Parabens Adsorption onto Activated Carbon: Relation with Chemical and Structural Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Activated Carbon Characterization
2.2. Adsorption Studies
2.3. Calorimetric Study
3. Materials and Methods
3.1. Preparation of Activated Carbons
3.2. Characterization of Activated Carbons
3.3. Adsorption Studies
3.4. Immersion Enthalpy Determination
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Haman, C.; Dauchy, X.; Rosin, C.; Munoz, J.F. Occurrence, fate and behavior of parabens in aquatic environments: A review. Water Res. 2015, 68, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Andersen, D.N.; Larsen, P.B. Survey of Parabens; Part of the LOUS-review Environmental Project No. 1474; The Danish Environmental Protection Agency: Copenhagen, Danmark, 2013. [Google Scholar]
- Masten, S.A. Butylparaben. Review of toxicological literature butylparaben. Rev. Toxicol. Lit. 2005, 1–64. [Google Scholar]
- Błędzka, D.; Gromadzińska, J.; Wąsowicz, W. Parabens. From environmental studies to human health. Environ. Int. 2014, 67, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Bolong, N.; Ismail, A.F.; Salim, M.R.; Matsuura, T. A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination 2009, 238, 229–246. [Google Scholar] [CrossRef]
- Chang, H.S.; Choo, K.H.; Lee, B.; Choi, S.J. The methods of identification, analysis, and removal of endocrine disrupting compounds (EDCs) in water. J. Hazard. Mater. 2009, 172, 1–12. [Google Scholar] [CrossRef]
- Muñoz Peña, M.J. Eliminación de Contaminantes Parabenos en Agua Mediante Procesos Físicos, Químicos y Electroquímicos; Universidad de Extremadura: Badajoz, Spain, 2015. [Google Scholar]
- Chen, Y.; Deng, P.; Xie, P.; Shang, R.; Wang, Z.; Wang, S. Heat-activated persulfate oxidation of methyl- and ethyl-parabens: Effect, kinetics, and mechanism. Chemosphere 2017, 168, 1628–1636. [Google Scholar] [CrossRef]
- Katsigiannis, A.; Noutsopoulos, C.; Mantziaras, J.; Gioldasi, M. Removal of emerging pollutants through Granular Activated Carbon. Chem. Eng. J. 2015, 280, 49–57. [Google Scholar] [CrossRef]
- Rossner, A.; Snyder, S.A.; Knappe, D.R.U. Removal of emerging contaminants of concern by alternative adsorbents. Water Res. 2009, 43, 3787–3796. [Google Scholar] [CrossRef]
- Goel, J.; Kadirvelu, K.; Rajagopal, C.; Kumar, V. Removal of lead (II) by adsorption using treated granular activated carbon: Batch and column studies. J. Hazard. Mater. 2005, 125, 211–220. [Google Scholar] [CrossRef]
- da Silva Lacerda, V.; López-Sotelo, J.B.; Correa-Guimarães, A.; Hernández-Navarro, S.; Sánchez-Báscones, M.; Navas-Gracia, L.M.; Martín-Ramos, P.; Martín-Gil, J. Rhodamine B removal with activated carbons obtained from lignocellulosic waste. J. Environ. Manage. 2015, 155, 67–76. [Google Scholar] [CrossRef]
- Wu, S.H.; Pendleton, P. Adsorption of Anionic Surfactant by Activated Carbon: Effect of Surface Chemistry, Ionic Strength, and Hydrophobicity. J. Colloid Interface Sci. 2001, 243, 306–315. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F.; Molina-Sabio, M. Textural and chemical characterization of microporous carbons. Adv. Colloid Interface Sci. 1998, 76–77, 271–294. [Google Scholar] [CrossRef]
- Dabrowski, A.; Podkoscielny, P.; Hubicki, Z.; Barczak, M. Adsorption of phenolic compounds by activated carbon–a critical review. Chemosphere 2005, 58, 1049–1070. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, R.W.; Ezra, F.S. Role of surface acidity in the adsorption of organic pollutants on the surface of carbon. Environ. Sci. Technol. 1968, 2, 291–297. [Google Scholar] [CrossRef]
- Mattson, J.S.; Mark, H.B.; Malbin, M.D.; Weber, W.J.; Crittenden, J.C. Surface chemistry of active carbon: Specific adsorption of phenols. J. Colloid Interface Sci. 1969, 31, 116–130. [Google Scholar] [CrossRef]
- Salame, I.I.; Bandosz, T.J. Role of surface chemistry in adsorption of phenol on activated carbons. J. Colloid Interface Sci. 2003, 264, 307–312. [Google Scholar] [CrossRef]
- Terzyk, A.P. Further insights into the role of carbon surface functionalities in the mechanism of phenol adsorption. J. Colloid Interface Sci. 2003, 268, 301–329. [Google Scholar] [CrossRef]
- Bottani, E.J.; Tascón, J.M.D. Adsorption by Carbons; Elsevier: Amsterdam, Netherlands, 2008; ISBN 978-0-08-044464-2. [Google Scholar]
- Stoeckli, F.; Centeno, T.A. On the characterization of microporous carbons by immersion calorimetry alone. Carbon N. Y. 1997, 35, 1097–1100. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Lewellyn, P.; Maurin, G.; Sing, K. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Molina-Sabio, M.; Rodríguez-Reinoso, F. Role of chemical activation in the development of carbon porosity. Colloids Surf. A Physicochem. Eng. Asp. 2004, 241, 15–25. [Google Scholar] [CrossRef]
- González-García, P. Activated carbon from lignocellulosics precursors: A review of the synthesis methods, characterization techniques and applications. Renew. Sustain. Energy Rev. 2018, 82, 1393–1414. [Google Scholar] [CrossRef]
- Schwarz, J.; Contescu, C. Surfaces of Nanoparticles and Porous Materials; Marcel Dekker: New York, NY, USA, 1999; ISBN 0824746686. [Google Scholar]
- Yalkowsky, S.H.; He, Y.; Jain, P. Handbook of Aqueous Solubility Data, 2nd ed.; CRC Press. Taylor & Francis Group: Boca Raton, FL, USA, 2010; ISBN 9781439802465. [Google Scholar]
- Giles, C.H.; Smith, D. A general treatment and classification of the solute adsorption isotherm. I. Theoretical. J. Colloid Interface Sci. 1974, 47, 766–778. [Google Scholar] [CrossRef]
- Do, D.D. Adsorption Analysis: Equilibria and Kinetics; Imperial College Press: London, UK, 1998; Volume 2, ISBN 978-1-86094-130-6. [Google Scholar]
- Boehm, H. Surface oxides on carbon and their analysis: A critical assessment. Carbon N. Y. 2002, 40, 145–149. [Google Scholar] [CrossRef]
- Daud, W.M.A.W.; Houshamnd, A.H. Textural characteristics, surface chemistry and oxidation of activated carbon. J. Nat. Gas. Chem. 2010, 19, 267–279. [Google Scholar] [CrossRef]
- Moreno-Castilla, C. Adsorption of organic molecules from aqueous solutions on carbon materials. Carbon N. Y. 2004, 42, 83–94. [Google Scholar] [CrossRef]
- Bernal, V.; Giraldo, L.; Moreno-Piraján, J.C.; Balsamo, M.; Erto, A. Mechanisms of Methylparaben Adsorption onto Activated Carbons: Removal Tests Supported by a Calorimetric Study of the Adsorbent–Adsorbate Interactions. Molecules 2019, 24, 413. [Google Scholar] [CrossRef]
- López-Ramón, M.V.; Stoeckli, F.; Moreno-Castilla, C.; Carrasco-Marín, F. Specific and non-specific interactions of water molecules with carbon surfaces from immersion calorimetry. Carbon N. Y. 2000, 38, 825–829. [Google Scholar] [CrossRef]
- Carvajal-Bernal, A.M.; Gómez-Granados, F.; Giraldo, L.; Moreno-Piraján, J. A study of the interactions of activated carbon-phenol in aqueous solution using the determination of immersion enthalpy. Appl. Sci. 2018, 8, 843. [Google Scholar] [CrossRef]
- Chen, H.W.; Chiou, C.S.; Chang, S.H. Comparison of methylparaben, ethylparaben and propylparaben adsorption onto magnetic nanoparticles with phenyl group. Powder Technol. 2017, 311, 426–431. [Google Scholar] [CrossRef]
- Chin, Y.P.; Mohamad, S.; Abas, M.R. Bin Removal of parabens from aqueous solution using B-cyclodextrin cross-linked polymer. Int. J. Mol. Sci. 2010, 11, 3459–3471. [Google Scholar] [CrossRef]
- Vargas, D.P.; Giraldo, L.; Moreno-Piraján, J.C. Calorimetric study of activated carbons impregnated with CaCl2. Open Chem. 2015, 13, 683–688. [Google Scholar] [CrossRef]
- Acevedo, S.; Giraldo, L.; Moreno-Piraján, J.C. Adsorption of CO2 onto activated carbons prepared by chemical activation with metallic salts. Int. J. Chem. React. Eng. 2017, 15, 1–11. [Google Scholar]
- Contescu, A.; Contescu, C.; Putyera, K.; Schwarz, J. Surface acidity of carbons characterized by their continuous pK distribution and Boehm titration. Carbon N. Y. 1997, 35, 83–94. [Google Scholar] [CrossRef]
- Álvarez, M.A.; Ruidíaz-Martínez, M.; Cruz-Quesada, G.; López-Ramón, M.V.; Rivera-Utrilla, J.; Sánchez-Polo, M.; Mota, A.J. Removal of parabens from water by UV-driven advanced oxidation processes. Chem. Eng. J. 2019, 379, 122334. [Google Scholar] [CrossRef]
- Giraldo, L.; Moreno-Piraján, J.C.; Huertas, J.I. Heat Conduction Micro-Calorimeter with Metallic Reaction Cell and Improved Heat Flux Sensing System. Instrum. Sci. Technol. 2002, 30, 177–186. [Google Scholar] [CrossRef]
- Rodríguez, G.A.; Giraldo, L.; Moreno, J.C. Calorimetric study of the immersion enthalpies of activated carbon cloths in different solvents and aqueous solutions. J. Therm. Anal. Calorim. 2009, 96, 547–552. [Google Scholar] [CrossRef]
- Bernal, V.; Giraldo, L.; Moreno-Piraján, J.C. Comparative calorimetry study of the phenol and acetaminophen adsorption on activated carbon in aqueous solution. Rev. Colomb. Cienc. Químicas Farm. 2015, 44, 90–106. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
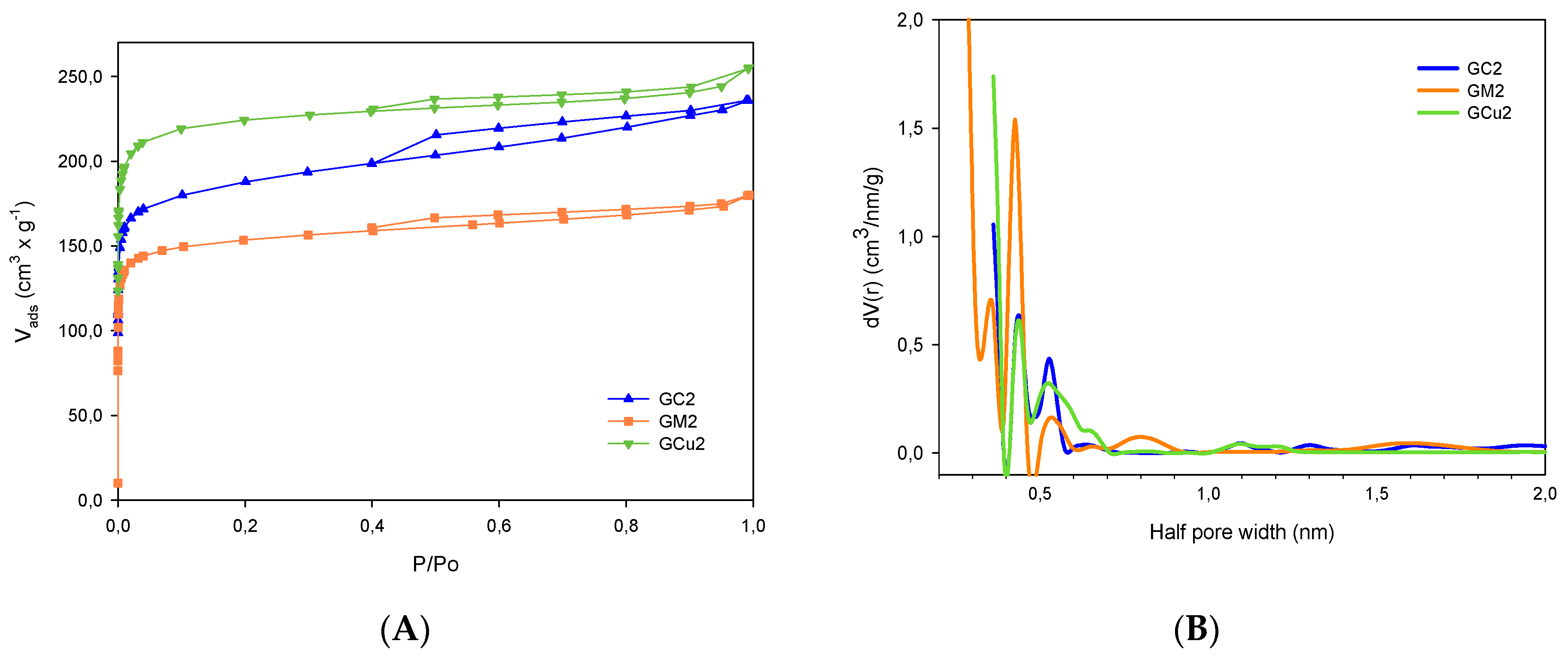
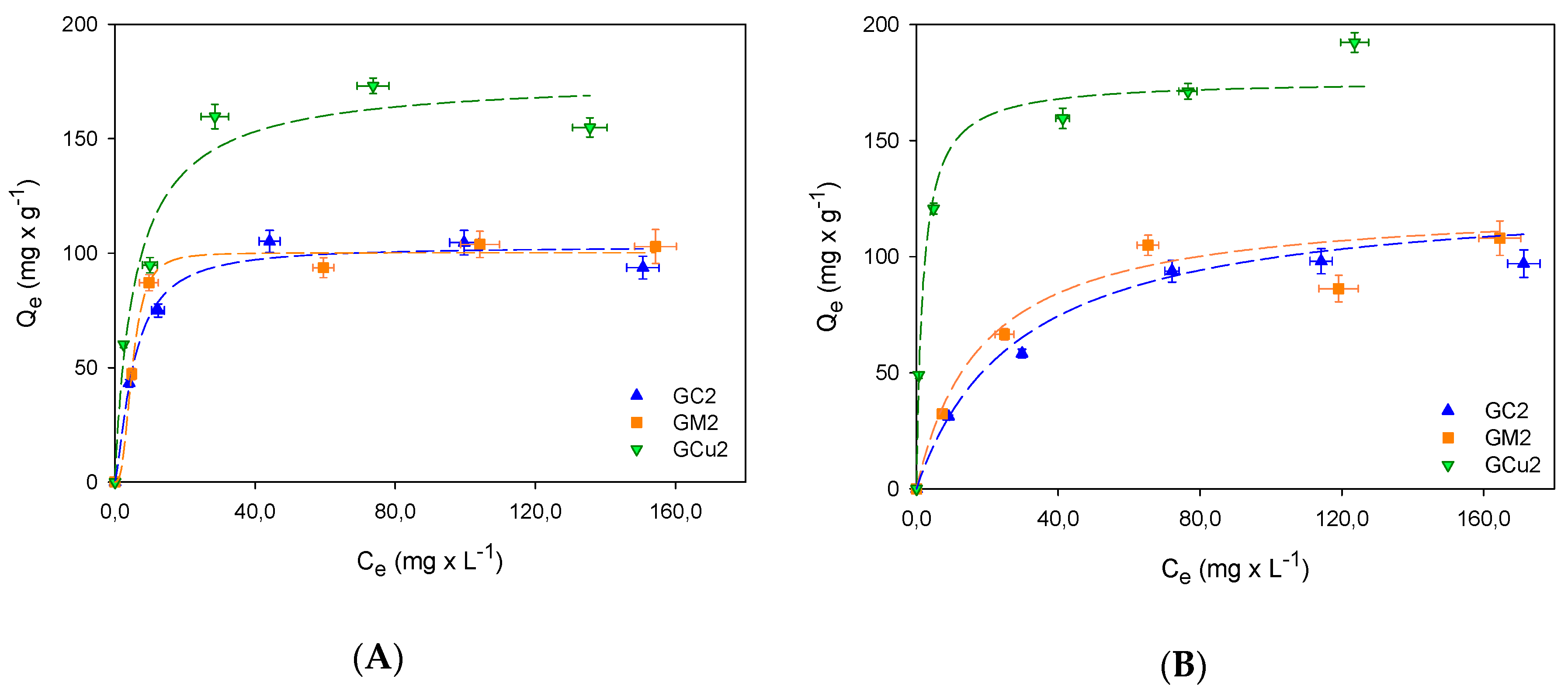
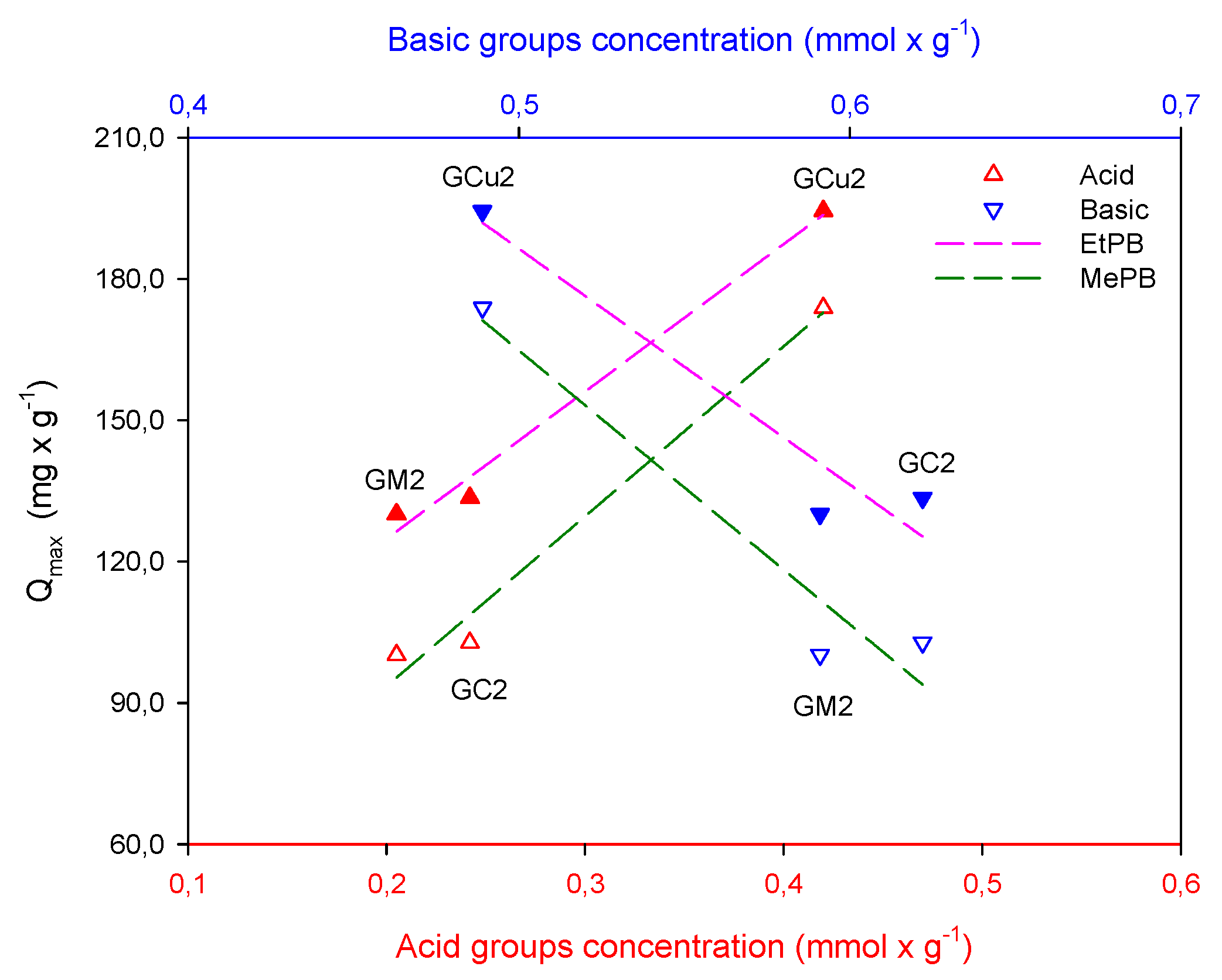
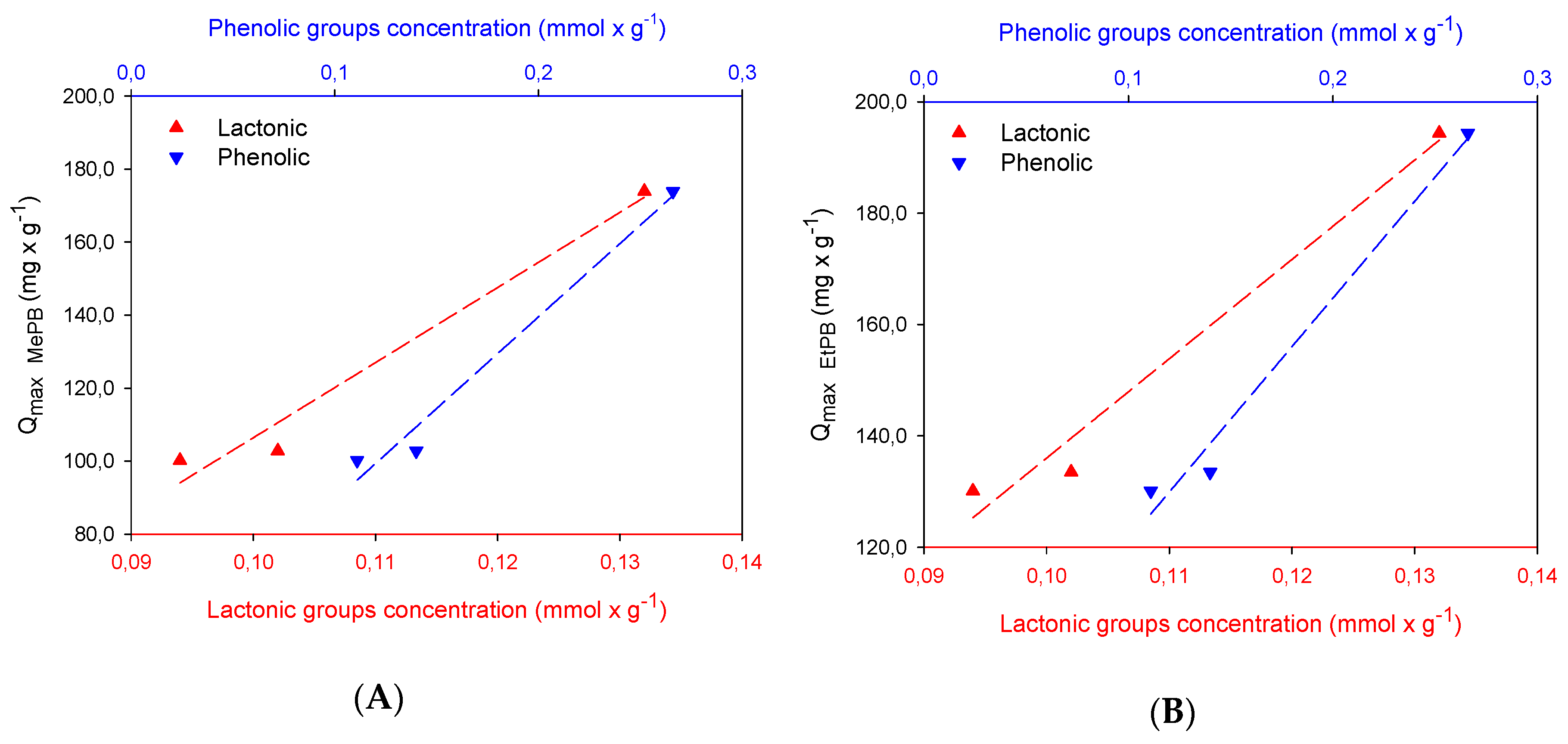
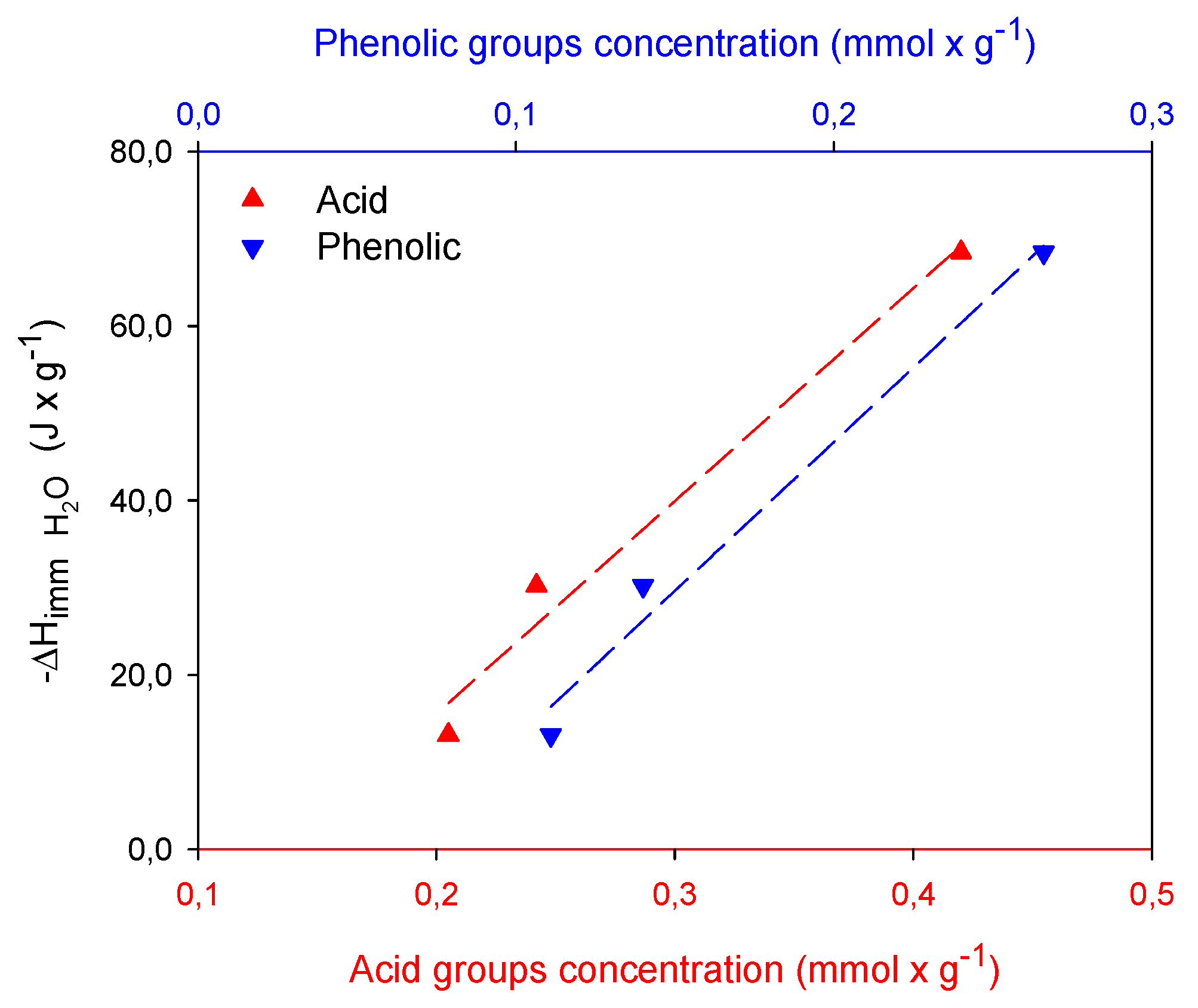
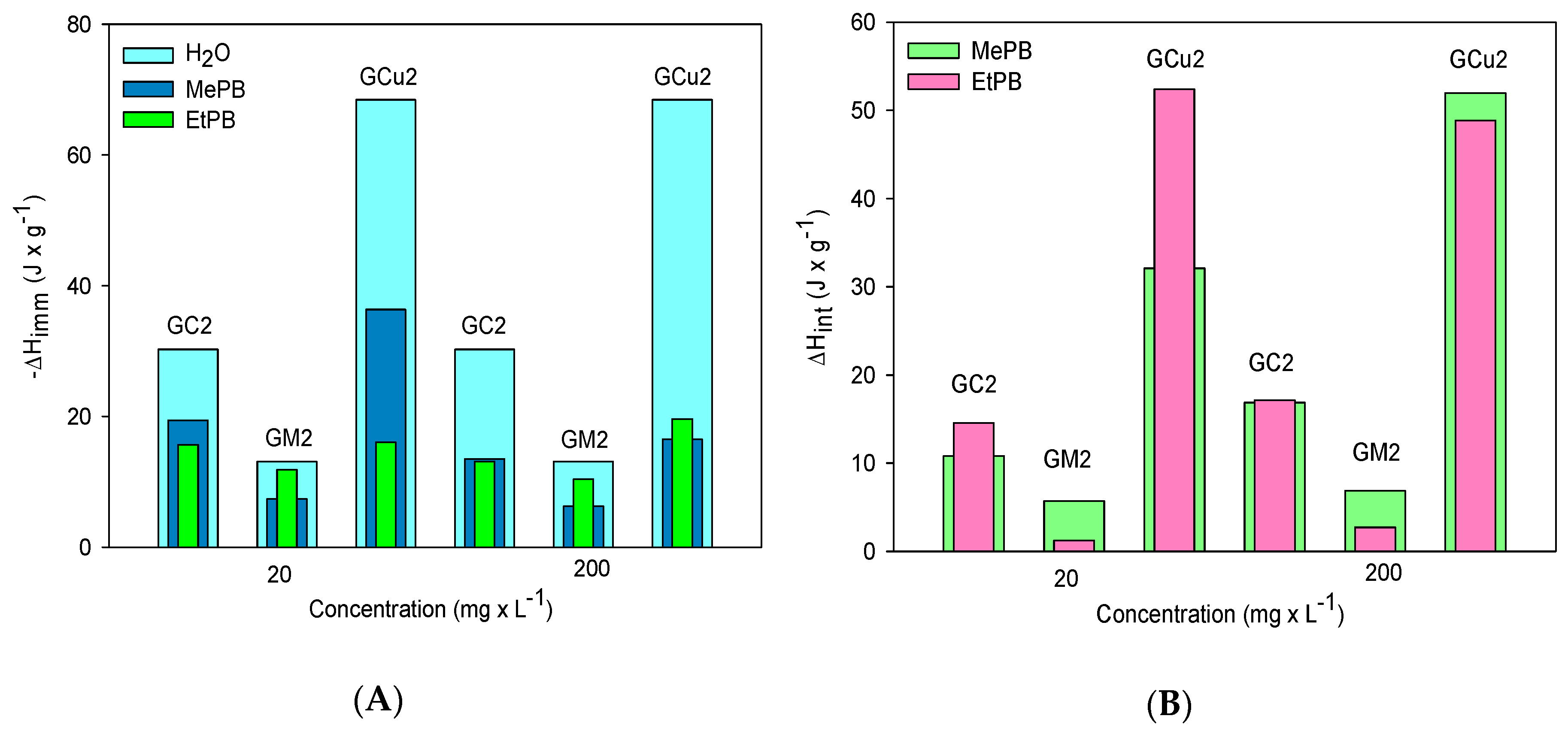
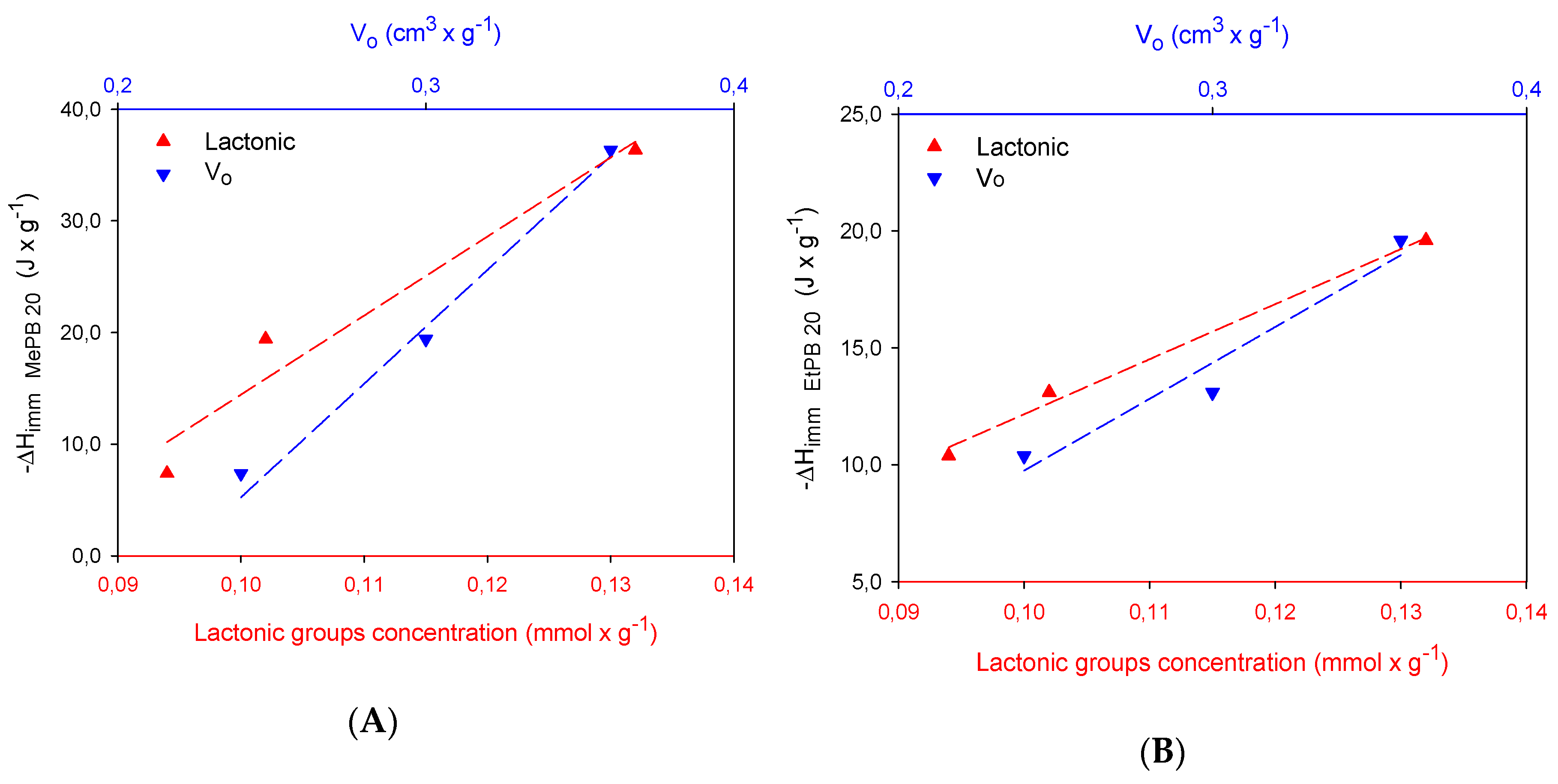
| Samples | SBET m2 × g−1 | Vmic cm3 × g−1 | Vtot QSDFT cm3 × g−1 |
|---|---|---|---|
| GC2 | 723 | 0.30 | 0.34 |
| GM2 | 608 | 0.24 | 0.25 |
| GCu2 | 896 | 0.36 | 0.35 |
| Samples | Carboxylic | Lactonic | Phenolic | Basic | Acidic | Total |
|---|---|---|---|---|---|---|
| GC2 | 0 | 0.10 | 0.14 | 0.62 | 0.24 | 0.86 |
| GM2 | 0 | 0.094 | 0.11 | 0.59 | 0.20 | 0.80 |
| GCu2 | 0.024 | 0.13 | 0.27 | 0.49 | 0.42 | 0.90 |
| Structures | 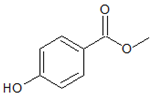 | 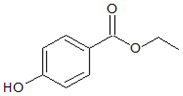 |
|---|---|---|
| Parameters | Methylparaben (MePB) | Ethylparaben (EtPB) |
| Molecular weight g × mol−1 | 152.2 | 166.2 |
| Molecular dimensions nm2 | 0.91 × 0.44 | 1.03 × 0.66 |
| Water solubility mg × L−1 at 25 °C | 2.50 × 10−3 | 8.85 × 10−2 |
| pKa | 8.17 | 8.22 |
| Parameters | MePB | EtPB | ||||
|---|---|---|---|---|---|---|
| GC2 | GM2 | GCu2 | GC2 | GM2 | GCu2 | |
| QmS mg × g−1 | 102.8 | 100.2 | 173.9 | 133.5 | 130.1 | 194.4 |
| KS L × mg−1 | 0.1 | 0.01 | 0.2 | 0.03 | 0.04 | 0.5 |
| nS | 0.54 | 0.70 | 0.96 | 1.0 | 0.97 | 1.5 |
| r2 | 0.98 | 0.99 | 0.98 | 0.99 | 0.97 | 0.99 |
| Samples | MePB | EtPB | |||
|---|---|---|---|---|---|
| GC2 | 30.23 | 19.41 | 13.52 | 15.68 | 13.10 |
| GM2 | 13.10 | 7.38 | 6.24 | 11.86 | 10.38 |
| GCu2 | 68.45 | 36.35 | 16.50 | 16.07 | 19.60 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Marenco, A.R.; Giraldo, L.; Moreno-Piraján, J.C. Parabens Adsorption onto Activated Carbon: Relation with Chemical and Structural Properties. Molecules 2019, 24, 4313. https://doi.org/10.3390/molecules24234313
Moreno-Marenco AR, Giraldo L, Moreno-Piraján JC. Parabens Adsorption onto Activated Carbon: Relation with Chemical and Structural Properties. Molecules. 2019; 24(23):4313. https://doi.org/10.3390/molecules24234313
Chicago/Turabian StyleMoreno-Marenco, Astrid Roxanna, Liliana Giraldo, and Juan Carlos Moreno-Piraján. 2019. "Parabens Adsorption onto Activated Carbon: Relation with Chemical and Structural Properties" Molecules 24, no. 23: 4313. https://doi.org/10.3390/molecules24234313
APA StyleMoreno-Marenco, A. R., Giraldo, L., & Moreno-Piraján, J. C. (2019). Parabens Adsorption onto Activated Carbon: Relation with Chemical and Structural Properties. Molecules, 24(23), 4313. https://doi.org/10.3390/molecules24234313




