Synthesis and In Vitro Growth Inhibition of 2-Allylphenol Derivatives Against Phythopthora cinnamomi Rands
Abstract
1. Introduction
2. Results and Discussion
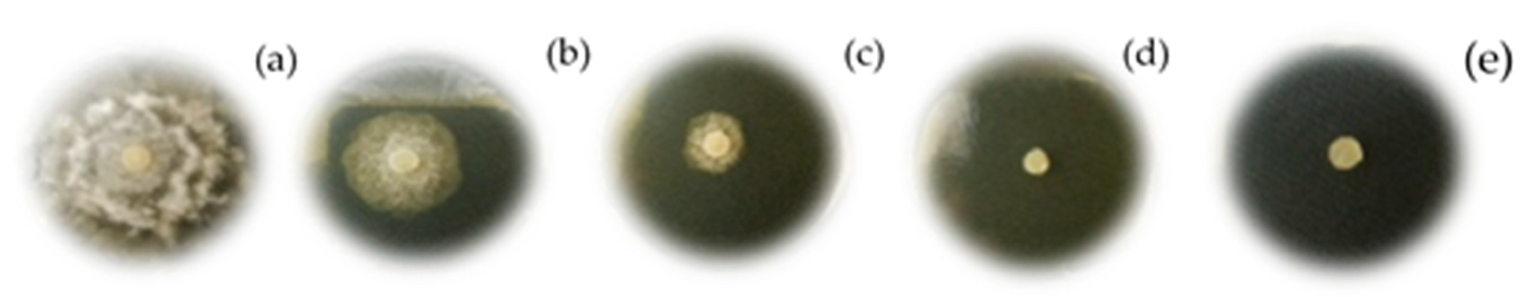
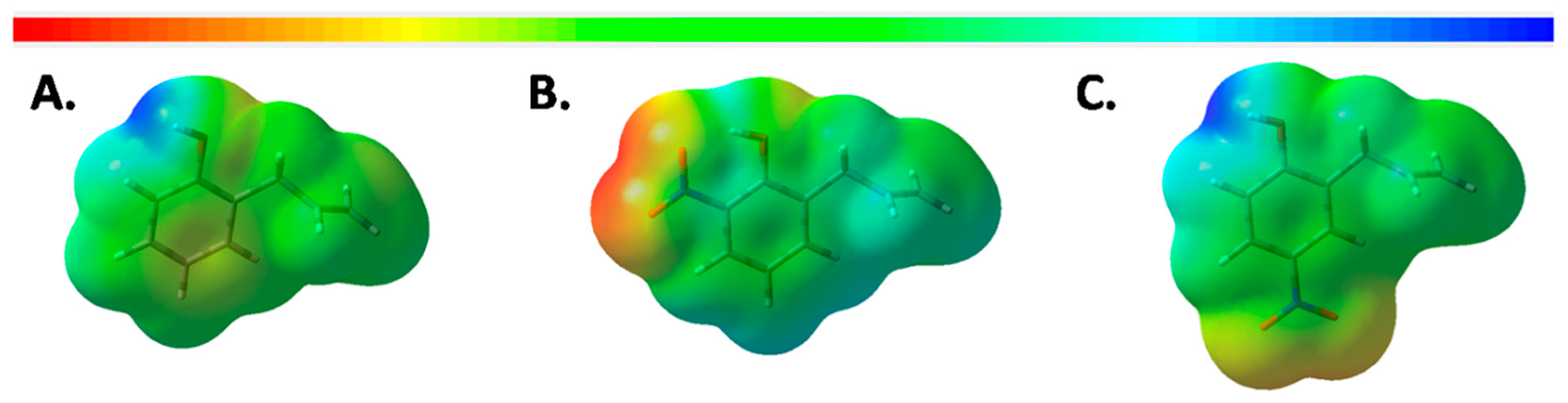
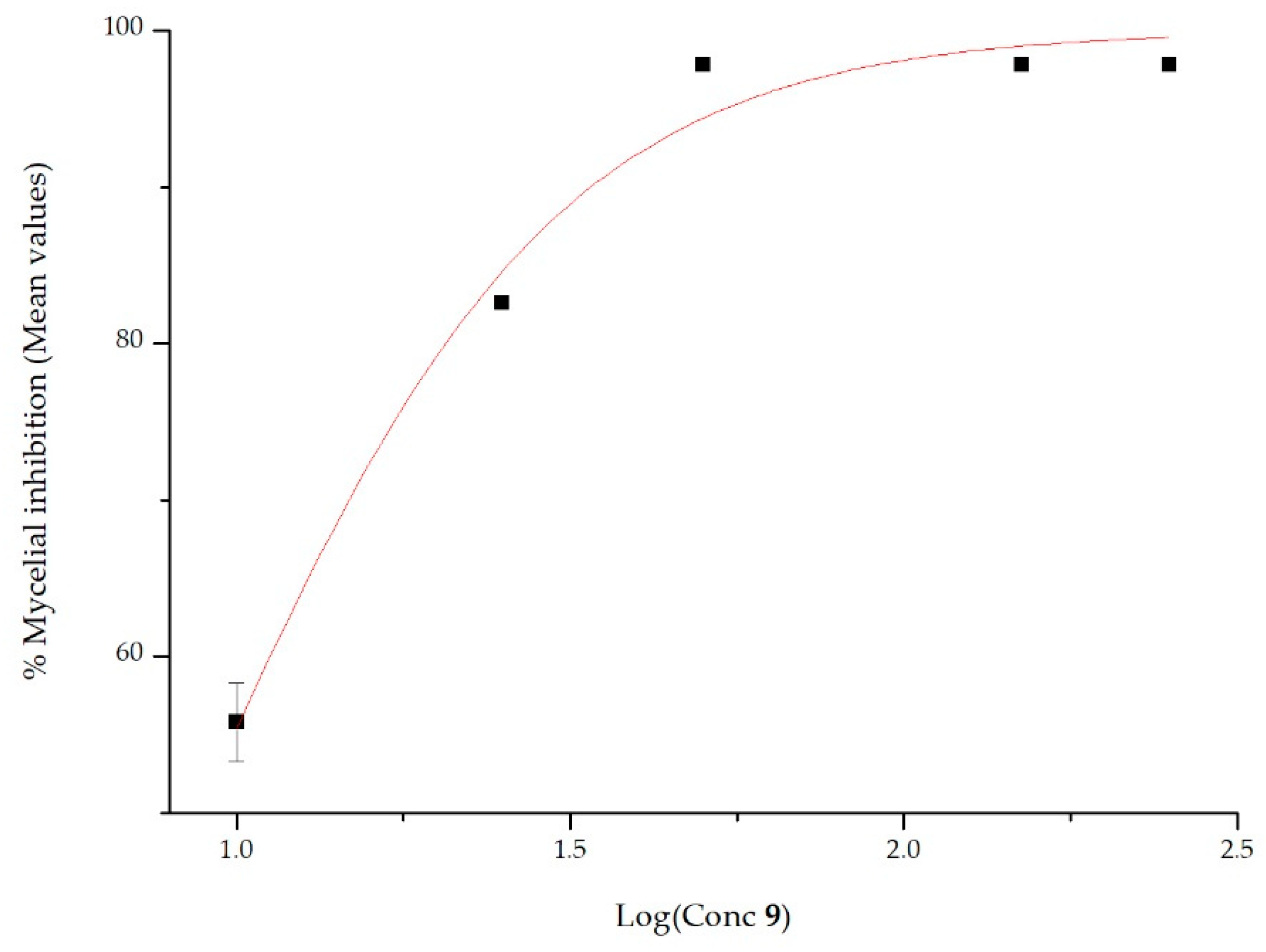
Growth Inhibition of 2-AP and Its Derivatives Against Phytophthora cinnamomi In Vitro
3. Experimental Section
3.1. General Information
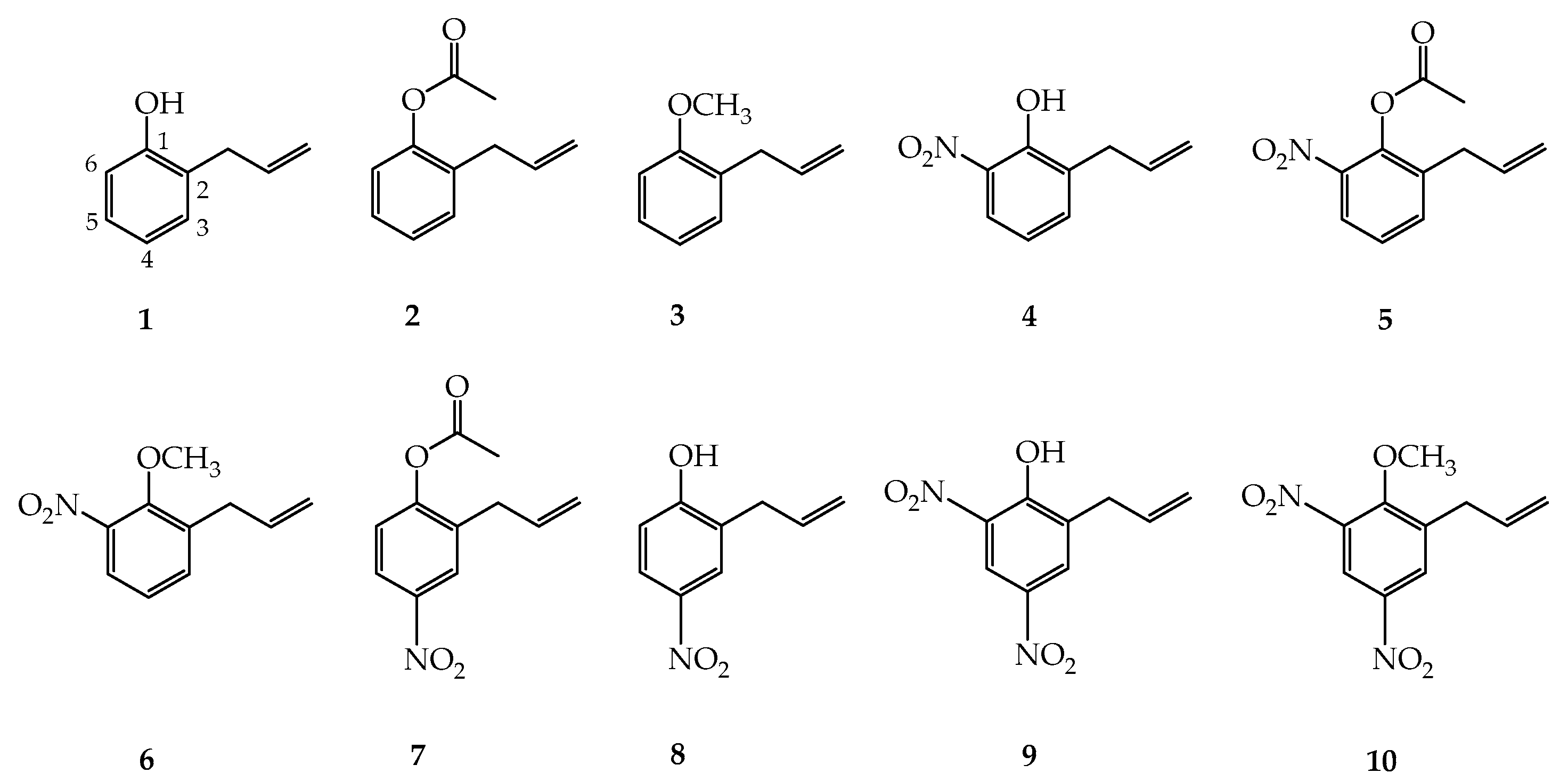
3.2. Synthesis of 2-AP Derivatives
3.2.1. Methylation Reactions
3.2.2. Nitration Procedure
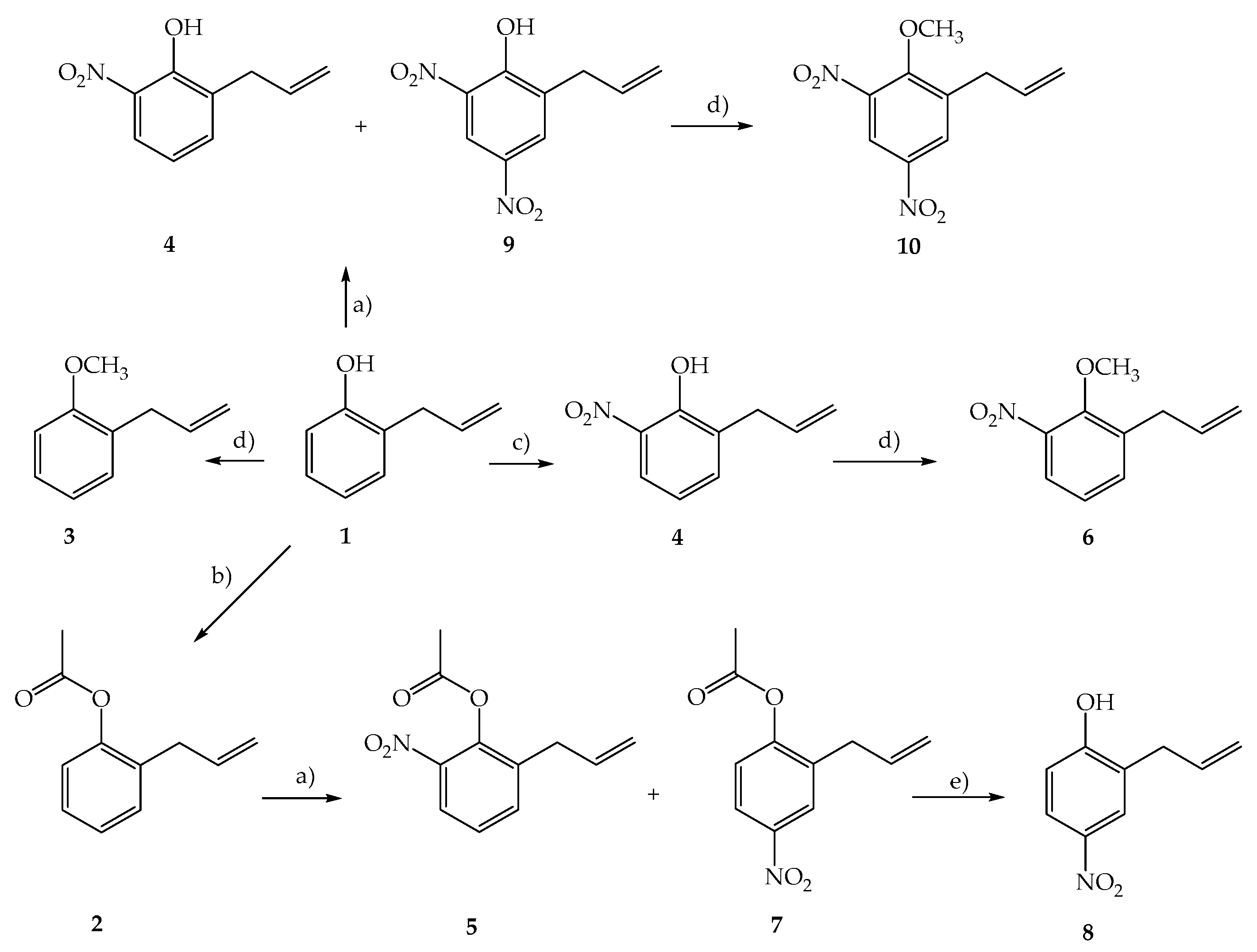
3.2.3. Preparation of 2-Allylphenyl Acetate (2)
3.2.4. Preparation of 1-Allyl-2-Methoxybenzene (3)
3.2.5. Preparation of 2-Allyl-6-Nitrophenol (4) and 2-Allyl-4,6-Dinitrophenol (9)
3.2.6. Preparation of 2-Allyl-6-Nitrophenyl Acetate (5) and 2-Allyl-4-Nitrophenyl Acetate (7)
3.2.7. Preparation of 1-Allyl-2-Methoxy-3-Nitrobenzene (6)
3.2.8. Preparation of 2-Allyl-4-Nitrophenol (8)
3.2.9. Preparation of 1-Allyl-6-Methoxy-3,5-Dinitrobenzene (10)
3.3. Antifungal Activity of 2-Allylphenol and Derivatives Against Phytophthora Cinnamomi In Vitro
3.4. Computational Details
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Whisson, S.C. Phytophthora . In Encyclopedia of Life Sciences (ELS); John Wiley & Sons: Chichester, UK, 2010. [Google Scholar]
- Cahill, D.M.; Rookes, J.E.; Wilson, B.A.; Gibson, L.; McDougall, K.L. Phytophthora cinnamomi and Australia’s biodiversity: Impacts, predictions and progress towards control. Aust. J. Bot. 2008, 56, 279–310. [Google Scholar] [CrossRef]
- Kamoun, S.; Furzer, O.; Jones, J.D.G.; Judelson, H.S.; Ali, G.S.; Dalio, R.J.D.; Roy, S.G.; Schena, L.; Zambounis, A.; Panabieres, F.; et al. The Top 10 Oomycete pathogens in molecular plant pathology. Mol. Plant. Pathol. 2015, 16, 413–434. [Google Scholar] [CrossRef] [PubMed]
- Serrazina, S.; Santos, C.; Machado, H.; Pesquita, C.; Vicentini, R.; Pais, M.S.; Sebastiana, M.; Costa, R. Castanea root transcriptome in response to Phytophthora cinnamomi challenge. Tree Genet. Genomes 2015, 11. [Google Scholar] [CrossRef]
- Dobrowolski, M.P.; Shearer, B.L.; Colquhoun, I.J.; O’Brien, P.A.; Hardy, G.E.S. Selection for decreased sensitivity to phosphite in Phytophthora cinnamomi with prolonged use of fungicide. Plant. Pathol. 2008, 57, 928–936. [Google Scholar] [CrossRef]
- Hu, J.H.; Hong, C.X.; Stromberg, E.L.; Moorman, G.W. Mefenoxam Sensitivity in Phytophthora cinnamomi Isolates. Plant. Dis. 2010, 94, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, L.; Taborga, L.; Diaz, K.; Olea, A.F.; Peña-Cortes, H. Synthesis of linear geranylphenols and their effect on mycelial growth of plant pathogen Botrytis cinerea. Molecules 2014, 19, 1512–1526. [Google Scholar] [CrossRef] [PubMed]
- Chavez, M.I.; Soto, M.; Cimino, F.A.; Olea, A.F.; Espinoza, L.; Diaz, K.; Taborga, L. In Vitro Antifungal Activity of New and Known Geranylated Phenols against Phytophthora cinnamomi Rands. Int. J. Mol. Sci. 2018, 19, 1601. [Google Scholar] [CrossRef] [PubMed]
- Taborga, L.; Diaz, K.; Olea, A.F.; Reyes-Bravo, P.; Flores, M.E.; Pena-Cortes, H.; Espinoza, L. Effect of Polymer Micelles on Antifungal Activity of Geranylorcinol Compounds against Botrytis cinerea. J. Agric. Food Chem. 2015, 63, 6890–6896. [Google Scholar] [CrossRef] [PubMed]
- Taborga, L.; Sortino, M.; Carrasco, H.; Butassi, E.; Zacchino, S.; Espinoza, L. Antifungal toxicity of linear geranylphenol. Influence of oxygenate substituents. Food Chem. Toxicol. 2017, 29, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, H.; Raimondi, M.; Svetaz, L.; Di Liberto, M.; Rodriguez, M.V.; Espinoza, L.; Madrid, A.; Zacchino, S. Antifungal activity of eugenol analogues. Influence of different substituents and studies on mechanism of action. Molecules 2012, 17, 1002–1024. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.J.; Hao, J.J.; Xia, Y.Y.; Liu, X.L.; Li, J.Q. Inhibitory effect of bionic fungicide 2-allylphenol on Botrytis cinerea (Pers. ex Fr.) in vitro. Pest. Manag. Sci. 2009, 65, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Qu, T.; Zhang, J.; Meng, Z.; Liu, X.; Cao, Y.; Li, J.; Hao, J.J. Metabolism of fungicide 2-allylphenol in Rhizoctonia cerealis. Ecotox. Environ. Safe. 2014, 102, 136–141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qu, T.; Gao, S.; Li, J.; Hao, J.J.; Ji, P. Synthesis and antifungal activity of 2-allylphenol derivatives against fungal plant pathogens. Pestic. Biochem. Physiol. 2017, 135, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Olea, A.F.; Bravo, A.; Martínez, R.; Thomas, M.; Sedan, C.; Espinoza, L.; Zambrano, E.; Carvajal, D.; Silva-Moreno, E.; Carrasco, H. Antifungal activity of eugenol derivatives against Botrytis Cinerea. Molecules 2019, 24, 1239. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Yang, R.; Zhang, C.; Zhu, L.; Miao, F.; Yang, X.; Zhou, L. 2-(Substituted phenyl)-3,4-dihydroisoquinolin-2-iums as Novel Antifungal Lead Compounds: Biological Evaluation and Structure-Activity Relationships. Molecules 2013, 18, 10413–10424. [Google Scholar] [CrossRef] [PubMed]
- Kerkenaar, A.; Sijpesteijn, A.K. Antifungal activity of metalaxyl and furalaxyl. Pestic. Biochem. Physiol. 1981, 15, 71–78. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Chen, H.; Fan, Y.; Shi, Z. Antifungal activity of eugenol against Botrytis cinerea. Trop. Plant. Pathol. 2010, 35, 137–143. [Google Scholar] [CrossRef]
- Voda, K.; Boh, B.; Vrtačnik, M. A quantitative structure–antifungal activity relationship study of oxygenated aromatic essential oil compounds using data structuring and PLS regression analysis. J. Mol. Model. 2004, 10, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Derita, M.; Montenegro, I.; Garibotto, F.; Enriz, R.; Cuellar, M.; Zacchino, S. Structural requirements for the antifungal activities of natural drimane sesquiterpenes and analogues, supported by conformational and electronic studies. Molecules 2013, 18, 2029–2051. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–10 are available from the authors. |
| Compounds | a EC50 (µg/mL) ± SD b | R c |
|---|---|---|
| 1 | 155 ± 1.45 | 0.9404 |
| 2 | 360 ± 1.27 | 0.9312 |
| 3 | I | i |
| 4 | 180 ± 1.42 | 0.9327 |
| 5 | 105 ± 1.70 | 0.9878 |
| 6 | I | i |
| 7 | 10 ± 1.43 | 0.9185 |
| 8 | 10 ± 0.15 | 0.9973 |
| 9 | 9 ± 1.77 | 0.9805 |
| 10 | 80 ± 2.00 | 0.9996 |
| Metalaxil MZ 48 WP (C+) | 7.5 ± 0.64 | 0.9999 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olea, A.F.; Espinoza, L.; Sedan, C.; Thomas, M.; Martínez, R.; Mellado, M.; Carrasco, H.; Díaz, K. Synthesis and In Vitro Growth Inhibition of 2-Allylphenol Derivatives Against Phythopthora cinnamomi Rands. Molecules 2019, 24, 4196. https://doi.org/10.3390/molecules24224196
Olea AF, Espinoza L, Sedan C, Thomas M, Martínez R, Mellado M, Carrasco H, Díaz K. Synthesis and In Vitro Growth Inhibition of 2-Allylphenol Derivatives Against Phythopthora cinnamomi Rands. Molecules. 2019; 24(22):4196. https://doi.org/10.3390/molecules24224196
Chicago/Turabian StyleOlea, Andrés F., Luis Espinoza, Claudia Sedan, Mario Thomas, Rolando Martínez, Marco Mellado, Héctor Carrasco, and Katy Díaz. 2019. "Synthesis and In Vitro Growth Inhibition of 2-Allylphenol Derivatives Against Phythopthora cinnamomi Rands" Molecules 24, no. 22: 4196. https://doi.org/10.3390/molecules24224196
APA StyleOlea, A. F., Espinoza, L., Sedan, C., Thomas, M., Martínez, R., Mellado, M., Carrasco, H., & Díaz, K. (2019). Synthesis and In Vitro Growth Inhibition of 2-Allylphenol Derivatives Against Phythopthora cinnamomi Rands. Molecules, 24(22), 4196. https://doi.org/10.3390/molecules24224196