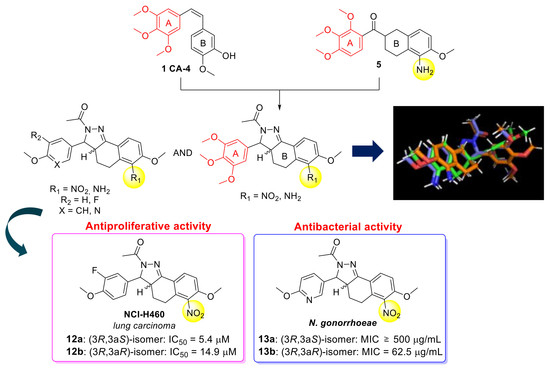
Design and Synthesis of New 6-Nitro and 6-Amino-3,3a,4,5-Tetrahydro-2H-Benzo[g]indazole Derivatives: Antiproliferative and Antibacterial Activity
Abstract
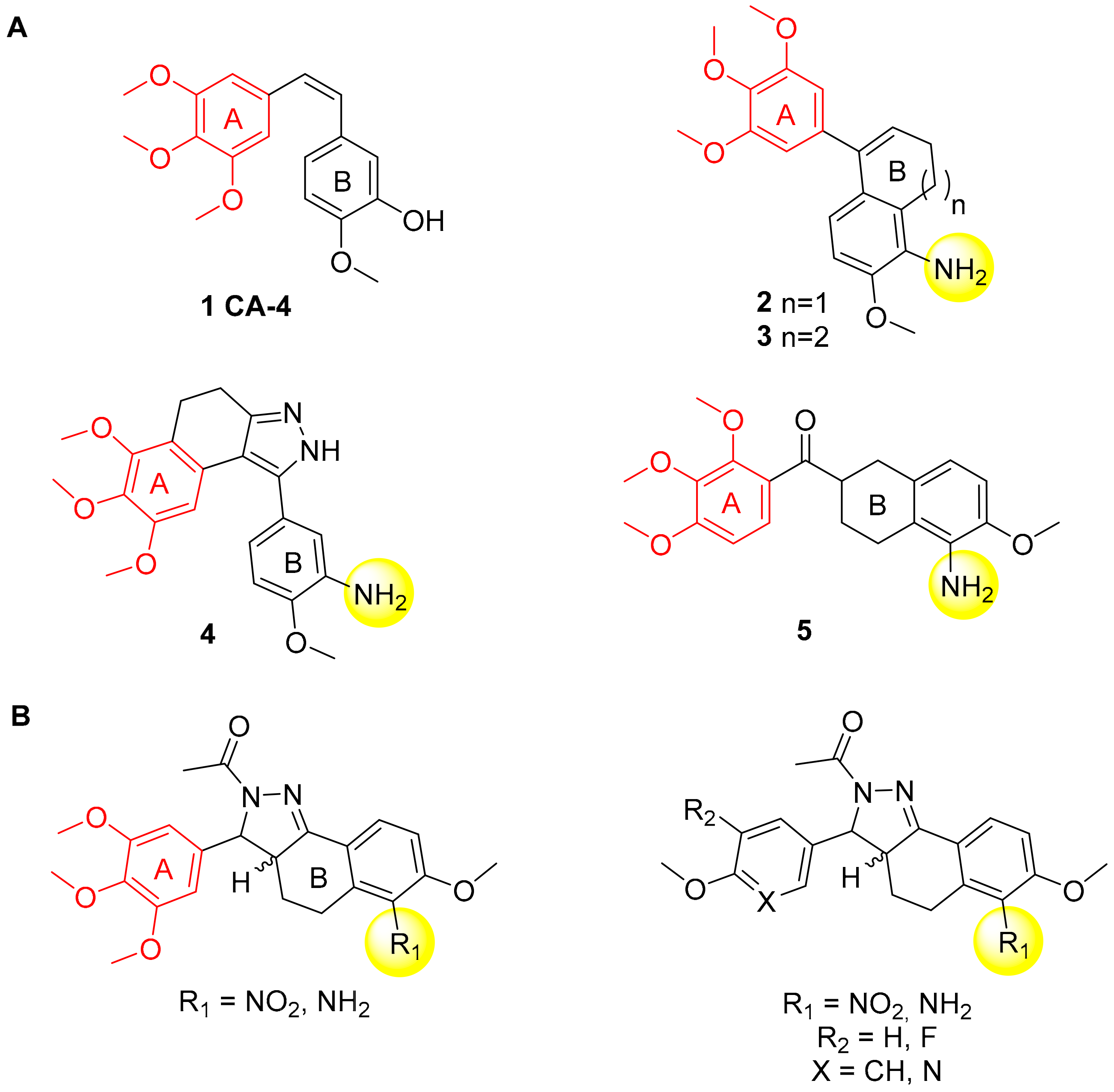
1. Introduction
2. Results and Discussion
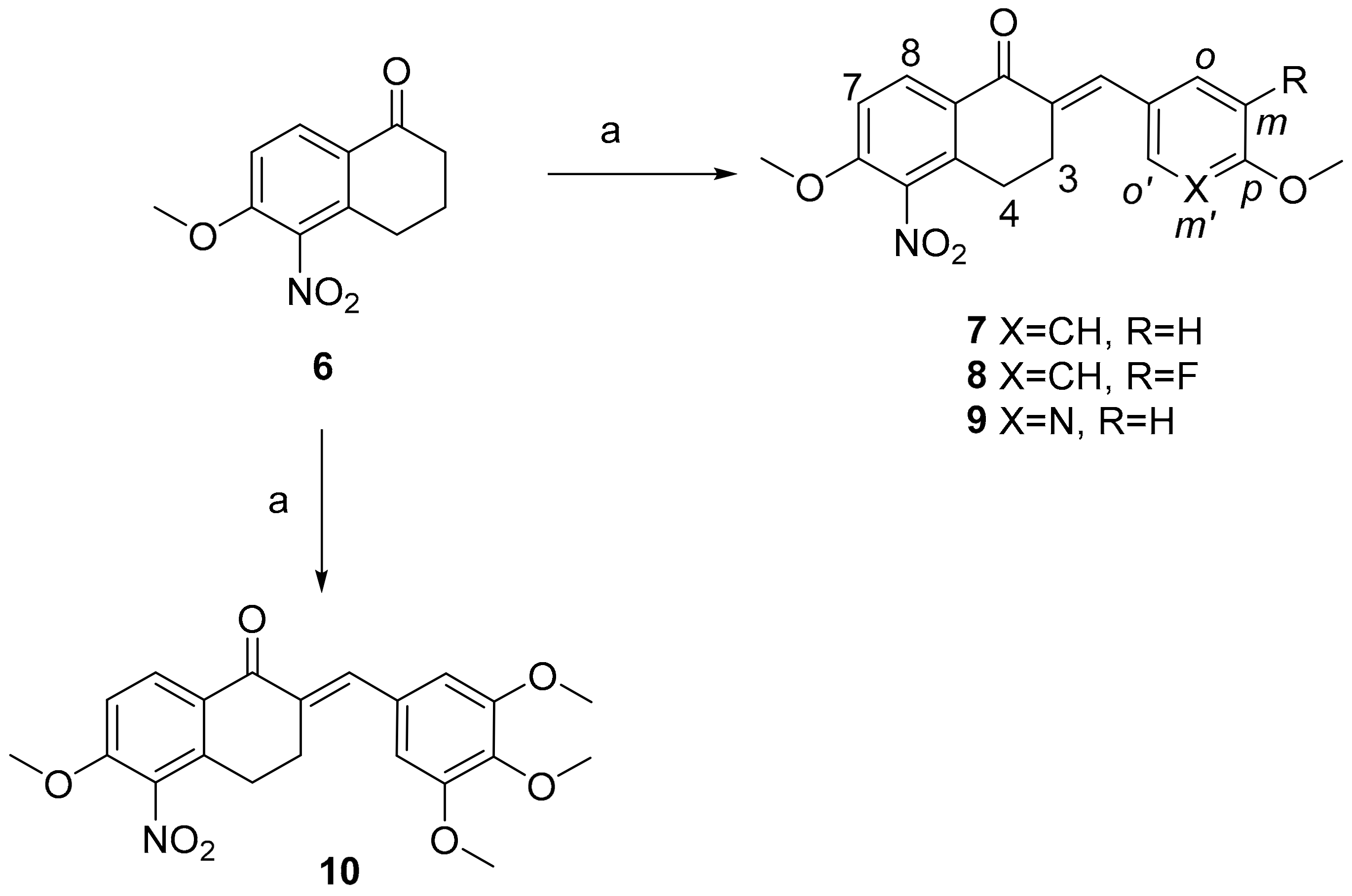
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. Antiproliferative Activity
2.2.2. Antibacterial Activity
3. Materials and Methods
3.1. General Information
3.2. Chemistry
3.2.1. Preparation of 6-Methoxy-5-nitro-1-tetralone (6)
3.2.2. General Procedure for the Synthesis of Chalcones 7–10 Derived from 6-Methoxy-5-Nitro-1-Tetralone (General Procedure A)
3.2.3. General Procedure for the Synthesis of N-Acetylpyrazolines 11–14a,b (General Procedure B)
3.2.4. General Procedure for the Synthesis of Pyrazolines Bearing 6-Amino-7-Methoxy-3,3a,4,5-Tetrahydro-2H-Benzo[g]indazole moieties 15–18a,b (General Procedure C)
3.3. Antiproliferative Activity
3.4. Antibacterial Activity
3.4.1. Microdilution Test
3.4.2. Hemolytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gaikwad, D.D.; Chapolikar, A.D.; Devkate, C.G.; Warad, K.D.; Tayade, A.P.; Pawar, R.P.; Domb, A.J. Synthesis of Indazole Motifs and Their Medicinal Importance: An Overview. Eur. J. Med. Chem. 2015, 90, 707–731. [Google Scholar] [CrossRef] [PubMed]
- Denya, I.; Malan, S.F.; Joubert, J. Indazole Derivatives and Their Therapeutic Applications: A Patent Review (2013–2017). Expert Opin. Ther. Pat. 2018, 28, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Lola, C.M.; Wang, M.; Miller, K.D.; Sledge, G.W.; Hutchins, G.D.; Zheng, Q.H. Synthesis of Carbon-11-Labeled Tricyclic Necroptosis Inhibitors as New Potential PET Agents for Imaging of Tumor Necrosis Factor α (TNF-α). Appl. Radiat. Isot. 2010, 68, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Keys, H.; Staples, R.J.; Yuan, J.; Degterev, A.; Cuny, G.D. Optimization of Tricyclic Nec-3 Necroptosis Inhibitors for in Vitro Liver Microsomal Stability. Bioorg. Med. Chem. Lett. 2012, 22, 5685–5688. [Google Scholar] [CrossRef] [PubMed]
- Collins, I.; Rowley, M.; Davey, W.B.; Emms, F.; Marwood, R.; Patel, S.; Patel, S.; Fletcher, A.; Ragan, I.C.; Leeson, P.D.; et al. 3-(1-Piperazinyl)-4,5-Dihydro-1H-Benzo[g]Indazoles: High Affinity Ligands for the Human Dopamine D4 Receptor with Improved Selectivity over Ion Channels. Bioorg. Med. Chem. 1998, 6, 743–753. [Google Scholar] [CrossRef]
- Reddy, S.T.; Shiva Kumar, K.; Meda, C.L.T.; Kandale, A.; Rambabu, D.; Rama Krishna, G.; Hariprasad, C.; Rao, V.; Venkataiah, S.; Malla Reddy, C.; et al. Conformationally Restricted Novel Pyrazole Derivatives: Synthesis of 1,8-Disubstituted 5,5-Dimethyl-4,5-Dihydro-1H-Benzo[g]Indazoles as a New Class of PDE4 Inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 3248–3255. [Google Scholar] [CrossRef] [PubMed]
- Deiana, V.; Gómez-Cañas, M.; Pazos, M.R.; Fernández-Ruiz, J.; Asproni, B.; Cichero, E.; Fossa, P.; Muñoz, E.; Deligia, F.; Murineddu, G.; et al. Tricyclic Pyrazoles. Part 8. Synthesis, Biological Evaluation and Modelling of Tricyclic Pyrazole Carboxamides as Potential CB2 receptor Ligands with Antagonist/Inverse Agonist Properties. Eur. J. Med. Chem. 2016, 112, 66–80. [Google Scholar] [CrossRef]
- Aparicio, D.A.; Lobo, G.M.; Sánchez, J.L.; Monasterios, M.C.; Acosta, M.E.; Lima, L.; Llovera, L.J.; Ángel, J.E.; Charris, J.E. Synthesis of Trans- and Cis-2-Acetyl-3-Phenyl-3,3a,4,5-Tetrahydro-2H-Benzo[g] Indazoles: Evaluation as Inhibitors of β-Hematin Formation. J. Chem. Res. 2017, 41, 668–672. [Google Scholar] [CrossRef]
- Jakob, C.G.; Upadhyay, A.K.; Donner, P.L.; Nicholl, E.; Addo, S.N.; Qiu, W.; Ling, C.; Gopalakrishnan, S.M.; Torrent, M.; Cepa, S.P.; et al. Novel Modes of Inhibition of Wild-Type Isocitrate Dehydrogenase 1 (IDH1): Direct Covalent Modification of His315. J. Med. Chem. 2018, 61, 6647–6657. [Google Scholar] [CrossRef]
- Gautam, P.; Gautam, D.; Chaudhary, R.P. Experimental and Theoretical Investigations on Acid Catalysed Stereoselective Synthesis of New Indazolyl-Thiazole Derivatives. J. Mol. Struct. 2018, 1160, 333–341. [Google Scholar] [CrossRef]
- Maurya, H.K.; Verma, R.; Alam, S.; Pandey, S.; Pathak, V.; Sharma, S.; Srivastava, K.K.; Negi, A.S.; Gupta, A. Studies on Substituted Benzo[h]Quinazolines, Benzo[g]Indazoles, Pyrazoles, 2,6-Diarylpyridines as Anti-Tubercular Agents. Bioorg. Med. Chem. Lett. 2013, 23, 5844–5849. [Google Scholar] [CrossRef] [PubMed]
- Gautam, D.; Chaudhary, R.P. Synthesis, Structure and Antimicrobial Evaluation of New 3,3a,4,5-Tetrahydro-2H-Benzo[g]Indazol-2-yl-Thiazol-4(5H)-Ones. Spectrochim. Acta.-Part. A Mol. Biomol. Spectrosc. 2015, 135, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Tzanetou, E.; Liekens, S.; Kasiotis, K.M.; Fokialakis, N.; Haroutounian, S.A. Novel Pyrazole and Indazole Derivatives: Synthesis and Evaluation of Their Anti-Proliferative and Anti-Angiogenic Activities. Arch. Pharm. 2012, 345, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Cai, J.; Chen, J.; Sun, C.; Li, L.; Ji, M. Discovery of 3,3a,4,5-Tetrahydro-2H-Benzo[g]Indazole Containing Quinoxaline Derivatives as Novel EGFR/HER-2 Dual Inhibitors. RSC Adv. 2015, 5, 24814–24823. [Google Scholar] [CrossRef]
- Kasiotis, K.M.; Tzanetou, E.N.; Stagos, D.; Fokialakis, N.; Koutsotheodorou, E.; Kouretas, D.; Haroutounian, S.A. Novel Conformationally Constrained Pyrazole Derivatives as Potential Anti-Cancer Agents. Z. Naturforsch. B. 2015, 70, 677–690. [Google Scholar] [CrossRef]
- Laroum, R.; Berrée, F.; Roisnel, T.; Dorcet, V.; Carboni, B.; Debache, A. AlCl3-Promoted Reaction of Cycloalkanones with Hydrazones: A Convenient Direct Synthesis of 4,5,6,7-Tetrahydro-1H-Indazoles and Their Analogues. Z. Tetrahedron Lett. 2019, 60, 150988. [Google Scholar] [CrossRef]
- Dark, G.; Hill, S.A.; Prise, S.; Tozer, G.; Pettit, G.; Chaplin, D. Combretastatin A-4, an Agent That Displays Selective Toxicity towards Tumour Vasculature. Cancer Res. 1997, 33, 832–835. [Google Scholar] [CrossRef]
- Porcù, E.; Bortolozzi, R.; Basso, G.; Viola, G. Recent Advances in Vascular Disrupting Agents in Cancer Therapy. Futur. Med. Chem. 2014, 6, 1485–1498. [Google Scholar] [CrossRef]
- Pérez-Pérez, M.J.; Priego, E.M.; Bueno, O.; Martins, M.S.; Canela, M.D.; Liekens, S. Blocking Blood Flow to Solid Tumors by Destabilizing Tubulin: An Approach to Targeting Tumor Growth. J. Med. Chem. 2016, 59, 8685–8711. [Google Scholar] [CrossRef]
- Devkota, L.; Lin, C.M.; Strecker, T.E.; Wang, Y.; Tidmore, J.K.; Chen, Z.; Guddneppanavar, R.; Jelinek, C.J.; Lopez, R.; Liu, L.; et al. Design, Synthesis, and Biological Evaluation of Water-Soluble Amino Acid Prodrug Conjugates Derived from Combretastatin, Dihydronaphthalene, and Benzosuberene-Based Parent Vascular Disrupting Agents. Bioorg. Med. Chem. 2016, 24, 938–956. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, H.; Wang, C.; Zhang, Q.; Fang, S.; Zhou, R.; Hu, J.; Zhu, J.; Zhou, Y.; Luo, C.; et al. 1-Phenyl-Dihydrobenzoindazoles as Novel Colchicine Site Inhibitors: Structural Basis and Antitumor Efficacy. Eur. J. Med. Chem. 2019, 177, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Bueno, O.; Tobajas, G.; Quesada, E.; Estévez-Gallego, J.; Noppen, S.; Camarasa, M.J.; Díaz, J.F.; Liekens, S.; Priego, E.M.; Pérez-Pérez, M.J. Conformational Mimetics of the α-Methyl Chalcone TUB091 Binding Tubulin: Design, Synthesis and Antiproliferative Activity. Eur. J. Med. Chem. 2018, 148, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Prada, J.; Robledo, S.M.; Vélez, I.D.; Crespo, M.d.P.; Quiroga, J.; Abonia, R.; Montoya, A.; Svetaz, L.; Zacchino, S.; Insuasty, B. Synthesis of Novel Quinoline-based 4,5-dihydro-1H-pyrazoles as Potential Anticancer, Antifungal, Antibacterial and Antiprotozoal Agents. Eur. J. Med. Chem. 2017, 131, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Romo, P.E.; Isaza, J.H.; Insuasty, B.; Abonia, R.; del Crespo, M.P.; Quiroga, J. Synthesis of Pyrazolo[3,4-b]Azepines and Their Antioxidant and Antibacterial Studies. Monatsh. Chem. 2019, 150, 1503–1511. [Google Scholar] [CrossRef]
- Crespo, M.P.; Avery, T.D.; Hanssen, E.; Fox, E.; Robinson, T.V.; Valente, P.; Taylor, D.K.; Tilley, L. Artemisinin and a Series of Novel Endoperoxide Antimalarials Exert Early Effects on Digestive Vacuole Morphology. Antimicrob. Agents Chemother. 2008, 52, 98–109. [Google Scholar] [CrossRef]
- Crespo, M.P.; Wei, M.Q. Antitumor Activity of Artemisinin and Its Derivatives: From a Well-Known Antimalarial Agent to a Potential Anticancer Drug. J. Biomed. Biotechnol. 2012, 2012, 93–102. [Google Scholar] [CrossRef]
- Meyer, M.D.; Altenbach, R.J.; Hancock, A.A.; Buckner, S.A.; Knepper, S.M.; Kerwin, J.F. Synthesis and in Vitro Characterization of N-[5-(4,5-Dihydro-1H-Imidazol-2-Yl)- 2-Hydroxy-5,6,7,8-Tetrahydronaphthalen-1-Yl]Methanesulfonamide and Its Enantiomers: A Novel Selective α1A Receptor Agonist. J. Med. Chem. 1996, 39, 4116–4119. [Google Scholar] [CrossRef]
- Jagtap, P.; Alexei, D.; Sungwoon, C.; Heather, K.; Junying, Y.; Cuny, G.D. Structure-Activity Relationship Study of Tricyclic Necroptosis Inhibitors. J. Med. Chem. 2007, 50, 1886–1895. [Google Scholar] [CrossRef]
- Stewart, J.J.P. MOPAC 2012; Stewart Computational Chemistry: Colorado Springs, CO, USA; Available online: http://OpenMOPCAC.net (accessed on 20 November 2019).
- Schmitt, D.M.; Connolly, K.L.; Jerse, A.E.; Detrick, M.S.; Horzempa, J. Antibacterial Activity of Resazurin-Based Compounds against Neisseria Gonorrhoeae in Vitro and in Vivo. Int. J. Antimicrob. Agents 2016, 48, 367–372. [Google Scholar] [CrossRef][Green Version]
- Tanaka, M.; Furuya, R.; Kobayashi, I.; Kanesaka, I.; Ohno, A.; Katsuse, A.K. Antimicrobial Resistance and Molecular Characterisation of Neisseria Gonorrhoeae Isolates in Fukuoka, Japan, 1996–2016. J. Glob. Antimicrob. Resist. 2019, 17, 3–7. [Google Scholar] [CrossRef]
- Costa-Lourenço, A.P.R.; Barros dos Santos, K.T.; Moreira, B.M.; Fracalanzza, S.E.L.; Bonelli, R.R. Antimicrobial Resistance in Neisseria Gonorrhoeae: History, Molecular Mechanisms and Epidemiological Aspects of an Emerging Global Threat. Braz. J. Microbiol. 2017, 48, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Leber, A.L. Antimicrobial Susceptibility Testing. In Clinical Microbiology Procedures Handbook, 4th ed.; American Society for Microbiology Press: Washington, DC, USA, 2016. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing: Twenty-third Informational Supplement, Document M100–S26, 26th ed.; CLSI: Wayne, PA, USA, 2008; pp. 1–250. [Google Scholar]
- Liaras, K.; Geronikaki, A.; Glamočlija, J.; Ćirić, A.; Soković, M. Thiazole-Based Chalcones as Potent Antimicrobial Agents. Synthesis and Biological Evaluation. Bioorg. Med. Chem. 2011, 19, 3135–3140. [Google Scholar] [CrossRef] [PubMed]
- Gonococcal Isolate Surveillance Project. Available online: http://www.cdc.gov/std/gisp/ (accessed on 10 september 2019).
- Conceição, K.; Konno, K.; Richardson, M.; Antoniazzi, M.M.; Jared, C.; Daffre, S.; Camargo, A.C.; Pimenta, D. Isolation and biochemical characterization of peptides presenting antimicrobial activity from the skin of Phyllomedusa hypochondrialis. Peptides 2006, 27, 3092–3099. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

| Comp. | Capan-1 | HCT-116 | NCI-H460 | DND-41 | HL-60 | K-562 | Z-138 |
|---|---|---|---|---|---|---|---|
| 11a | 40.8 ± 5.0 | 29.9 ± 9.0 | 10.8 ± 1.6 | >100 | 30.9 ± 14.8 | 46.5 ± 0.2 | >100 |
| 11b | 27.0 ± 4.0 | 25.3 ± 18.9 | 11.8 ± 1.6 | >100 | 38.0 ± 10.1 | 34.5 ± 5.9 | 70.4 ± 14.8 |
| 12a | 42.9 ± 1.6 | 26.9 ± 4.8 | 5.4 ± 0.5 | >100 | 42.5 ± 0.5 | 64.3 ± 17.9 | 49.7 ± 25.2 |
| 12b | 17.9 ± 3.5 | 36.0 ± 6.8 | 14.9 ± 2.9 | >100 | 38.9 ± 2.2 | 59.7 ± 4.8 | 45.1 ± 27.5 |
| 13a | 73.4 ± 26.6 | 36.5 ± 1.2 | 30.9 ± 10.0 | 51.0 ± 7.6 | 42.7 ± 3.9 | 73.1 ± 3.1 | 39.7 ± 6.9 |
| 13b | 32.3 ± 11.1 | 48.9 ± 6.8 | 45.0 ± 1.7 | 54.4 ± 10.2 | 42.9 ± 1.9 | 77.6 ± 9.1 | 42.3 ± 8.4 |
| 14a | 57.8 ± 2.4 | 82.0 ± 9.0 | 43.7 ± 4.7 | >100 | 28.6 ± 35.7 | >100 | >100 |
| 14b | 42.9 ± 12.3 | >100 | 44.6 ± 0.8 | >100 | 26.8 ± 36.6 | >100 | >100 |
| 15a | 45.1 ± 20.6 | 43.5 ± 6.0 | 42.1 ± 7.5 | 38.6 ± 7.2 | 55.9 ± 20.8 | 51.0 ± 29.2 | 43.9 ± 28.1 |
| 15b | 53.5 ± 18.8 | >100 | 54.1 ± 4.6 | 50.1 ± 25.0 | 44.8 ± 27.6 | 85.9 ± 7.1 | 50.1 ± 25.0 |
| 16a | 36.7 ± 14.4 | 45.0 ± 11.5 | 39.3 ± 8.5 | 30.3 ± 7.4 | 38.7 ± 7.5 | 44.9 ± 21.8 | 50.2 ± 0.9 |
| 16b | 42.6 ± 3.9 | 66.1 ± 31.3 | 33.7 ± 10.88 | 37.7 ± 6.4 | 65.9 ± 20.2 | 75.7 ± 21.0 | 48.0 ± 26.0 |
| 17a | 56.4 ± 11.6 | 62.2 ± 10.9 | 47.3 ± 15.3 | 42.8 ± 28.6 | 49.0 ± 25.5 | >100 | >100 |
| 17b | 60.7 ± 1.7 | 80.6 ± 9.7 | 43.9 ± 9.6 | 66.1 ± 18.6 | 65.8 ± 17.1 | >100 | >100 |
| 18a | 30.3 ± 9.0 | 45.2 ± 2.6 | 36.6 ± 10.4 | 50.6 ± 8.5 | 52.5 ± 16.3 | >100 | 89.9 ± 5.0 |
| 18b | 32.7 ± 22.0 | 52.1 ± 15.3 | 44.4 ± 2.2 | 40.8 ± 3.3 | 41.6 ± 5.0 | 69.2 ± 4.3 | 60.1 ± 1.8 |
| Docetaxel | 0.0042 ± 0.0021 | 0.0009 ± 0.0008 | 0.0038 ± 0.0029 | 0.0033 ± 0.0014 | 0.0023 ± 0.0003 | 0.0037 ± 0.0003 | 0.0011 ± 0.0008 |
| Stauroporine | 0.0007 ± 0.0002 | 0.00010 ± 0.0000 | 0.0015 ± 0.0004 | 0.0043 ± 0.0022 | 0.0074 ± 0.0017 | 0.0224 ± 0.0074 | 0.0003 ± 0.0001 |
| Comp. | S. aureus ATCC 25923 | S. aureus ATCC 43300 | VISA | E. coli ATCC 25922 | P. aeruginosa ATCC 27853 | K. pneumoniae ATCC700603 | K. pneumoniae BAA 1705 | N. gonorrhoeae ATCC 49226 |
|---|---|---|---|---|---|---|---|---|
| 7 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | ≥500 |
| 8 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| 9 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| 10 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| 11a | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | ≥500 |
| 11b | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | ≥500 |
| 12a | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | 250 |
| 12b | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| 13a | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | ≥500 |
| 13b | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | 62.5 |
| 14a | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | ≥500 |
| 14b | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | ≥500 |
| GEN b | <1 | <0.25 | <0.25 | <0.625 | 1 | 0.625 | <0.312 | 1.25–2.5 |
| TET c | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuartas, V.; Crespo, M.d.P.; Priego, E.-M.; Persoons, L.; Daelemans, D.; Camarasa, M.-J.; Insuasty, B.; Pérez-Pérez, M.-J. Design and Synthesis of New 6-Nitro and 6-Amino-3,3a,4,5-Tetrahydro-2H-Benzo[g]indazole Derivatives: Antiproliferative and Antibacterial Activity. Molecules 2019, 24, 4236. https://doi.org/10.3390/molecules24234236
Cuartas V, Crespo MdP, Priego E-M, Persoons L, Daelemans D, Camarasa M-J, Insuasty B, Pérez-Pérez M-J. Design and Synthesis of New 6-Nitro and 6-Amino-3,3a,4,5-Tetrahydro-2H-Benzo[g]indazole Derivatives: Antiproliferative and Antibacterial Activity. Molecules. 2019; 24(23):4236. https://doi.org/10.3390/molecules24234236
Chicago/Turabian StyleCuartas, Viviana, María del Pilar Crespo, Eva-María Priego, Leentje Persoons, Dirk Daelemans, María-José Camarasa, Braulio Insuasty, and María-Jesús Pérez-Pérez. 2019. "Design and Synthesis of New 6-Nitro and 6-Amino-3,3a,4,5-Tetrahydro-2H-Benzo[g]indazole Derivatives: Antiproliferative and Antibacterial Activity" Molecules 24, no. 23: 4236. https://doi.org/10.3390/molecules24234236
APA StyleCuartas, V., Crespo, M. d. P., Priego, E.-M., Persoons, L., Daelemans, D., Camarasa, M.-J., Insuasty, B., & Pérez-Pérez, M.-J. (2019). Design and Synthesis of New 6-Nitro and 6-Amino-3,3a,4,5-Tetrahydro-2H-Benzo[g]indazole Derivatives: Antiproliferative and Antibacterial Activity. Molecules, 24(23), 4236. https://doi.org/10.3390/molecules24234236