The Effects of Different Varieties of Aurantii Fructus Immaturus on the Potential Toxicity of Zhi-Zi-Hou-Po Decoction Based on Spectrum-Toxicity Correlation Analysis
Abstract
1. Introduction
2. Results
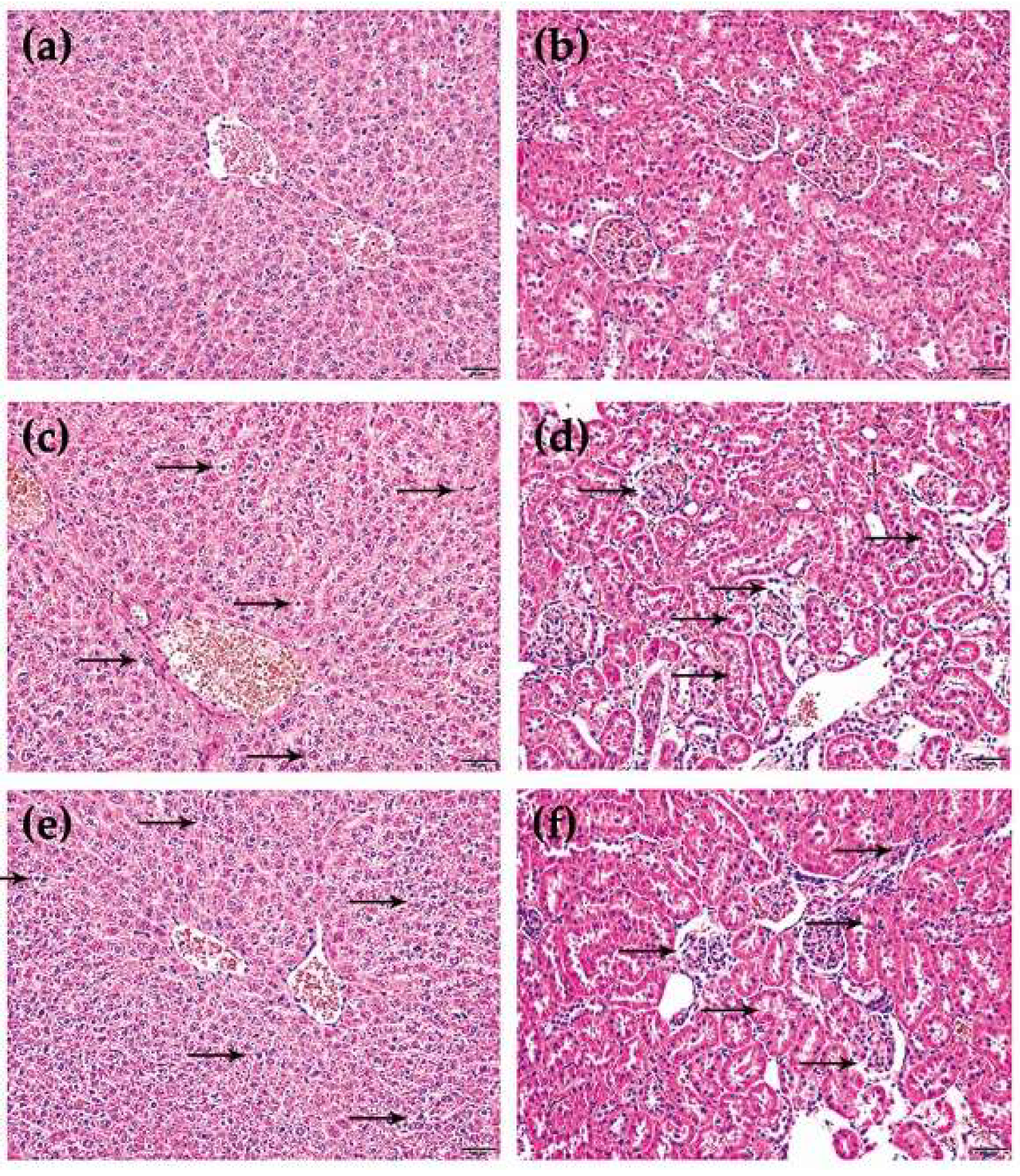
2.1. Verification of Toxicity Difference
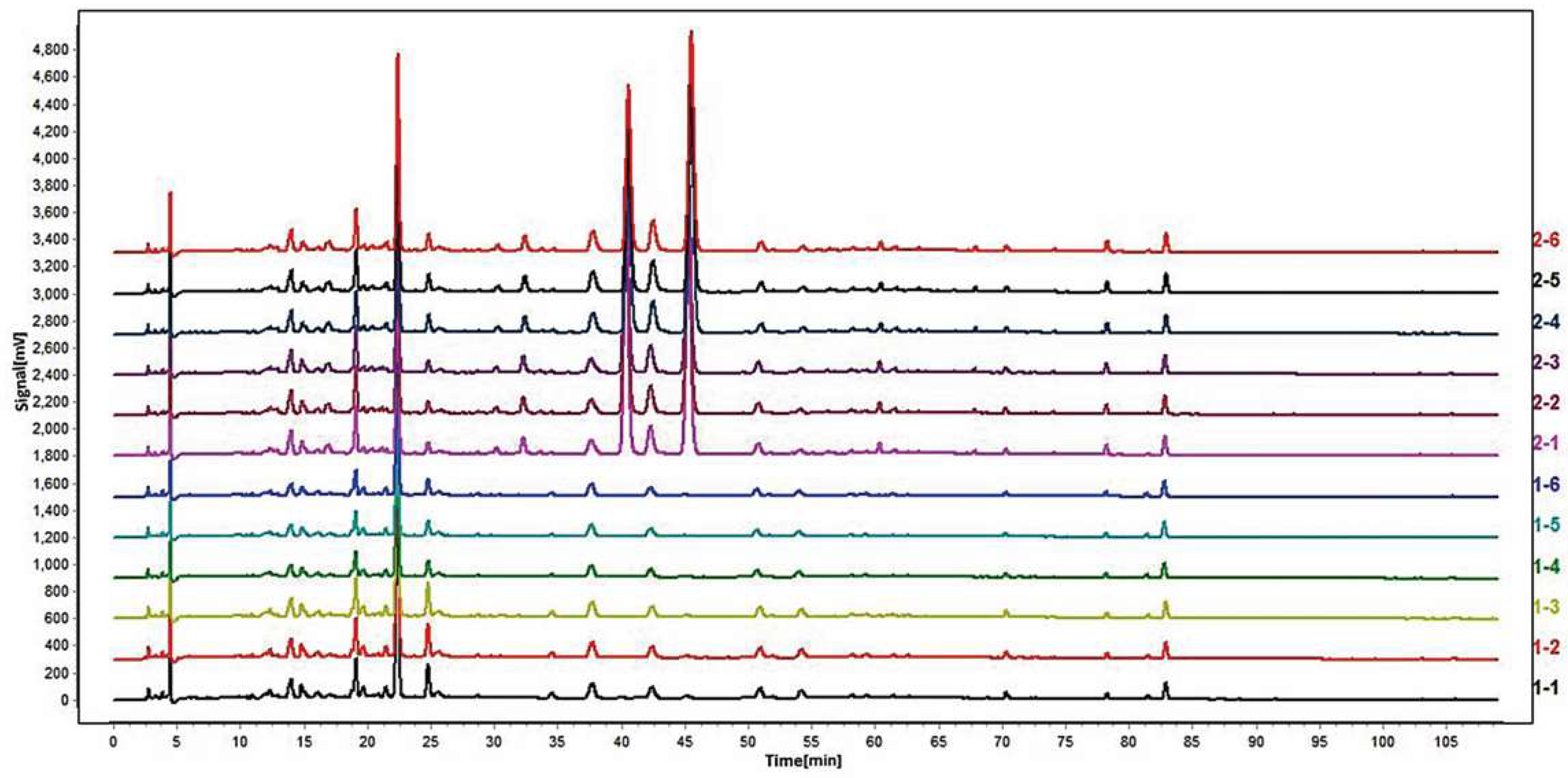
2.2. Similarity Analysis by Chromatographic Fingerprint
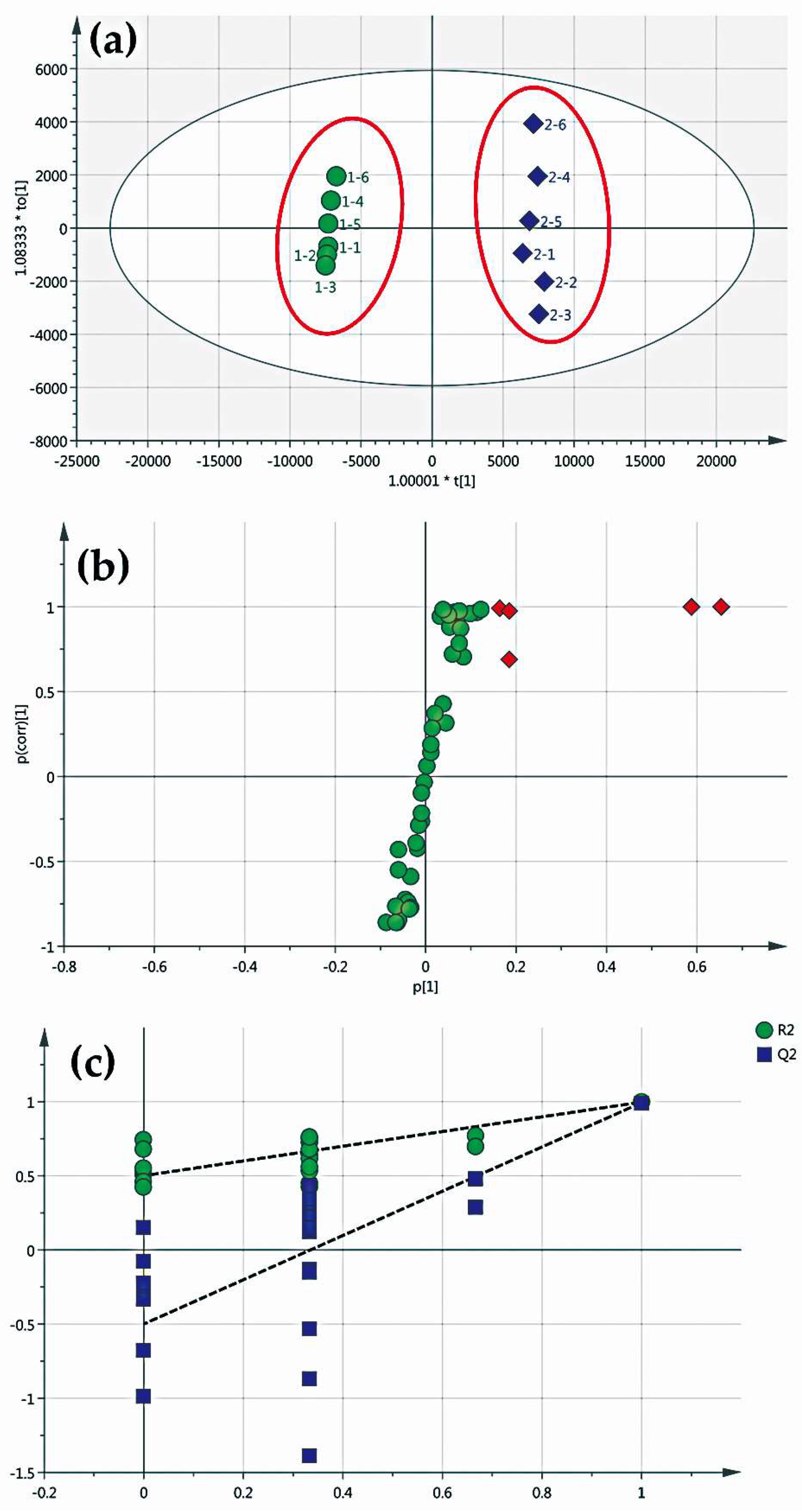
2.3. Screening of Differential Components
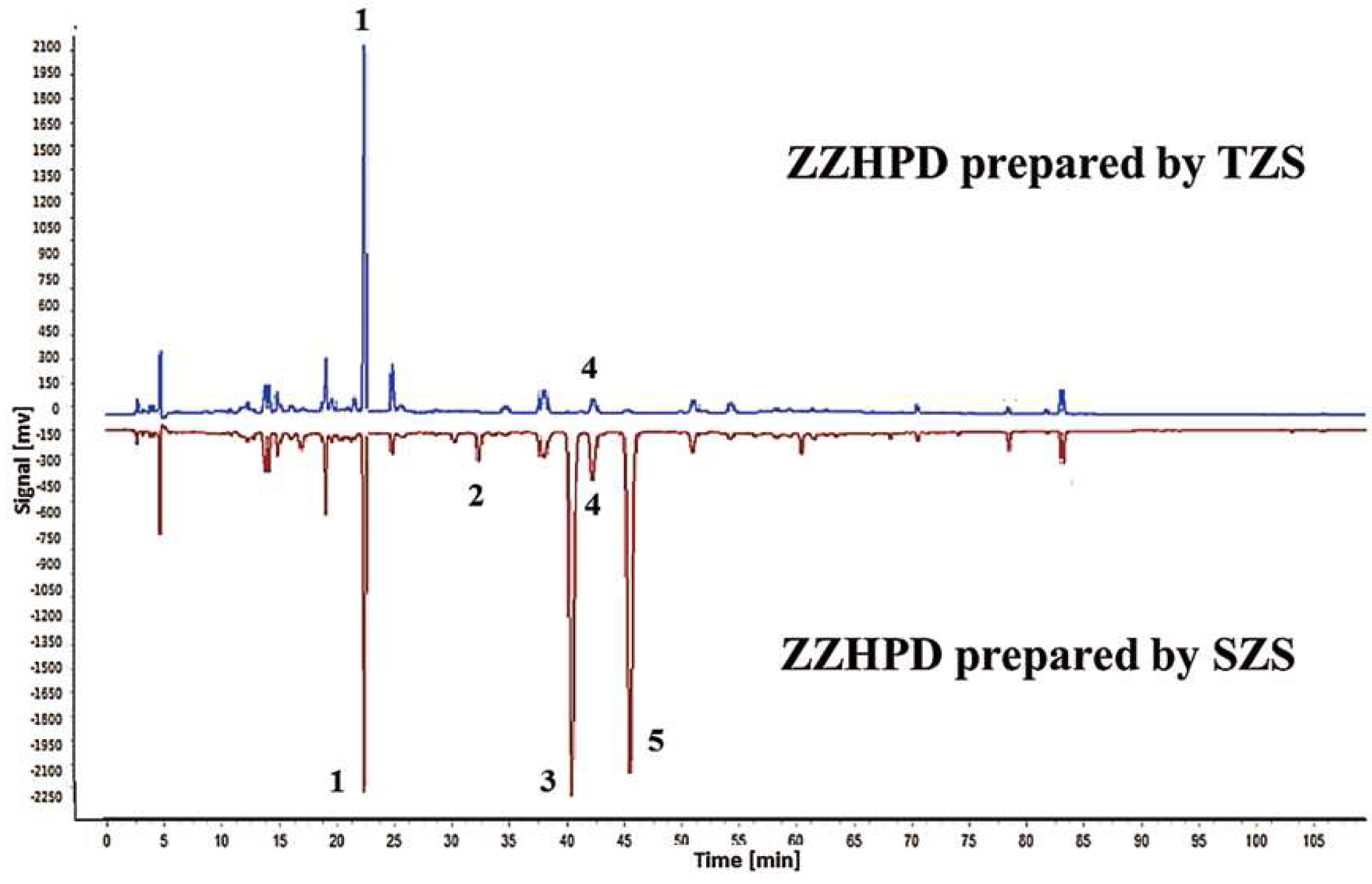
2.4. Identification of Differential Component Peaks
2.4.1. Identification of Iridoid Glycosides
2.4.2. Identification of Flavonoids
3. Discussion
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Preparation of ZZHPD Sample
4.3. Animal Treatments and Sample Collection
4.4. Serum Biochemistry Assay and Histopathology
4.5. LC–MS Analysis
4.5.1. Sample Preparation
4.5.2. Method Development
4.5.3. Method Validation
4.5.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, S.; Bao, Y.R.; Li, T.J.; Yu, T.; Chang, X.; Yang, G.L.; Meng, X.S. Mechanism of fructus aurantii flavonoids promoting gastrointestinal motility: From organic and inorganic endogenous substances combination point of view. Pharmacogn. Mag. 2017, 13, 372–377. [Google Scholar]
- Jia, S.; Hu, Y.; Zhang, W.N.; Zhao, X.Y.; Chen, Y.H.; Sun, C.D.; Li, X.; Chen, K.S. Hypoglycemic and hypolipidemic effects of neohesperidin derived from Citrus aurantium L. in diabetic KK-Ay mice. Food Funct. 2015, 6, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Ana, C.C.; Jesus, P.V.; Hugo, E.A.; Teresa, A.T.; Ulises, G.C.; Neith, P. Antioxidant capacity and UPLC–PDA ESI–MS polyphenolic profile of Citrus aurantium extracts obtained by ultrasound assisted extraction. J. Food Sci. Technol. 2018, 55, 5106–5114. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.Y.; Wan, L.; Wang, T.X.; Jiang, J.G. Citrus aurantium L. var. amara Engl. inhibited lipid accumulation in 3T3-L1 cells and Caenorhabditis elegans and prevented obesity in high-fat diet-fed mice. Pharmacol. Res. 2019, 147, 104347. [Google Scholar] [CrossRef]
- Chuang, C.C.; Wen, W.C.; Sheu, S.J. Origin identification on the commercial samples of Aurantii Fructus. J. Sep. Sci. 2007, 30, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Liu, Y.Y.; Wang, C.; Song, Z.Q.; Zha, Q.L.; Lu, C.; Wang, C.; Lu, A.P. Discrimination of Zhishi from different species using rapid-resolution liquid chromatography-diode array detection/ultraviolet (RRLC-DAD/UV) coupled with multivariate statistical analysis. J. Med. Plants Res. 2012, 6, 866–875. [Google Scholar]
- Daviesa, J.; Read, J. A systematic review into the incidence, severity and duration of antidepressant withdrawal effects: Are guidelines evidence-based? Addict. Behav. Mol. Mankind 2019, 97, 111–121. [Google Scholar] [CrossRef]
- Xing, H.; Zhang, K.; Zhang, R.W.; Shi, H.Y.; Bi, K.S.; Chen, X.H. Antidepressant-like effect of the water extract of the fixed combination of Gardenia jasminoides, Citrus aurantium and Magnolia officinalis in a rat model of chronic unpredictable mildstress. Phytomedicine 2015, 22, 1178–1185. [Google Scholar] [CrossRef]
- Wang, Y.J.; Feng, F. Evaluation of the hepatotoxicity of the Zhi-Zi-Hou-Po decoction by combining UPLC-Q-exactive-MS-based metabolomics and HPLC-MS/MS-based geniposide tissue distribution. Molecules 2019, 24, 511. [Google Scholar] [CrossRef]
- Yu, J.G.; Liu, Y.; Guo, J.M.; Tao, W.W.; Chen, Y.Y.; Fan, X.H.; Shen, J.; Duan, J.A. Health risk of Licorice-Yuanhua combination through induction of colonic H2S metabolism. J. Ethnopharmacol. 2019, 236, 136–146. [Google Scholar] [CrossRef]
- Cui, X.; Shen, Y.M.; Jiang, S.; Qian, D.W.; Shang, E.X.; Zhu, Z.H.; Duan, J.A. Comparative analysis of the main active components and hypoglycemic effects after the compatibility of Scutellariae Radix and Coptidis Rhizoma. J. Sep. Sci. 2019, 42, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.M.; Hong, Y.L.; Lin, X.; Shen, L.; Feng, Y. Recent pharmaceutical evidence on the compatibility rationality of traditional Chinese medicine. J. Ethnopharmacol. 2017, 206, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Yang, F.L.; Zhang, J.; Sun, G.X.; Wang, C.; Guo, Y.; Wen, R.; Sun, W.Y. Quantitative fingerprint and quality control analysis of Compound Liquorice Tablet combined with antioxidant activities and chemometrics methods. Phytomedicine 2019, 59, 152790. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xu, J.N.; Zhu, R.; Zhu, Y.Q.; Zhao, Y.; Chen, P.; Pan, C.; Yao, W.B.; Gao, X.D. Fingerprinting profile of polysaccharides from Lycium barbarum usingmultiplex approaches and chemometrics. Int. J. Biol. Macromol. 2015, 78, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, L.M.; Peng, J.J.; Zhao, Y.L.; Li, D.X.; Xie, X.M.; Tong, L.; Yu, Z.G. Screening bioactive quality control markers of QiShenYiQi dripping pills based on the relationship between the ultra-high performance liquid chromatography fingerprint and vascular protective activity. J. Sep. Sci. 2017, 40, 4076–4084. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, X.L.; Zhu, T.T.; Zhu, H.; Zhu, X.C.; Cai, H.; Cao, G.; Xu, X.Y.; Niu, M.J.; Cai, B.C. Study on spectrum-effect correlation for screening the effective components in Fangji Huangqi Tang basing on ultra-high performance liquid chromatography-mass spectrometry. Phytomedicine 2018, 47, 81–92. [Google Scholar] [CrossRef]
- Shen, C.H.; Liu, C.T.; Song, X.J.; Zeng, W.Y.; Lu, X.Y.; Zheng, Z.L.; Pan, J.; Zhan, R.T.; Yan, P. Evaluation of analgesic and anti-inflammatory activities of Rubia cordifolia L. by spectrum-effect relationships. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2018, 1090, 73–80. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, C.; Zhao, D.S.; Wang, L.L.; Li, P.; Li, H.J. Discovery of hepatotoxic equivalent combinatorial markers from dioscorea bulbifera tuber by fingerprint-toxicity relationship modeling. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhang, S.J.; Gao, B.B.; Qian, Z.; Liu, J.J.; Wu, S.H.; Si, J.P. Identification and quantitative analysis of phenolic glycosides with antioxidant activity in methanolic extract of Dendrobium catenatum flowers and selection of quality control herb-markers. Food Res. Int. 2019, 123, 732–745. [Google Scholar] [CrossRef]
- Luo, K.W.; Feng, F. Identification of absorbed components and metabolites of Zhi-Zi-Hou-Po decoction in rat plasma after oral administration by an untargeted metabolomics-driven strategy based on LC-MS. Anal. Bioanal. Chem. 2016, 408, 5723–5735. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.J.; Feng, F. Integrated chemical profiling of Zhi-Zi-Hou-Po decoction by liquid chromatography-diode array detector-time of flight mass analyzer and liquid chromatography-triple stage quadrupole mass analyzer combined with chemometrics. Anal. Methods. 2016, 8, 4689–4710. [Google Scholar] [CrossRef]
- Zhu, L.; Feng, F.; Yan, X.M.; Wang, Z.; Zhang, L.L. LC Characterization of the major constituents in Zhi-Zi-Hou-Pu decoction using various detection approaches. Chromatographia 2009, 70, 975–980. [Google Scholar] [CrossRef]
- Cui, Y.Z.; Sun, R.; Wang, Q.J.; Wang, M.Z. Hepatotoxicity induced by intragastrically administrated with Gardenia decoction in mice. Nat. Prod. Res. 2017, 31, 2824–2827. [Google Scholar] [CrossRef]
- Wang, D.; Feng, F. Effect of different formula compatibility of Zhi-Zi-Hou-Pu decoction on the intestinal absorption of geniposide. Pharm. Clin. Res. 2012, 20, 177–180. (In Chinese) [Google Scholar]
- Lin, Y.T.; Hsiu, S.L.; Hou, Y.C.; Chen, H.Y.; Chao, P.D.L. Degradation of flavonoid aglycones by rabbit, rat and human fecal flora. Biol. Pharm. Bull. 2003, 26, 747–751. [Google Scholar] [CrossRef]
- Simons, A.L.; Renouf, M.; Murphy, P.A.; Hendrich, S. Greater apparent absorption of flavonoids is associated with lesser human fecal flavonoid disappearance rates. J. Agric. Food Chem. 2010, 58, 141–147. [Google Scholar] [CrossRef][Green Version]
- Kawata, Y.; Hattori, M.; Akao, T.; Kobashi, K.; Namba, T. Formation of nitrogen-containing metaboliths from geniposide and gardenoside by human intestinal bacteria. Planta Med. 1991, 57, 536–542. [Google Scholar] [CrossRef]
- Fujita, T.; Kawase, A.; Niwa, T.; Tomohiro, N.; Masuda, M.; Matsuda, H.; Iwaki, M. Comparative evaluation of 12 immature citrus fruit extracts for the inhibition of cytochrome P450 isoform activities. Biol. Pharm. Bull. 2008, 31, 925–930. [Google Scholar] [CrossRef]
- Burkina, V.; Zlabek, V.; Halsne, R.; Ropstad, E.; Zamaratskaia, G. In vitro effects of the citrus flavonoids diosmin, naringenin and naringin on the hepatic drug-metabolizing CYP3A enzyme in human, pig, mouse and fish. Biochem. Pharmacol. 2016, 110–111, 109–116. [Google Scholar] [CrossRef]
Sample Availability: Samples of the herbs are available from the authors. |
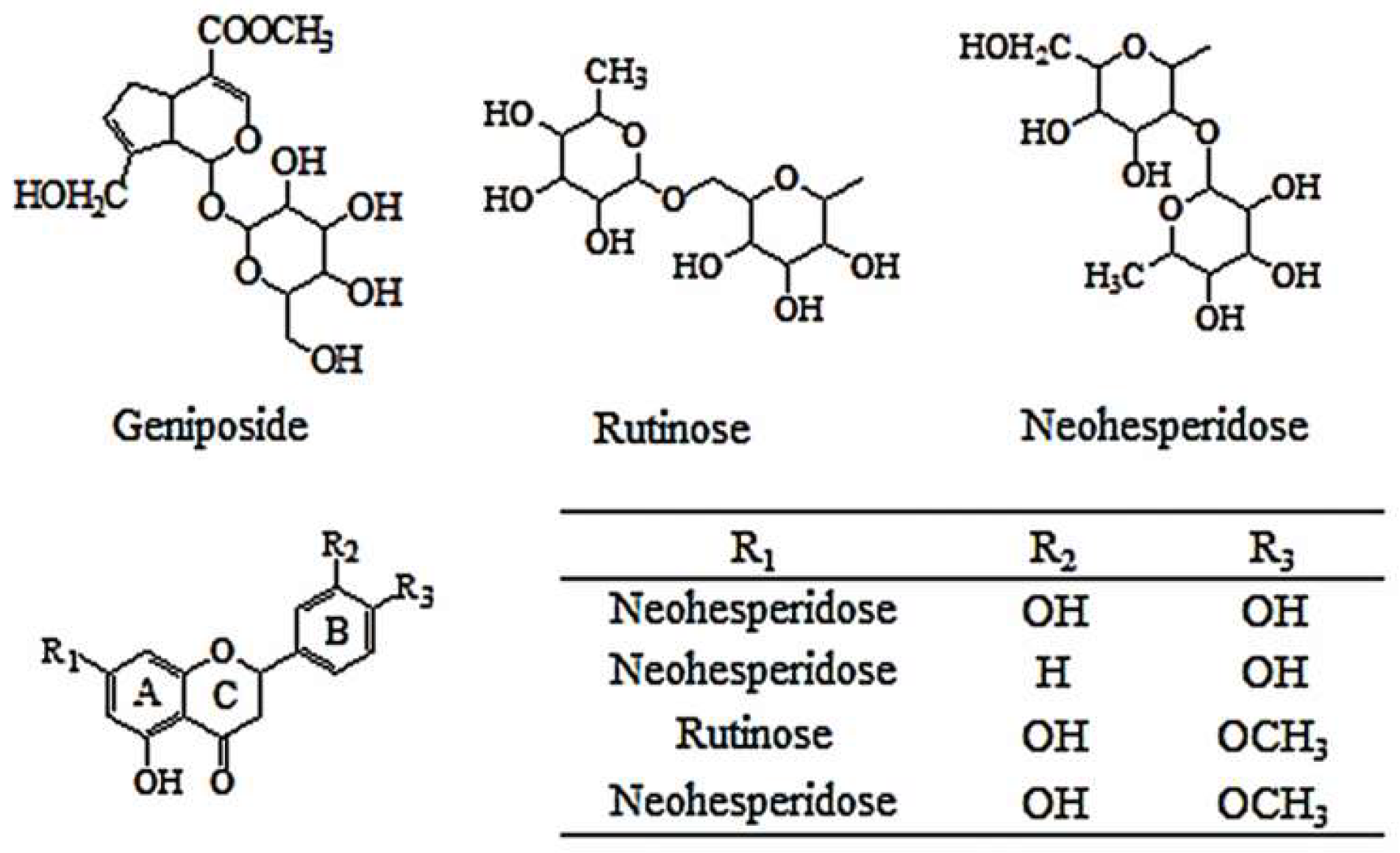
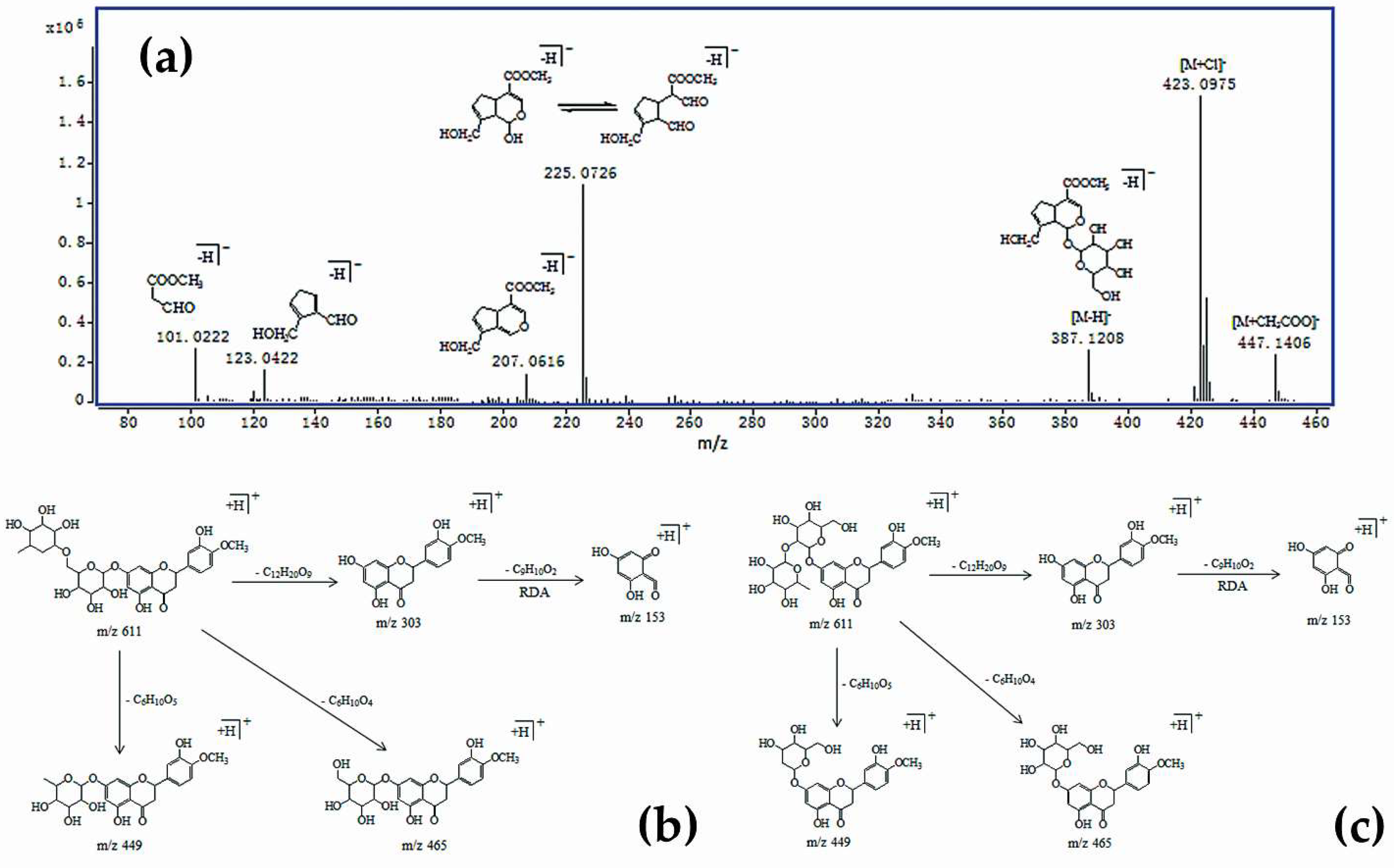
| No. | tR (min) | HPLC-TOF/MS (m/z) | HPLC-QqQ-MS/MS | UV (nm) | Mass Weight | Formula | Identification | VIP | |
|---|---|---|---|---|---|---|---|---|---|
| + | − | ||||||||
| 1 | 22.381 | 411.1253 [M + Na]+ 423.0975 [M + Cl]− 447.1406 [M + CH3COO]− 387.1208 [M − H]− 775.2473 [2M − H]− | 227, 209, 203 | 225, 207, 123, 101 | 240 | 388 | C17H24O10 | Geniposide | 1.54751 |
| 2 | 32.293 | 619.1629 [M + Na]+ 597.1806 [M + H]+ 595.1662 [M − H]− | 451, 435, 289, 153 | 449, 287, 151 | 284 | 596 | C27H32O15 | Neoeriocitrin | 1.06007 |
| 3 | 40.389 | 603.1739 [M + Na]+ 581.1900 [M + H]+ 579.1750 [M − H]− | 435, 419, 273, 153 | 459, 271, 151 | 283 | 580 | C27H32O14 | Naringin | 4.06373 |
| 4 | 42.310 | 633.1789 [M + Na]+ 611.1964 [M + H]+ 609.1819 [M − H]− | 465, 449, 303, 153 | 645, 301, 151 | 284 | 610 | C28H34O15 | Hesperidin | 1.29501 |
| 5 | 45.319 | 633.1847 [M + Na]+ 611.0051 [M + H]+ 609.1861 [M − H]− | 465, 449, 303, 153 | 609, 642, 151 | 284 | 610 | C28H34O15 | Neohesperidin | 4.30420 |
| ZZHPD | Geniposide (%) | Neoeriocitrin (%) | Naringin (%) | Hesperidin (%) | Neohesperidin (%) |
|---|---|---|---|---|---|
| TZS | 3.70508 | - 1 | - 1 | 0.40404 | - 1 |
| SZS | 3.88513 | 0.54788 | 9.23816 | 1.12065 | 7.98138 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Feng, F. The Effects of Different Varieties of Aurantii Fructus Immaturus on the Potential Toxicity of Zhi-Zi-Hou-Po Decoction Based on Spectrum-Toxicity Correlation Analysis. Molecules 2019, 24, 4254. https://doi.org/10.3390/molecules24234254
Zhang Q, Feng F. The Effects of Different Varieties of Aurantii Fructus Immaturus on the Potential Toxicity of Zhi-Zi-Hou-Po Decoction Based on Spectrum-Toxicity Correlation Analysis. Molecules. 2019; 24(23):4254. https://doi.org/10.3390/molecules24234254
Chicago/Turabian StyleZhang, Qianqian, and Fang Feng. 2019. "The Effects of Different Varieties of Aurantii Fructus Immaturus on the Potential Toxicity of Zhi-Zi-Hou-Po Decoction Based on Spectrum-Toxicity Correlation Analysis" Molecules 24, no. 23: 4254. https://doi.org/10.3390/molecules24234254
APA StyleZhang, Q., & Feng, F. (2019). The Effects of Different Varieties of Aurantii Fructus Immaturus on the Potential Toxicity of Zhi-Zi-Hou-Po Decoction Based on Spectrum-Toxicity Correlation Analysis. Molecules, 24(23), 4254. https://doi.org/10.3390/molecules24234254






