. Kinet. Cata. 2014, 55, 47–55. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
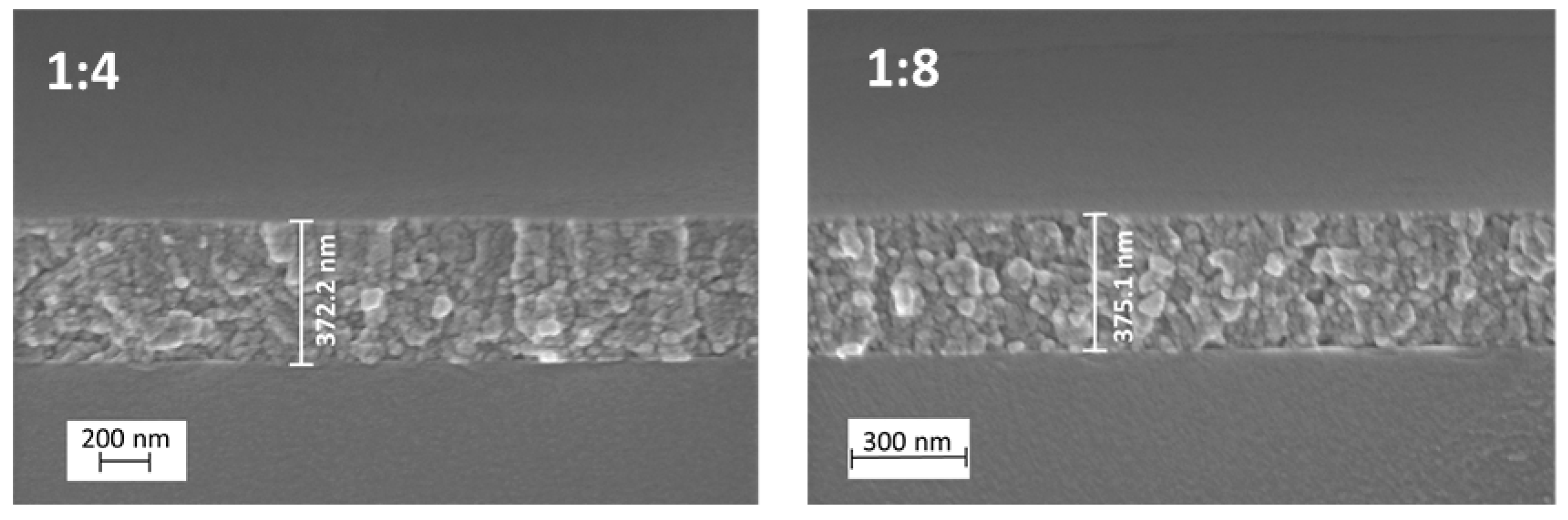


| TTIP:AcacH Molar Ratio | Thickness, nm | Band Gap, eV |
|---|---|---|
| 1:3 | 380 | 3.3 |
| 1:4 | 370 | 3.4 |
| 1:5 | 370 | 3.4 |
| 1:6 | 410 | 3.4 |
| 1:7 | 370 | 3.4 |
| 1:8 | 375 | 3.4 |
| 1:10 | 370 | 3.4 |
| 1:12 | 390 | 3.4 |
| 1:20 | 280 | 3.5 |
| TTIP:AcacH Molar Ratio | (Vo)/(Ti-O) (at%/at%) | (OH–)/(Ti-O) (at%/at%) | (C-O)/(C-H) (at%/at%) | (Ti-O-C)/(C-H) (at%/at%) | (C=C)/(C-H) (at%/at%) |
|---|---|---|---|---|---|
| 1:4 | 0.08 | 0.07 | 0.8 | 0.38 | 5.43 |
| 1:8 | 0.06 | 0.07 | 1.35 | 0.44 | 7.80 |
| TTIP:AcacH Molar Ratio | Contact Angle° | ||
|---|---|---|---|
| As-Deposited | One-month Aged | UV-A 15 min | |
| 1:4 | 20 | 55 | 4 |
| 1:8 | 20 | 30 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiridonova, J.; Katerski, A.; Danilson, M.; Krichevskaya, M.; Krunks, M.; Oja Acik, I. Effect of the Titanium Isopropoxide:Acetylacetone Molar Ratio on the Photocatalytic Activity of TiO2 Thin Films. Molecules 2019, 24, 4326. https://doi.org/10.3390/molecules24234326
Spiridonova J, Katerski A, Danilson M, Krichevskaya M, Krunks M, Oja Acik I. Effect of the Titanium Isopropoxide:Acetylacetone Molar Ratio on the Photocatalytic Activity of TiO2 Thin Films. Molecules. 2019; 24(23):4326. https://doi.org/10.3390/molecules24234326
Chicago/Turabian StyleSpiridonova, Jekaterina, Atanas Katerski, Mati Danilson, Marina Krichevskaya, Malle Krunks, and Ilona Oja Acik. 2019. "Effect of the Titanium Isopropoxide:Acetylacetone Molar Ratio on the Photocatalytic Activity of TiO2 Thin Films" Molecules 24, no. 23: 4326. https://doi.org/10.3390/molecules24234326
APA StyleSpiridonova, J., Katerski, A., Danilson, M., Krichevskaya, M., Krunks, M., & Oja Acik, I. (2019). Effect of the Titanium Isopropoxide:Acetylacetone Molar Ratio on the Photocatalytic Activity of TiO2 Thin Films. Molecules, 24(23), 4326. https://doi.org/10.3390/molecules24234326









