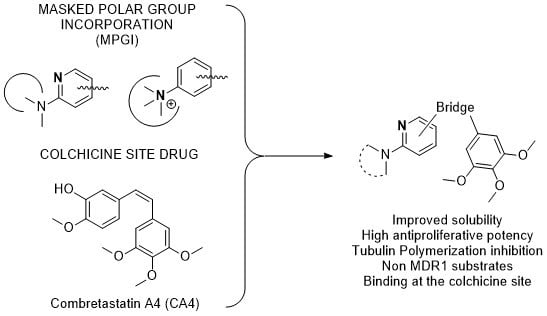
Reagents were used as purchased without further purification. Solvents (THF, DMF, CH2Cl2) were dried and freshly distilled before use according to procedures reported in the literature. Chromatographic separations were performed on silica gel columns by flash (Kieselgel 40, 0.040–0.063; Merck). TLC was performed on precoated silica gel polyester plates (0.25 mm thickness, with UV 254 fluorescent indicator). Melting points were determined on a Buchi 510 apparatus and are uncorrected. 1H NMR and 13C NMR spectra were recorded on a Bruker AC200 spectrometer at 200/50 MHz, on a Bruker SY spectrometer at 400/100 MHz or on a Varian Mercury 400/100 MHz, using CDCl3 as solvent (unless otherwise stated). Chemical shifts (δ) are given in ppm downfield from tetramethylsilane, and coupling constants (J values) are in Hertz. Electrospray-ionisation (ESI) high-resolution mass spectra (HRMS) were obtained on a VG-TS250 apparatus (70 eV). A Helios Alfa UV-320 from Thermo-Spectronic was used for UV spectra and turbidimetric measurements. IR spectra were acquired in a FTIR Nicolet Impact 410 equipment (film, unless otherwise stated) and the absorptions are expressed in cm−1. The aqueous solubility of the compound was determined in a Helios Alfa Spectrophotometer.
4.1. Synthesis
4.1.1. General Procedure to Obtain Diarylketones by One-step Synthesis (1a, 1b, 1f, 1g)
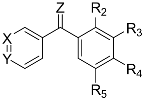
1.5 mmol per mmol of 1.6 M nBuLi in hexane was added to a stirred 0.2 M solution of the corresponding bromo derivative in dry THF at −78 °C under an inert atmosphere. After 1 h, the colored solution was added to a 0.2 M suspension of the corresponding sodium benzoate in dry THF at 0 °C, previously formed by adding 3 mmol per mmol of sodium hydride over 2 mmol per mmol of the benzoic acid. After 24 h at room temperature, the reaction was poured onto ammonium chloride, extracted with ethyl acetate and the organic layers were dried over anhydrous sodium sulfate, filtered and dried under vacuum. The reaction crude was purified by column chromatography.
(4-(Dimethylamino)phenyl)(3,4,5-trimethoxyphenyl)methanone (
1a) [
16]. 3.50 g (17.5 mmol) of
p-bromodimethylaniline in THF treated with 16.4 mL (26.2 mmol) of
nBuLi 1.6 M in hexane at −78 °C were added to 35.0 mmol of 3,4,5-trimethoxybenzoate in dry THF to obtain 4.22 g of reaction crude. After flash chromatography Hex/EtOAc 1:1, 2.50 g (7.93 mmol, 46%) of compound
1a were isolated.
1H-NMR δ: 3.06 (6H, s); 3.85 (6H, s); 3.90 (3H, s); 6.66 (2H, d, 8.6); 6.98 (2H, s); 7.80 (2H, d, 8.6).
13C-NMR δ: 40.1 (2) (CH
3); 56.3 (2) (CH
3); 61.0 (CH
3); 107.1 (2) (CH); 110.6 (2) (CH); 124.8 (C); 132.7 (2) (CH); 134.5 (C); 140.8 (C); 152.8 (2) (C); 153.3 (C); 194.4 (C). IR (film): 1681; 1583; 1411; 1328; 1121 cm
−1. Mp (Diisopropylether): 107–109 °C. HRMS: calculated for C
18H
21NO
4 [M + Na]
+: 338.1368; found: 338.1363.
(6-(Dimethylamino)pyridin-3-yl)(3,4,5-trimethoxyphenyl)methanone (1b). 627 mg (3.12 mmol) of 5-bromo-N,N-dimethylpiridin-2-amine in THF treated with 1.9 mL (3.1 mmol) of nBuLi 1.6 M in hexane at −78 °C were added to 6.22 mmol of 3,4,5-trimethoxybenzoate in dry THF to obtain 1.25 g of reaction crude. After flash chromatography Hex/EtOAc 9:1, 833 mg (2.63 mmol, 84%) of compound 1b were isolated. 1H-NMR δ: 3.20 (6H, s); 3.88 (6H, s); 3.91 (3H, s); 6.56 (1H, d, 9,2); 6.99 (2H, s); 8.01 (1H, dd, 9.2, 2.4); 8.63 (1H, d, 2.4). 13C-NMR δ: 38.2 (2) (CH3); 56.3 (2) (CH3); 60.9 (CH3); 105.1 (CH); 107.0 (2) (CH); 121.2 (C); 133.7 (C); 138.8 (CH); 143.4 (C); 152.4 (CH); 152.8 (2) (C); 160.4 (C); 187.6 (C). IR (KBr): 1686; 1608; 1584; 1508; 1403; 1337; 1132 cm−1. Mp (Diisopropylether): 119–120 °C. HRMS: calculated for C17H21N2O4 [M + H]+: 317.1501; found: 317.1507.
(3-(Dimethylamino)phenyl)(3,4,5-trimethoxyphenyl)methanone (1f). 2.78 g (13.9 mmol) of 3-bromodimethylaniline in THF treated with 9.5 mL (15 mmol) of nBuLi 1.6 M in hexane at −78 °C were added to 20.8 mmol of 3,4,5-trimethoxybenzoate in dry THF to obtain 3.27 g of reaction crude. After flash chromatography Hex/EtOAc 7:3, 3.27 g (10.4 mmol, 75%) of compound 1f were isolated. 1H-NMR δ: 2.89 (6H, s); 3.76 (6H, s); 3.83 (3H, s); 6.82 (1H, dd, 8.0, 2.8); 6.95 (1H, bd, 8.0); 6.99 (2H, s); 7.04 (1H, bs); 7.29 (1H, t, 8.0). 13C-NMR δ: 40.5 (2) (CH3); 56.3 (2) (CH3); 61.0 (CH3); 107.8 (2) (CH); 113.2 (CH); 116.2 (CH); 118.3 (CH); 128.8 (CH); 133.0 (C); 138.5 (C); 141.8 (C); 150.4 (C); 152.8 (2) (C); 196.4 (C). IR (KBr): 1650; 1589; 1412; 1334; 1230; 1124 cm−1. Mp (methanol): 77–78 °C. HRMS: calculated for C18H21NO4 [M + Na]+: 338.1368; found: 338.1361.
(2-(Dimethylamino)pyridin-4-yl)(3,4,5-trimethoxyphenyl)methanone (1g). 713 mg (3.54 mmol) of 4-bromo-N,N-dimethylpyridin-2-amine in THF treated with 2.9 mL (4.6 mmol) of nBuLi 1.6 M in hexane at −78 °C were added to 7.07 mmol of 3,4,5-trimethoxybenzoate in dry THF to obtain 874 mg of reaction crude. After flash chromatography Hex/EtOAc 7:3, 282 mg (0.90 mmol, 26%) of compound 1g were isolated. 1H-NMR δ: 3.13 (6H, s); 3.86 (6H, s); 3.93 (3H, s); 6.72 (1H, d, 9.4); 6.75 (1H, d, 2.2); 7.10 (2H, s); 8.27 (1H, dd, 9.4, 2.2). 13C-NMR δ: 37.9 (2) (CH3); 56.1 (2) (CH3); 60.7 (CH3); 105.0 (CH); 107.6 (2) (CH); 110.4 (CH); 131.2 (C); 142.5 (C); 146.1 (C); 148.1 (CH); 152.8 (2) (C); 159.3 (C); 194.9 (C). IR (film): 1659; 1595; 1411; 1328; 1125 cm−1. Mp (Hexane/Ethyl acetate): 101–102 °C. HRMS: calculated for C17H21N2O4 [M + H]+: 317.1501; found: 317.1498
4.1.2. General Procedure to Obtain Diarylketones by Two-step Synthesis (1c, 1d, 1e, 1h)
1.2 mmol per mmol of 1.6 M nBuLi in hexane was added to a stirred 0.2 M suspension of the corresponding bromo derivative in dry THF at −78 °C under inert atmosphere. After 1–2 h, the corresponding aromatic aldehyde was slowly added to the resulting yellowish solution, allowing the reaction to slowly reach room temperature. After 12–24 h, the mixture was poured over ice and extracted with CH2Cl2. The organic layers were dried over anhydrous Na2SO4, filtered, and evaporated under vacuum. Then, 1 mmol of PDC per mmol of crude and a small amount of tetrabutylammonium hydrogen sulfate were added in the dark to a 0.15–0.25 M solution of the alcohol in dry CH2Cl2 at 0 °C. The reaction was allowed to reach room temperature and proceed until completion (TLC) for 4–24 h. The reaction crude was filtered through silica, using CH2Cl2 and ethyl acetate as eluents, and the organic layers evaporated under vacuum to yield the diarylketones that were purified by column chromatography.
(6-(Pyrrolidin-1-yl)pyridin-3-yl)(3,4,5-trimethoxyphenyl)methanone (1c). 500 mg (2.84 mmol) of 6-(pyrrolidin-1-yl)nicotinaldehyde were added to a suspension of 840 mg (3.40 mmol) of 5-bromo-1,2,3-trimethoxybenzene and 2.6 mL (4.2 mmol) of nBuLi (1.6 M in hexane) in dry THF. After quenching and extraction of the crude, 393 mg (1.76 mmol) of PDC were added. After 4 h the crude was chromatographed CH2Cl2/EtOAc 1:1 to obtain 450 mg (1.31 mmol, 75%) of 1c. 1H-NMR δ: 2.03 (4H, m); 3.54 (4H, m); 3.86 (6H, s); 3.89 (3H, s); 6.40 (1H, d, 8.8); 6.98 (2H, s); 7.98 (1H, d, 8.8); 8.62 (1H, s). 13C-NMR δ: 25.4 (2) (CH2); 47.0 (2) (CH2); 56.2 (2) (CH3); 60.9 (CH3); 106.0 (CH); 107.0 (2) (CH); 121.1 (C); 133.7 (C); 138.5 (CH); 141.0 (C); 152.8 (2) (C); 152.9 (CH); 158.3 (C); 193.1 (C). IR (KBr): 1640; 1548; 1330; 1127 cm−1. Mp (diethyl ether): 140–141 °C. HRMS: calculated for C19H23N2O4 [M + H]+: 343.1658; found: 343.1651.
(4-(Dimethylamino)phenyl)(2,3,4-trimethoxyphenyl)methanone (1d). 4.43 g (29.7 mmol) of 4-(dimethylamine)benzaldehyde were added to a suspension of 5.00 g (29.7 mmol) of 6-bromo-1,2,3-trimethoxybenzene and 18.6 mL (29.7 mmol) of nBuLi (1.6 M in hexane) in dry THF. After quenching and extraction of the crude, 16.8 g (44.7 mmol) of PDC were added. After 24 h the crude was chromatographed Hex/EtOAc 8:2 to obtain 1.86 g (5.90 mmol, 20%) of 1d. 1H-NMR δ: 3.02 (6H, s); 3.76 (3H, s); 3.87 (6H, s); 6.60 (2H, d, 9.0); 6.67 (1H, d, 8.6); 7.01 (1H, d, 8.6); 7.72 (2H, d, 9.0). 13C-NMR δ: 40.1 (2) (CH3); 56.1 (CH3); 61.1 (CH3); 61.9 (CH3); 106.8 (CH); 110.5 (2) (CH); 124.2 (CH); 125.5 (C); 127.7 (C); 132.4 (2) (CH); 142.0 (C); 152.0 (C); 153.5 (C); 155.0 (C); 193.6 (C). IR (KBr): 1636; 1588 cm−1. Mp (Hexane/CH2Cl2): 205–207 °C. HRMS: calculated for C18H21NO4 [M + Na]+: 338.1368; found: 338.1360.
(2,5-dimethoxyphenyl)(4-(dimethylamino)phenyl)methanone (1e). 5.40 g (36.2 mmol) of 4-(dimethylamine)benzaldehyde were added to a suspension of 5.02 g (36.2 mmol) of 2-bromo-1,4-dimethoxybenzene and 22.6 mL (36.2 mmol) of nBuLi (1.6 M in hexane) in dry THF. After quenching and extraction of the crude, 22.05 g (58.6 mmol) of PDC were added. After 24 h the crude was chromatographed Hex/EtOAc 8:2 to obtain 3.52 g (12.40 mmol, 32%) of 1e. 1H-NMR δ: 3.03 (6H, s); 3.68 (3H, s); 3.75 (3H, s); 6.61 (2H, d, 8.8); 6.85 (1H, d, 2.5); 6.90 (1H, dd, 8.8, 2.5); 6.92 (1H, d, 8.8); 7.74 (2H, d, 8.8). 13C-NMR δ: 40.0 (2) (CH3); 55.8 (CH3); 56.5 (CH3); 110.4 (2) (CH); 113.0 (CH); 114.1 (CH); 115.9 (CH); 125.1 (C); 130.9 (C); 132.3 (2) (CH); 150.9 (C); 153.3 (C); 153.5 (C); 194.0 (C). IR (KBr): 1637; 1588; 1544 cm−1. Mp (t-butylmethyl ether): 78–80 °C. HRMS: calculated for C17H20NO3 [M + H]+: 286.1443; found: 286.1420.
(2-(Pyrrolidin-1-yl)pyridin-4-yl)(3,4,5-trimethoxyphenyl)methanone (1h). 1.00 g (5.67 mmol) of 2-(pyrrolidin-1-yl)isonicotinaldehyde were added to a suspension of 2.15 g (8.51 mmol) of 5-bromo-1,2,3-trimethoxybenzene and 6.40 mL (10.2 mmol) of nBuLi (1.6 M in hexane) in dry THF. After quenching and extraction of the crude, 0.78 g (14.2 mmol) of PDC were added. After 4 h the crude was chromatographed with CH2Cl2/MeOH 98:2 to obtain 735 mg (2.15 mmol, 38%) of 1h. 1H-NMR δ: 1.98 (4H, m); 3.43 (4H, m); 3.82 (6H, s); 3.89 (3H, s); 6.55 (1H, s); 6.60 (1H, d, 5.6); 7.07 (2H, s); 8.20 (1H, d, 5.6). 13C-NMR δ: 25.4 (2) (CH2); 46.8 (2) (CH2); 56.2 (2) (CH3); 60.9 (CH3); 106.0 (CH); 107.7 (2) (CH); 110.1 (CH); 131.2 (C); 146.1 (C); 148.1 (CH); 152.9 (2) (C); 155.0 (C); 157.1 (C); 195.1 (C). IR (film): 1650; 1598; 1538; 1456; 1328; 1127 cm−1. HRMS: calculated for C19H23N2O4 [M + H]+: 343.1658; found: 343.1642.
4.1.3. General Procedure to Obtain Oxime Derivatives (2a–h)
3–15 mmol of NH2OH·HCl per mmol of diarylketone and 2–4 droplets of pyridine were added to a 0.02–0.06 M solution of the corresponding diarylketone in 10–25 mL of MeOH, and the reaction was refluxed for 12–48 h. After cooling, the solvent was evaporated off, the residue was re-dissolved in CH2Cl2, washed with brine, and the organic layers were dried, filtered, and evaporated.
(4-(Dimethylamino)phenyl)(3,4,5-trimethoxyphenyl)methanone oxime (
2a) [
16]. 560 mg (7.93 mmol) of hydroxylamine hydrochloride were added to 500 mg (1.59 mmol) of ketone
1a in methanol to obtain, after crystallization in MeOH/EtOAc, 300 mg (0.91 mmol, 57%) of a 45:55
Z/E mixture of
2a. While isomer
E crystals are yellow, isomer
Z crystals are green, so they were manually separated.
2a (
E):
1H-NMR (CD
3OD) δ: 2.89 (6H, s); 3.65 (6H, s); 3.67 (3H, s); 6.61 (2H, s); 6.63 (2H, d, 8.9); 7.25 (2H, d, 8.9).
13C-NMR (CD
3OD) δ: 40.5 (2) (CH
3); 56.4 (2) (CH
3); 61.1 (CH
3); 107.0 (2) (CH); 111.7 (2) (CH); 121.6 (C); 132.4 (2) (CH); 135.0 (C); 139.8 (C); 152.1 (C); 154.1 (2) (C); 158.1 (C). Mp. (MeOH/EtOAc): 148–149 °C. HRMS: calculated for C
18H
22N
2O
4 [M + Na]
+: 353.1472; found: 353.1477.
2a (
E):
1H-NMR (CD
3OD) δ: 2.90 (6H, s); 3.68 (6H, s); 3.71 (3H, s); 6.49 (2H, s); 6.77 (2H, d, 8.9); 7.24 (2H, d, 8.9).
13C-NMR (CD
3OD) δ: 40.5 (2) (CH
3); 56.4 (2) (CH
3); 61.1 (CH
3); 107.8 (2) (CH); 112.3 (2) (CH); 121.6 (C); 129.7 (2) (CH); 135.0 (C); 139.8 (C); 152.1 (C); 154.1 (2) (C); 158.1 (C). IR (KBr): 3410; 1604; 1527; 1126 cm
−1. Mp (MeOH/EtOAc): 163–164 °C. HRMS: calculated for C
18H
22N
2O
4 [M+Na]+: 353.1477; found: 353.1470.
(6-(Pyrrolidin-1-yl)pyridin-3-yl)(3,4,5-trimethoxyphenyl)methanone oxime (2c). 170 mg (2.46 mmol) of hydroxylamine hydrochloride were added to 56 mg (0.16 mmol) of ketone 1c in methanol to obtain, after crystallization in MeOH, 15 mg (0.04 mmol, 25%) of 2c. 1H-NMR δ: 2.13 (4H, m); 3.80 (4H, m); 3.88 (6H, s); 3.92 (3H, s); 6.68 (1H, d, 8.8); 6.99 (2H, s); 8.22 (1H, dd, 8.8, 2.0); 8.65 (1H, d, 2.0). IR (KBr): 3490; 1610; 1590; 1430; 1380; 1210 cm−1.
(4-(Dimethylamino)phenyl)(2,3,4-trimethoxyphenyl)methanone oxime (2d). 862 mg (12.4 mmol) of hydroxylamine hydrochloride were added to 346 mg (1.10 mmol) of ketone 1d in methanol to obtain, after column chromatography Hex/EtOAc 7:3, 158 mg (0.48 mmol, 44%) of 2d. 1H-NMR δ: 2.96 (6H, s); 3.77 (3H, s); 3.91 (6H, s); 6.65 (2H, d, 7.6); 6.77 (1H, d, 7.4); 6.87 (1H, d, 7.4); 7.37 (2H, d, 7.6). 13C-NMR δ: 40.3 (2) (CH3); 55.9 (CH3); 60.8 (CH3); 60.9 (CH3); 107.2 (CH); 108.3 (C); 112.1 (2) (CH); 120.2 (C); 123.7 (CH); 128.0 (2) (CH); 142.2 (C); 151.0 (C); 151.2 (C); 154.05 (C); 155.96(C). IR (KBr): 3490; 1523; 1601 cm−1. Mp (CH2Cl2/Hexane): 179–181 °C. HRMS: calculated for C18H22N2O4 [M + Na]+: 353.1477; found: 353.1469.
(2,5-Dimethoxyphenyl)(4-(dimethylamino)phenyl)methanone oxime (2e). 494 mg (7.1 mmol) of hydroxylamine hydrochloride were added to 223 mg (0.80 mmol) of ketone 1e in methanol to obtain, after column chromatography Hex/EtOAc 8:2, 128 mg (0.40 mmol, 50%) of 2e. 1H-NMR δ: 2.99 (6H, s, NCH3); 3.73 (3H, s, OCH3); 3.78 (3H, s, OCH3); 6.63 (2H, d, 8.0); 6.74 (1H, d, 7.6); 6.95 (1H, d, 7.6); 7.38 (2H, d, 9.0). 13C-NMR δ: 40.2 (2)(CH3); 55.7 (CH3); 56.7 (CH3); 111.7 (2)(CH); 113.0 (CH); 114.8 (CH); 115.0 (CH); 123.1 (C); 123.8 (C); 127.9 (2)(CH); 150.6 (C); 151.0 (C); 153.6 (C); 155.7 (C). IR (KBr): 3485, 1525, 1606 cm−1. Mp (CH2Cl2/hexane): 163–165 °C. HRMS: calculated for C17H21N2O3 [M + H]+: 301.1552; found: 301.1556.
(3-(Dimethylamino)phenyl)(3,4,5-trimethoxyphenyl)methanone oxime (2f). 220 mg (3.17 mmol) of hydroxylamine hydrochloride were added to 100 mg (0.32 mmol) of ketone 1f in methanol to obtain 96 mg (0.29 mmol, 91%) of a 1:1 mixture of Z/E isomers of 2f. (Z): 1H-NMR δ: 2.87 (6H, s); 3.72 (6H, s); 3.80 (3H, s); 6.55 (2H, s); 6.61 (1H, d, 8.0); 6.65 (1H, d, 8.0); 6.72 (1H, s); 7.26 (1H, t, 8.0). 13C-NMR δ: 40.6 (2) (CH3); 56.2 (2) (CH3); 60.9 (CH3); 106.7 (2) (CH); 113.1 (CH); 114.0 (CH); 117.3 (CH); 128.3 (C); 128.9 (CH); 133.3 (C); 136.9 (C); 150.3 (C); 152.9 (2) (C); 158.5 (C). (E): 1H-NMR δ: 2.90 (6H, s); 3.76 (6H, s); 3.84 (3H, s); 6.62 (1H, s); 6.71 (2H, s); 6.74 (1H, d, 8.0); 6.90 (1H, d, 8.0); 7.13 (1H, t, 8.0). 13C-NMR δ: 40.6 (2) (CH3); 56.2 (2) (CH3); 60.9 (CH3); 105.3 (2) (CH); 111.8 (CH); 113.4 (CH); 117.1 (CH); 128.3 (C); 128.9 (CH); 133.3 (C); 136.9 (C); 150.3 (C); 152.9 (2) (C); 158.1 (C). IR (film): 3428; 1582; 1503; 1411; 1344; 1236; 1127 cm−1. HRMS: calculated for C18H23N2O4 [M + H]+: 331.1658; found: 331.1650.
(2-(Dimethylamino)pyridin-4-yl)(3,4,5-trimethoxyphenyl)methanone oxime (2g). 40 mg (0.54 mmol) of hydroxylamine hydrochloride were added to 55 mg (0.17 mmol) of ketone 1g in methanol to obtain, after column chromatography with Hex/EtOAc 1:1, 12 mg (0.04 mmol, 23%) of a 4:6 mixture of Z/E isomers of 2g. (Z): 1H-NMR δ: 3.11 (6H, s); 3.86 (6H, s); 3.91 (3H, s); 6.61 (2H, s); 6.66 (1H, s); 8.13 (1H, d, 5.4); 8.25 (1H, d, 5.4). (E): 1H-NMR δ: 3.16 (6H, s); 3.79 (6H, s); 3.83 (3H, s); 6.47 (1H, s); 6.50 (1H, d, 5.4); 6.74 (2H, s); 8.22 (1H, d, 5.4). 13C-NMR δ: 38.6 (2) (CH3); 56.2 (2) (CH3); 60.9 (CH3); 104.1 (CH); 104.8 (2) (CH); 110.0 (CH); 126.7 (C); 130.4 (C); 146.1 (C); 142.3 (CH); 153.1 (2) (C); 156.3 (C); 158.9 (C). IR (film): 3205; 1650; 1586; 1458; 1236; 1125 cm−1. HRMS: calculated for C17H22N3O4 [M + H]+: 332.1610; found: 332.1609.
(2-(Pyrrolidin-1-yl)pyridin-4-yl)(3,4,5-trimethoxyphenyl)methanone oxime (2h). 167 mg (2.41 mmol) of hydroxylamine hydrochloride were added to 275 mg (0.80 mmol) of ketone 1h in methanol to obtain, after column chromatography Hex/EtOAc 6:4, 120 mg (0.34 mmol, 42%) of a 4:6 mixture of Z/E isomers of 2h. (Z): 1H-NMR δ: 1.60 (4H, m); 3.43 (4H, m); 3.83 (6H, s); 3.91 (3H, s); 6.46 (1H, d, 4.6); 6.60 (1H, s); 6.61 (2H, s); 8.14 (1H, d, 4.6). (E): 1H-NMR δ: 2.02 (4H, m); 3.47 (4H, m); 3.80 (6H, s); 3.87 (3H, s); 6.30 (1H, s); 6.42 (1H, d, 4.6); 6.76 (2H, s); 8.26 (1H, d, 4.6). 13C-NMR δ: 25.5 (2) (CH2); 46.9 (2) (CH2); 56.2 (2) (CH3); 60.9 (CH3); 104.9 (2) (CH); 114.6 (CH); 119.5 (CH); 130.2 (C); 139.6 (C); 142.7 (C); 147.7 (CH); 153.0 (2) (C); 155.4 (C); 155.4 (C). IR (KBr): 3433; 1605; 1542; 1238; 1127 cm−1. HRMS: calculated for C19H24N3O4 [M + H]+: 358.1767; found: 358.1769.
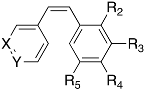
4.1.4. General Procedure to Obtain Isocombretastatines by Wittig Reactions (3a–h)
The required methyltriphenylphosphonium iodide (3 mmol/mmol of diarylketone) was suspended in dry THF (30–60 mL) and cooled to −40 °C under inert atmosphere, nButyllithium (1.6 M in hexane, 0.9 equivalents respect to the salt) was added drop wise, and the resulting solution was stirred at this temperature for 60 min turning from deep-red to yellow-red. Then, a solution of the diarylketone in dry THF (15 mL) was added and warmed to room temperature. Once completed, the reaction mixture was poured onto ammonium chloride and extracted with ethyl acetate. The organic layers were washed with brine, dried over sodium sulfate and concentrated under vacuum. The residue was purified by column chromatography on silica gel.
N,N-Dimethyl-4-(1-(3,4,5-trimethoxyphenyl)vinyl)aniline (
3a) [
16]. A solution of 1.40 g (4.44 mmol) of
1a in THF was added to a suspension of 5.40 g (13.3 mmol) of methyltriphenylphosphonium iodide and 6.90 mL (11.0 mmol) of
nBuLi (1.6 M in hexane) in dry THF at −40 °C to obtain, after column chromatography with Hex/EtOAc 6:4, 1.05 g (3.35 mmol, 75%) of compound
3a.
1H-NMR δ: 2.93 (6H, s); 3.80 (6H, s); 3.90 (3H, s); 5.24 (1H, d, 1.4); 5.36 (1H, d, 1.4); 6.62 (2H, s); 6.65 (2H, d, 8.6); 7.23 (2H, d, 8.6).
13C-NMR δ: 40.4 (2) (CH
3); 56.1 (2) (CH
3); 60.9 (CH
3); 105.8 (2) (CH); 110.9 (CH
2); 111.9 (2) (CH); 129.1 (2) (CH); 137.6 (C); 138.1 (2) (C); 149.9 (C); 150.3(C); 152.9 (2) (C). IR (film): 1605; 1579; 1516; 1350; 1126; 1008 cm
−1. HRMS: calculated for C
19H
24NO
3 [M + H]
+: 314.1756; found: 314.1755.
N,N-Dimethyl-5-(1-(3,4,5-trimethoxyphenyl)vinyl)pyridin-2-amine (3b). A solution of 127 mg (0.40 mmol) of 1b in THF was added to a suspension of 485 mg (1.20 mmol) of methyltriphenylphosphonium iodide and 0.23 mL (1.0 mmol) of nBuLi (1.6 M in hexane) in dry THF at −40 °C to obtain, after column chromatography with Hex/EtOAc 7:3, 51 mg (0.2 mmol, 41%) of compound 3b. 1H-NMR δ: 3.12 (6H, s); 3.82 (6H, s); 3.86 (3H, s); 5.25 (1H, s); 5.33 (1H, s); 6.47 (1H, d, 8.8); 6.55 (2H, s); 7.40 (1H, dd, 8.8, 2.4); 8.20 (1H, d, 2.4). 13C-NMR δ: 38.3 (2) (CH3); 56.2 (2) (CH3); 61.0 (CH3); 105.2 (CH); 105.4 (2) (CH); 111.6 (C); 111.8 (CH2); 124.7 (C); 137.2 (C); 137.6 (CH); 146.5 (C); 147.0 (CH); 153.0 (2) (C); 158.3 (C). IR (KBr): 1603; 1577; 1510; 1454; 1388; 1125 cm−1. Mp (Hex/EtOAc): 92–93 °C. HRMS: calculated for C18H23N2O3 [M + H]+: 315.1709; found: 315.1715.
2-(Pyrrolidin-1-yl)-5-(1-(3,4,5-trimethoxyphenyl)vinyl)pyridine (3c). A solution of 176 mg (0.51 mmol) of 1c in THF was added to a suspension of 623 mg (1.54 mmol) of methyltriphenylphosphonium iodide and 1.0 mL (1.6 mmol) of nBuLi (1.6 M in hexane) in dry THF at −40 °C to obtain, after column chromatography with Hex/EtOAc 1:1, 80 mg (0.23 mmol, 46%) of compound 3c. 1H-NMR δ: 2.00 (4H, m); 3.47 (4H, m); 3.79 (6H, s); 3.85 (3H, s); 5.23 (1H, s); 5.31 (1H, s); 6.33 (1H, d, 8.8); 6.54 (2H, s); 7.41 (1H, dd, 8.8, 2.2); 8.18 (1H, d, 2.2). 13C-NMR δ: 25.5 (2) (CH2); 46.9 (2) (CH2); 56.1 (2) (OCH3); 60.8 (OCH3); 105.5 (2) (CH); 106.0 (CH); 111.2 (CH2); 124.3 (C); 137.1 (CH); 134.5 (C); 137.8 (C); 147.0 (CH); 147.2 (C); 152.8 (2) (C); 156.3 (C). IR (KBr): 1600; 1506; 1235; 1126; 1006 cm−1. HRMS: calculated for C20H25N2O3 [M + H]+: 341.1865; found 341.1870.
N,N-dimethyl-4-(1-(2,3,4-trimethoxyphenyl)vinyl)aniline (3d). A solution of 500 mg (1.58 mmol) of 1d in THF was added to a suspension of 1.93 g (4.80 mmol) of methyltriphenylphosphonium iodide and 2.5 mL (4.0 mmol) of nBuLi (1.6 M in hexane) in dry THF at −40 °C to obtain, after column chromatography with Hex/EtOAc 9:1, 349 mg (1.11 mmol, 70%) of compound 3d. 1H-NMR δ: 2.95 (6H, s); 3.59 (3H, s); 3.87 (3H, s); 3.89 (3H, s); 5.09 (1H, d, 1.6); 5.54 (1H, d, 1.6); 6.64 (2H, d, 8.8); 6.67 (1H, d, 8.4); 6.92 (1H, d, 8.4); 7.19 (2H, d, 8.8). 13C-NMR δ: 40.5 (2) (CH3); 56.0 (CH3); 60.8 (CH3); 60.9 (CH3); 106.9 (CH); 111.8 (CH2); 112.1 (2) (CH); 125.4 (CH); 127.6 (2) (CH); 129.8 (C); 129.9 (C); 142.4 (C); 146.5 (C); 150.1 (C); 151.8 (C); 153.3 (C). IR (KBr): 1602; 1521 cm−1. Mp (t-butylmethylether): 63–65 °C. HRMS: calculated for C19H24NO3 [M + H]+: 314.1756; found: 314.1748.
4-(1-(2,5-Dimethoxyphenyl)vinyl)-N,N-dimethylaniline (3e). A solution of 500 mg (1.75 mmol) of 1e in THF was added to a suspension of 2.12 g (4.80 mmol) of methyltriphenylphosphonium iodide and 2.7 mL (4.38 mmol) of nBuLi (1.6 M in hexane) in dry THF at −40 °C to obtain, after column chromatography with Hex/EtOAc 6:4, 245 mg (0.87mmol, 50%) of compound 3e. 1H-NMR δ: 2.99 (6H, s); 3.68 (3H, s); 3.82 (3H, s); 5.18 (1H, s); 5.70 (1H, s); 6.70 (2H, d, 9.0); 6.90 (3H, s); 7.28 (2H, d, 9.0). 13C-NMR δ: 40.6 (2)(CH3); 55.8 (CH3); 56.7 (CH3); 111.7 (CH2); 112.1 (2) (CH); 112.8 (CH); 113.2 (CH); 117.1 (CH); 127.3 (2) (CH); 128.8 (C); 133.0 (C); 146.4 (C); 150.1 (C); 151.6 (C); 153.7 (C). IR (KBr): 1525, 1605 cm−1. Mp (t-butylmethylether): 68–70 °C. HRMS: calculated for C18H22NO2 [M + H]+: 284.1651; found: 284.1645.
N,N-Dimethyl-3-(1-(3,4,5-trimethoxyphenyl)vinyl)aniline (3f). A solution of 300 mg (0.95 mmol) of 1f in THF was added to a suspension of 1.15 g (2.85 mmol) of methyltriphenylphosphonium iodide and 1.2 mL (1.9 mmol) of nBuLi (1.6 M in hexane) in dry THF at −40 °C to obtain, after column chromatography with Hex/EtOAc 7:3, 280 mg (0.89mmol, 94%) of compound 3f. 1H-NMR (CD3OD) δ: 2.90 (6H, s); 3.75 (6H, s); 3.78 (3H, s); 5.38 (1H, d, 1.2); 5.41 (1H, d, 1.2); 6.61 (2H, s); 6.65 (1H, d, 8.0); 6.73 (1H, s); 6.75 (1H, dd, 8.0); 7.16 (1H, t, 8.0). 13C-NMR (CD3OD) δ: 40.8 (2) (CH3); 56.2 (2) (CH3); 60.9 (CH3); 105.7 (2) (CH); 112.3 (CH); 112.8 (CH); 113.6 (CH2); 117.3 (CH); 128.8 (CH); 137.5 (C); 137.7 (C); 142.1 (C); 150.5 (C); 150.8 (C); 152.8 (2) (C). IR (film): 1596; 1578; 1503; 1411; 1353; 1235; 1127; 898 cm−1. HRMS: calculated for C19H24NO3 [M + H]+: 314.1756; found: 314.1750
N,N-Dimethyl-4-(1-(3,4,5-trimethoxyphenyl)vinyl)pyridin-2-amine (3g). A solution of 141 mg (0.44 mmol) of 1g in THF was added to a suspension of 510 mg (1.26 mmol) of methyltriphenylphosphonium iodide and 1.0 mL (1.6 mmol) of nBuLi (1.6 M in hexane) in dry THF at −40 °C to obtain, after column chromatography with CH2Cl2/EtOAc 9:1, 102 mg (0.32 mmol, 73%) of compound 3g. 1H-NMR (CD3OD) δ: 3.10 (6H, s); 3.82 (6H, s); 3.88 (3H, s); 5.51 (1H, s); 5.53 (1H, s); 6.49 (1H, d, 8.2); 6.52 (1H, s); 6.53 (2H, s); 8.12 (1H, d, 8.2). 13C-NMR (CD3OD) δ: 37.1 (2) (CH3); 55.0 (2) (CH3); 59.5 (CH3); 105.1 (2) (CH); 110.9 (CH); 111.9 (CH); 114.9 (CH2); 135.8 (C); 137.6 (C); 146.5 (CH); 148.6 (C); 150.3 (C); 152.6 (2) (C); 159.3 (C). IR (film): 1594; 1541; 1502; 1409; 1234; 1127 cm−1. HRMS: calculated for C18H23N2O3 [M + H]+: 315.1709; found: 315.1712.
2-(Pyrrolidin-1-yl)-4-(1-(3,4,5-trimethoxyphenyl)vinyl)pyridine (3h). A solution of 360 mg (1.05 mmol) of 1g in THF was added to a suspension of 1.27 g (3.15 mmol) of methyltriphenylphosphonium iodide and 1.6 mL (2.6 mmol) of nBuLi (1.6 M in hexane) in dry THF at −40 °C to obtain, after column chromatography with Hex/EtOAc 8:2, 295 mg (0.87 mmol, 83%) of compound 3h. 1H-NMR δ: 1.98 (4H, m); 3.43 (4H, m); 3.80 (6H, s); 3.86 (3H, s); 5.47 (1H, s); 5.50 (1H, s); 6.31 (2H, s); 6.46 (1H, d, 5.0); 6.53 (1H, s); 8.10 (1H, d, 5.0). 13C-NMR δ: 25.5 (2) (CH2); 46.8 (2) (CH2); 56.1 (2) (CH3); 60.9 (CH3); 105.5 (2) (CH); 105.8 (CH); 111.1 (CH); 115.3 (CH2); 135.9 (C); 137.9 (C); 147.9 (C); 149 (CH); 149.7 (C); 152.9 (2) (C); 157.6 (C). IR (film): 1593; 1535; 1492; 1454; 1236; 1126 cm−1. HRMS: calculated for C20H25N2O3 [M + H]+: 341.1865; found: 341.1862.
4.1.5. General Procedure to Obtain Dihydro Derivatives by Reduction of Isocombretastatins (4d, 4e, 4f)
Catalytic amounts of Pd/C were added over a 0.01–0.02 M solution of the isocombretastatins in ethanol. After 24 h stirring at room temperature and under hydrogen atmosphere, the residue was filtered over silica gel and concentrated under vacuum.
N,N-Dimethyl-4-(1-(2,3,4-trimethoxyphenyl)ethyl)aniline (4d). Ten mg of Pd/C were added to a solution of 84 mg (0.30 mmol) of 3d in EtOH under hydrogen atmosphere to obtain, after column chromatography with Hex/EtOAc 95:5, 56 mg (0.20 mmol; 67%) of 4d. 1H-NMR δ: 1.55 (3H, d, 7.3); 2.91 (6H, s); 3.70 (3H, s); 3.84 (3H, s); 3.87 (3H, s); 4.40 (1H, q, 7.3); 6.63 (1H, d, 8.6); 6.71 (2H, d, 8.6); 6.88 (1H, d, 8.6); 7.12 (2H, d,8.6). 13C-NMR δ: 21.8 (CH); 36.7 (CH3); 40.8 (CH); 55.9 (CH); 60.6 (CH); 60.8 (CH); 107.0 (CH3); 112.8 (CH3); 121.7 (CH3); 128.1 (2)(CH3); 133.3 (C); 135.2 (C); 142.2 (C); 148.7 (C); 151.4 (C); 151.7 (C). IR (film): 1519; 1610 cm−1. HRMS: calculated for C19H26NO3 [M + H]+: 316.1913; found: 316.1915.
4-(1-(2,5-Dimethoxyphenyl)ethyl)-N,N-dimethylaniline (4e). Ten mg of Pd/C were added to a solution of 63.5 mg (0.22 mmol) of 3e in EtOH under hydrogen atmosphere to obtain, after column chromatography with Hex/EtOAc 95:5, 44 mg (0.15 mmol; 68%) of 4e. 1H-NMR δ: 1.55 (3H, d, 7.2); 2.91 (6H, s); 3.73 (3H, s); 3.75 (3H, s); 4.49 (1H, q, 7.2); 6.56–6.79 (5H, m); 7.14 (2H, d, 8.6). 13C-NMR δ: 20.9 (CH3); 36.5 (CH); 40.9 (CH3); 55.5 (CH3); 56.2 (CH3); 77.0 (c); 110.2 (CH); 111.6 (CH); 112.9 (CH); 114.6 (CH); 128.2 (2) (CH); 137.2 (C); 148.7 (C); 151.2 (C); 153.6 (C). IR (film): 1517; 1613 cm−1. HRMS: calculated for C18H24NO2 [M + H]+: 286.1807; found: 286.1816.
N,N-Dimethyl-3-(1-(3,4,5-trimethoxyphenyl)ethyl)aniline (4f). Ten mg of Pd/C were added to a solution of 50 mg (0.16 mmol) of 3f in EtOH under hydrogen atmosphere to obtain 47 mg (0.15 mmol; 94%) of 4f. 1H-NMR δ: 1.63 (3H, d, 7.5); 2.93 (6H, s); 3.99 (9H, s); 4.10 (1H, q, 7.5); 6.48 (2H, s); 6.60 (1H, d, 6.5); 6.62 (1H, d, 6.5); 6.66 (1H, bs); 7.22 (1H, t, 6.5). 13C-NMR δ: 22.2 (CH3); 41.1 (2) (CH3); 45.5 (CH); 56.1 (2) (CH3); 60.9 (CH3); 104.7 (2) (CH); 111.1 (CH); 112.7 (CH); 129.2 (2) (CH); 136.2 (C); 142.2 (C); 147.2 (C); 150.2 (C); 153.0 (2) (C). IR (film): 1589; 1499; 1458; 1327; 1233; 1126 cm−1. HRMS: calculated for C19H26NO3 [M + H]+: 316.1913; found: 316.1909.
4.1.6. General Procedure to Obtain Combretastatins by Wittig Reactions (8a–c and 8g–i)
0.75 to 1.10 mmol per mmol of nBuLi (1.6 M in hexane) were added to a 0.08 M suspension of triphenyl(3,4,5-trimethoxybenzyl)phosphonium bromide in dry THF at −78 °C under inert atmosphere. After one hour stirring, 0.25 to 0.90 mmol of the corresponding aromatic aldehyde per mmol of the phosphonium salt were added and reaction was allowed to reach room temperature. After 24 h, the reaction was poured onto ice and extracted with CH2Cl2. Organic layers were washed with brine, dried over sodium sulphate and concentrated under vacuum. The residue was purified by column chromatography on silica gel.
N,N-dimethyl-4-(3,4,5-trimethoxystyryl)aniline (
8a) [
15]. A solution of 1.00 g (6.70 mmol) of
p-dimethylaminebenzaldehyde in THF was added to a suspension of 4.20 g (8.04 mmol) of triphenyl(3,4,5-trimethoxybenzyl)phosphonium bromide and 6.10 mL (9.65 mmol) of
nBuLi (1.6 M in hexane) in dry THF at −78 °C to obtain, after column chromatography with Hex/EtOAc 7:3, 290 mg (0.92 mmol, 14%) of isomer (
Z)-
8a, 113 mg (0.36 mmol, 5%) of isomer (
E)-
8b and 1.20 g (3.83 mmol, 57%) of a 1:1
Z/
E mixture. (
Z)-
8a: 1H-NMR δ: 2.93 (6H, s); 3.71 (6H, s); 3.85 (3H, s); 6.32 (1H, d, 12.2); 6.46 (1H, d, 12.2); 6.57 (2H, s); 6.60 (2H, d; 8.6); 7.20 (2H, d, 8.6).
13C-NMR δ
: 40.5 (2) (CH
3); 55.9 (2) (CH
3); 60.9 (CH
3); 105.8 (2) (CH); 112.0 (2) (CH); 125.1 (C); 126.8 (CH); 130.1 (3) (CH); 133.6 (C); 136.7 (C); 149.7 (C); 152.9 (2) (C). IR (film): 1606; 1585; 1516; 1236; 1126; 767 cm
−1. HRMS: calculated for C
19H
24NO
3 [M + H]
+: 314.1751; found: 314.1758. (
E)-
8a: 1H-NMR δ: 2.99 (6H, s); 3.87 (3H, s); 3.92 (6H, s); 6.74 (2H, s); 6.76 (2H, d, 8.8); 6.82 (1H, d, 15.4); 6.96 (1H, d, 15.4); 7.40 (2H, d, 8.8).
13C-NMR δ
: 40.6 (2) (CH
3); 56.1 (2) (CH
3); 61.0 (CH
3); 103.0 (2) (CH); 112.6 (2) (CH); 124.4 (CH); 125.8 (C); 127.6 (2) (CH); 128.4 (CH); 134.0 (C); 137.2 (C); 150.0 (C); 153.4 (2) (C). IR (film): 1606; 1585; 1516; 1236; 1126; 767 cm
−1. Mp (diethyl ether): 94–95 °C. HRMS: calculated for C
19H
24NO
3 [M + H]
+: 314.1756; found: 314.1760.
N,N-dimethyl-5-(3,4,5-trimethoxystyryl)pyridin-2-amine (8b). A solution of 230 mg (1.53 mmol) of 6-(dimethylamino)nicotinaldehyde in THF was added to a suspension of 1.30 g (2.14 mmol) of triphenyl(3,4,5-trimethoxybenzyl)phosphonium bromide and 1.5 mL (2.10 mmol) of nBuLi (1.6 M in hexane) in dry THF at −40 °C to obtain, after column chromatography with Hex/EtOAc 1:1, 65 mg (0.21 mmol, 14%) of isomer (Z)-8b, and 213 mg (0.68 mmol, 44%) of a 1:1 Z/E mixture. (Z)-8b: 1H-NMR δ: 3.05 (6H, s); 3.72 (6H, s); 3.83 (3H, s); 6.32 (1H, d, 8.8); 6.38 (2H, s); 6.51 (2H, s); 7.38 (1H, dd, 8.8, 2.2); 8.09 (1H, d, 2.2). 13C-NMR δ: 38.1 (2) (CH3). 56.0 (2) (CH3). 60.8 (CH3). 104.7 (CH). 105.7 (2) (CH). 109.5 (C). 120.4 (C). 126.9 (CH). 127.8 (CH). 133.2 (C). 133.6 (C). 137.2 (CH). 148.9 (CH). 153.3 (2) (C). 158.5 (C). IR (film): 1606; 1600; 1508; 1457; 1389; 1236; 1126 cm−1. HRMS: calculated for C18H23N2O3 [M + H]+: 315.1709; found: 315.1708. (E)-8b: 1H-NMR δ: 3.11 (6H, s); 3.86 (3H, s); 3.92 (6H, s); 6.52 (1H, d, 8.8); 6.69 (2H, s); 6.80 (1H, d, 16.4); 6.89 (1H, d, 16.4); 7.67 (1H, dd, 8.8, 2.2); 8.22 (1H, d, 2.2). 13C-NMR δ: 38.5 (2) (CH3); 56.4 (2) (CH3); 61.2 (CH3); 103.2 (2) (CH); 106.2 (CH); 121.3 (C); 125.5 (CH); 128.1 (CH); 133.8 (C); 134.1 (CH); 137.6 (CH); 147.5 (C); 153.6 (2) (C); 158.8 (C). IR (film): 1606; 1585; 1516; 1236; 1126; 767 cm−1.
2-(Pyrrolidin-1-yl)-5-(3,4,5-trimethoxystyryl)pyridine (8c). A solution of 57 mg (0.32 mmol) of 6-(pyrrolidin-1-yl)nicotinaldehyde in THF was added to a suspension of 156 mg (0.27 mmol) of triphenyl(3,4,5-trimethoxybenzyl)phosphonium bromide and 0.2 mL (0.12 mmol) of nBuLi (1.6 M in hexane) in dry THF at −40 °C to obtain, after column chromatography with Hex/EtOAc 1:1, 40 mg (0.12 mmol, 38%) of a 1:1 Z/E mixture of compound 8c. (Z)-8c: 1H-NMR δ: 2.04 (4H, m); 3.50 (4H, m); 3.78 (6H, s); 3.92 (3H, s); 6.23 (1H, d, 8.8); 6.33 (1H, d, 12.0); 6.43 (1H, d, 12.0); 6.49 (2H, s); 7.40 (1H, dd, 8.8, 2.2); 8.11 (1H, d, 2.2). (E)-8c: 2.02 (4H, m); 3.50 (4H, m); 3.85 (6H, s); 3.93 (3H, s); 6.36 (1H, s); 6.70 (2H, s); 6.71 (1H, d, 4.4); 6.84 (1H, d, 2.2); 6.94 (1H, s); 8.22 (1H, d, 2.2). HRMS: calculated for C20H25N2O3 [M + H]+: 341.1865; found: 341.1868.
2-(Pyrrolidin-1-yl)-4-(3,4,5-trimethoxystyryl)pyridine (8h). A solution of 400 mg (2.27 mmol) of 6-(dimethylamino)nicotinaldehyde in THF was added to a suspension of 1.66 g (3.18 mmol) of triphenyl(3,4,5-trimethoxybenzyl)phosphonium bromide and 2.0 mL (3.18 mmol) of nBuLi (1.6 M in hexane) in dry THF at −40 °C to obtain, after column chromatography with Hex/EtOAc 6:4, 123 mg (0.23 mmol, 16%) of isomer (Z)-8h, and 181 mg (0.53 mmol, 17%) of a 1:1 Z/E mixture. (Z)-8h: 1H-NMR δ: 1.95 (4H, m); 3.35 (4H, m); 3.68 (6H, s); 3.82 (3H, s); 6.24 (1H, d, 1.4); 6.40 (1H, d, 12.2); 6.41 (1H, dd, 5.2, 1.4); 6.50 (2H, s); 6.54 (1H, d, 12.2); 8.02 (1H, d, 5.2).13C-NMR δ: 25.5 (2) (CH2); 46.6 (2) (CH2); 56.0 (2) (CH3); 60.9 (CH3); 106.1 (2) (CH); 106.1 (CH); 111.3 (CH); 128.5 (CH); 132.2 (C); 132.3 (CH); 137.5 (C) 145.9 (C); 147.9 (2) (C); 152.9 (CH); 157.6 (C). IR (film): 1650; 1598; 1538; 1329; 1229; 1127 cm−1. HRMS: calculated for C20H25N2O3 [M + H]+: 341.1865; found: 341.1868. (E)-8h: 1H-NMR δ: 1.98 (4H, m); 3.45 (4H, m); 3.80 (6H, s); 3.84 (3H, s); 6.48 (1H, s); 6.66 (1H, d, 5.4); 6.88 (1H, d, 16.1); 6.72 (2H, s); 7.08 (1H, d, 16.1); 8.07 (1H, d, 5.4). 13C-NMR δ: 25.6 (2) (CH2); 46.8 (2) (CH2); 56.1 (2) (CH3); 60.9 (CH3); 104.0 (2) (CH); 104.5 (CH); 106.2 (CH); 126.6 (C); 126.7 (CH); 131.8 (C); 132.0 (CH); 137.5 (CH) 145.6 (C); 152.8 (2) (C); 155.2 (C). IR (film): 1650; 1598; 1538; 1329; 1229; 1127 cm−1.
1-(4-(3,4,5-Trimethoxystyryl)phenyl)pyrrolidine (8i). A solution of 2.00 g (11.4 mmol) of 4-(pyrrolidin-1-yl)benzaldehyde in THF was added to a suspension of 7.10 g (13.70 mmol) of triphenyl(3,4,5-trimethoxybenzyl)phosphonium bromide and 9.8 mL (13.70 mmol) of nBuLi (1.6 M in hexane) in dry THF at −40 °C to obtain, after column chromatography with Hex/EtOAc 7:3, 1.83 g (5.40 mmol, 47%) of isomer (Z)-8i, and 1.20 g (0.53 mmol, 31%) of isomer (E)-8i. (Z)-8i: 1H-NMR δ: 1.99 (4H, m); 3.26 (4H, m, NCH2); 3.73 (6H, s, OCH3); 3.86 (3H, s, OCH3); 6.30 (1H, d; 12); 6,41 (2H, d; 8.2); 6.45 (1H, d, 12); 6.62 (2H, s); 7.21 (2H, d, 8.2). 13C-NMR δ: 25.5 (2) (CH2); 47.6 (2) (CH2); 56.0 (2) (OCH3); 60.9 (OCH3); 105.8 (2) (CH); 111.2 (2) (CH); 124.0 (C); 126.0 (CH); 130.3 (2) (CH); 130.4 (CH); 133.9 (C); 136.7 (C); 147.1 (C); 153.0 (2) (C). IR (film): 1604; 1237; 1126; 772 cm−1. HRMS: calculated for C21H26NO3 [M + H]+: 340.1913; found: 341.1902. (E)-8i: 1H-NMR δ: 2.00 (4H, m); 3.31 (4H, m, NCH2); 3.87 (3H, s, OCH3); 3.91 (6H, s, OCH3); 6.56 (2H, d, 8.6); 6.70 (2H, s); 6.80 (1H, d, 16.1); 6.98 (1H, d, 16.1); 7.40 (2H, d, 8.6). 13C-NMR δ: 25.5 (2) (CH2); 47.7 (2) (CH2); 56.1 (2) (OCH3); 61.0 (OCH3); 102.9 (2) (CH); 111.9 (2) (CH); 123.6 (CH); 124.7 (C); 127.7 (2) (CH); 128.7 (CH); 134.2 (C); 137.1 (C); 147.5 (C); 153.4 (2) (C). IR (film): 1585, 1233, 1126, 818 cm−1. Mp (EtOAc): 118–119 °C.
4.1.7. General Procedure to Obtain the Ammonium Salt Derivatives (5a, 5d, 5e, 6a, 7a, 7d, 9a, 9b and 9i)
3.0–10.0 mmol per mmol of iodomethane were added to a 0.05 M solution of the corresponding amino derivatives in dry acetone and the reaction was refluxed in a sealed tube for 24 h. After cooling, the precipitate was filtered off and washed with ether.
N,N,N-Trimethyl-4-(3,4,5-trimethoxybenzoyl)benzenaminiumiodide (5a). 200 mg (0.63 mmol) of diarylketone 1a and 0.237 mL (3.81 mmol) of iodomethane led to 130 mg (0.39 mmol, 62%) of 5a. 1H-NMR (CD3OD) δ: 3.68 (9H, s); 3.75 (6H, s); 3.77 (3H, s); 6.99 (2H, s); 7.88 (2H, d, 6.8); 8.01 (2H, d, 6.8). 13C-NMR (CD3OD) δ: 56.9 (3) (CH3); 57.3 (2) (CH3); 64.5 (CH3); 109.1 (2) (CH); 121.5 (2) (CH); 132.7 (2) (CH); 141.0 (C); 147.8 (C); 150.8 (C); 153.8 (C); 154.5 (2) (C); 190.0 (C). IR (KBr): 3002; 1661; 1582; 1332; 1125 cm−1. HRMS: calculated for C19H24NO4 [M]+: 330.1700; found: 330.1690.
N,N,N-Trimethyl-4-(2,3,4-trimethoxybenzoyl)benzenaminium iodide (5d). 250 mg (0.79 mmol) of diarylketone 1d and 0.495 mL (7.90 mmol) of iodomethane led to 180 mg (0.39 mmol, 69%) of 5d, after crystallization in methanol. 1H-NMR (Pyridine) δ: 3.35 (3H, s); 3.40 (3H, s); 3.45 (3H, s); 3.74 (9H, s); 6.47 (1H, d, 8.8); 7.02 (1H, d, 8.8); 7.70 (2H, d, 8.4); 7.72 (2H, d, 8.4). 13C-NMR (Pyridine) δ: 54.8 (CH3); 56.8 (3) (CH3); 59.4 (CH3); 60.6 (CH3); 106.6 (CH); 119.6 (2) (CH); 124.1 (CH); 124.6 (C); 130.2 (2) (CH); 139.4 (C); 141.0 (C); 148.7 (C); 151.9 (C); 156.2 (C); 192.2 (C). IR (KBr): 1648; 1592; 1461; 1412; 1296; 1094 cm−1. Mp (methanol): 172–173 °C. HRMS: calculated for C19H24NO4 [M]+: 330.1700; found: 330.1705.
4-(2,5-Dimethoxybenzoyl)-N,N,N-trimethylbenzenaminium iodide (5e). 260 mg (0.91 mmol) of diarylketone 1e and 0.170 mL (2.71 mmol) of iodomethane led to 135 mg (0.47 mmol, 52%) of 5e, after crystallization in methanol. 1H-NMR (DMSO-d6) δ: 3.60 (3H, s); 3.63 (9H, s); 3.73 (3H, s); 6.93 (1H, t, 1.8); 7.15 (2H, d, 1.8); 7.84 (2H, d, 9.0); 8.08 (2H, d, 9.0). 13C-NMR (DMSO-d6) δ: 40.4 (3) (CH3); 56.3 (CH3); 56.4 (CH3); 113.6 (CH); 114.0 (CH); 117.8 (CH); 121.3 (2) (CH); 127.9 (C); 130.6 (2) (CH); 138.0 (C); 150.2 (C); 150.8 (C); 153.1 (C); 194.1 (C). IR (KBr): 1662; 1600; 1496; 1413; 1226; 1035 cm−1. Mp (diethyl ether): 162–163 °C. HRMS: calculated for C18H22NO3 [M]+: 300.1594; found: 330.1600.
4-((Hydroxyimino)(3,4,5-trimethoxyphenyl)methyl)-N,N,N-trimethylbenzenaminium iodide (6a). 100 mg (0.30 mmol) of oxime 2a and 0.200 mL (3.03 mmol) of iodomethane led to 31 mg (0.09 mmol, 30%) of 6a, after crystallization in methanol/ethyl acetate. (Z): 1H-NMR (CD3OD) δ: 3.28 (9H, s); 3.73 (6H, s); 3.78 (3H, s); 6.71 (2H, s); 7.62 (2H, d, 8.8); 8.01 (2H, d, 8.8). (E): 1H-NMR (CD3OD) δ: 3.30 (9H, s); 3.68 (6H, s); 3.78 (3H, s); 6.60 (2H, s); 7.71 (2H, d, 8.8); 7.88 (2H, d, 8.8). IR (KBr): 3470; 1582; 1505; 1344; 1130 cm−1. Mp (EtOAc/MeOH): 179–180 °C. HRMS: calculated for C19H25N2O4 [M]+: 345.1808; found: 345.1806.
N,N,N-trimethyl-4-(1-(3,4,5-trimethoxyphenyl)vinyl)benzenaminium iodide (7a). 250 mg (0.83 mmol) of isocombretastatine 3a and 0.500 mL (7.97 mmol) of iodomethane led to 260 mg (0.79 mmol, 95%) of 7a. 1H-NMR (CD3OD) δ: 3.62 (9H, s); 3.68 (6H, s); 3.73 (3H, s); 5.59 (1H, s); 5.63 (1H, s); 6.53 (2H, s); 7.53 (2H, d, 8.3); 7.93 (2H, d, 8.3). 13C-NMR (CD3OD) δ: 56.7 (3) (CH3); 57.8 (2) (CH3); 61.1 (CH3); 106.8 (2) (CH); 116.9 (CH2); 121.2 (2) (CH); 131.0 (2) (CH); 137.7 (C); 144.8 (C); 145.9 (C); 147.7 (C); 149.5 (C); 154.4 (2) (C). IR (KBr): 1662; 1600; 1496; 1413; 1226; 1035 cm−1. Mp (acetone): 176–177 °C. HRMS: calculated for C20H26NO3 [M]+: 328.1907; found: 328.1899.
N,N,N-Trimethyl-4-(1-(2,3,4-trimethoxyphenyl)vinyl)benzenaminium iodide (7d). 170 mg (0.54 mmol) of isocombretastatine 3d and 0.170 mL (2.71 mmol) of iodomethane led to 160 mg (0.49 mmol, 91%) of 7d, after crystallization in methanol. 1H-NMR (DMSO-d6) δ: 3.35 (3H, s); 3.51 (9H, s); 3.66 (3H, s); 3.76 (3H, s); 5.32 (1H, s); 5.70 (1H, s); 6.79 (1H, d, 8.2); 6.89 (1H, d, 8.2); 7.37 (2H, d, 8.6); 7.76 (2H, d, 8.6). 13C-NMR (DMSO-d6) δ: 55.9 (CH3); 56.4 (3) (CH3); 60.4 (2) (CH3); 107.9 (CH); 117.7 (CH2); 120.3 (2) (CH); 124.8 (CH); 127.2 (C); 127.4 (2) (CH); 141.8 (C); 142.6 (C); 144.6 (C); 146.1 (C); 150.9 (C); 153.6 (C). IR (KBr): 1594; 1496; 1459; 1406; 1295; 1090 cm−1. Mp (methanol): 168–169 °C. HRMS: calculated for C20H26NO3 [M]+: 328.1907; found: 328.1913.
N,N,N-trimethyl-4-(3,4,5-trimethoxystyryl)benzenaminium iodide (9a). 290 mg (0.92 mmol) of a 1:1 Z/E mixture of combretastatine 8a and 0.500 mL (7.97 mmol) of iodomethane led to 260 mg (0.79 mmol, 86%) of a 7:3 Z/E mixture of compound 9a. After crystallization in acetone/ethyl acetate, 61 mg (0.19 mmol, 21%) of isomer Z were isolated. 1H-NMR δ: 3.65 (6H, s); 3.79 (3H, s); 3.95 (9H, s); 6.37 (2H, s); 6.48 (1H, d, 12.2); 6.64 (1H, d, 12.2); 7.45 (2H, d, 8.6); 7.84 (2H, d, 8.6). 13C-NMR δ: 56.2 (2) (CH3); 57.9 (3) (CH3); 60.9 (CH3); 105.8 (2) (CH); 120.0 (2) (CH); 127.2 (CH); 131.0 (2) (CH). 132.9 (CH); 138.4 (C); 139.6 (C); 139.8 (C); 145.4 (C); 153.4 (2) (C). IR (KBr): 1581; 1240; 1125; 844 cm−1. Mp (acetone/ethylacetate): 75 °C. HRMS: calculated for C20H26NO3 [M]+: 328.1907; found: 328.1916.
N,N,N-trimethyl-5-(3,4,5-trimethoxystyryl)pyridin-2-aminium iodide (9b). 160 mg (0.51 mmol) of a 1:1 Z/E mixture of combretastatin 8b and 0.320 mL (5.1 mmol) of iodomethane led to 213 mg of reaction crude. After crystallization in acetone/methanol, 35 mg (0.11 mmol, 22%) of isomer (Z)-9b were isolated. 1H-NMR (CD3OD) δ: 3.56 (9H, s); 3.75 (3H, s); 3.79 (6H, s); 6.84 (2H, s); 7.18 (1H, d, 1.0); 7.34 (1H, d, 16.0); 7.79 (1H, d, 8.6); 8.17 (1H, dd, 8.6, 2.2); 8.64 (1H, d, 2.2). IR (KBr): 1579; 1462; 1244; 1122 cm−1. HRMS: calculated for C19H25N2O3 [M]+: 329.1860; found: 329.1850.
1-Methyl-1-(4-(3,4,5-trimethoxystyryl)phenyl)pyrrolidin-1-ium iodide (9i). 100 mg (0.29 mmol) of combretastatin (Z)-8i and 0.182 mL (2.9 mmol) of iodomethane led to 92 mg (0.26 mmol, 89%) of isomer (E)-9i. 1H-NMR δ: 2.33 (4H, m); 3.64 (3H, s); 3.82 (3H, s); 3.88 (6H, s); 4.06 (2H, m); 4.61 (2H, m); 6.74 (2H, s); 6.96 (1H, d, 16.0); 7.05 (1H, d, 16.0); 7.61 (2H, d, 8.6); 7.84 (2H, d, 8.6). 13C-NMR δ: 21.2 (2) (CH2); 55.7 (CH3); 56.4 (2) (CH3); 61.0 (CH3); 66.7 (2) (CH2); 104.0 (2) (CH); 121.6 (2) (CH); 125.3 (CH); 128.1 (2) (CH); 131.8 (C); 132.1 (CH); 138.4 (C); 139.6 (C); 144.5 (C); 153.4 (2) (C). IR (KBr): 1583; 1247; 1125; 845 cm−1. Mp (acetone): 158–159 °C. HRMS: calculated for C22H28NO3 [M]+: 354.2069; found: 354.2044.