A Newly Discovered Phenylethanoid Glycoside from Stevia rebaudiana Bertoni Affects Insulin Secretion in Rat INS-1 Islet β Cells
Abstract
1. Introduction
2. Results and Discussion
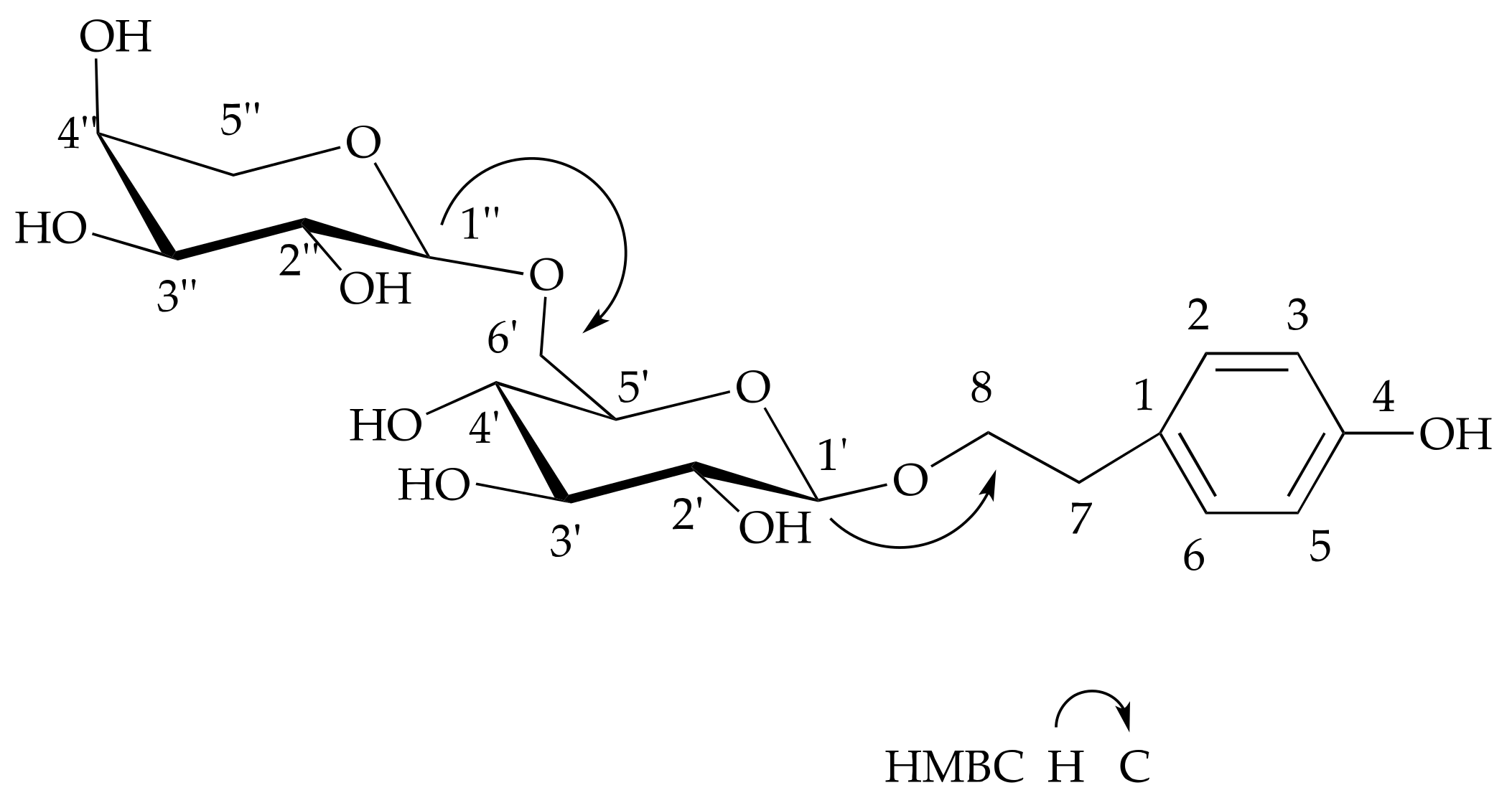
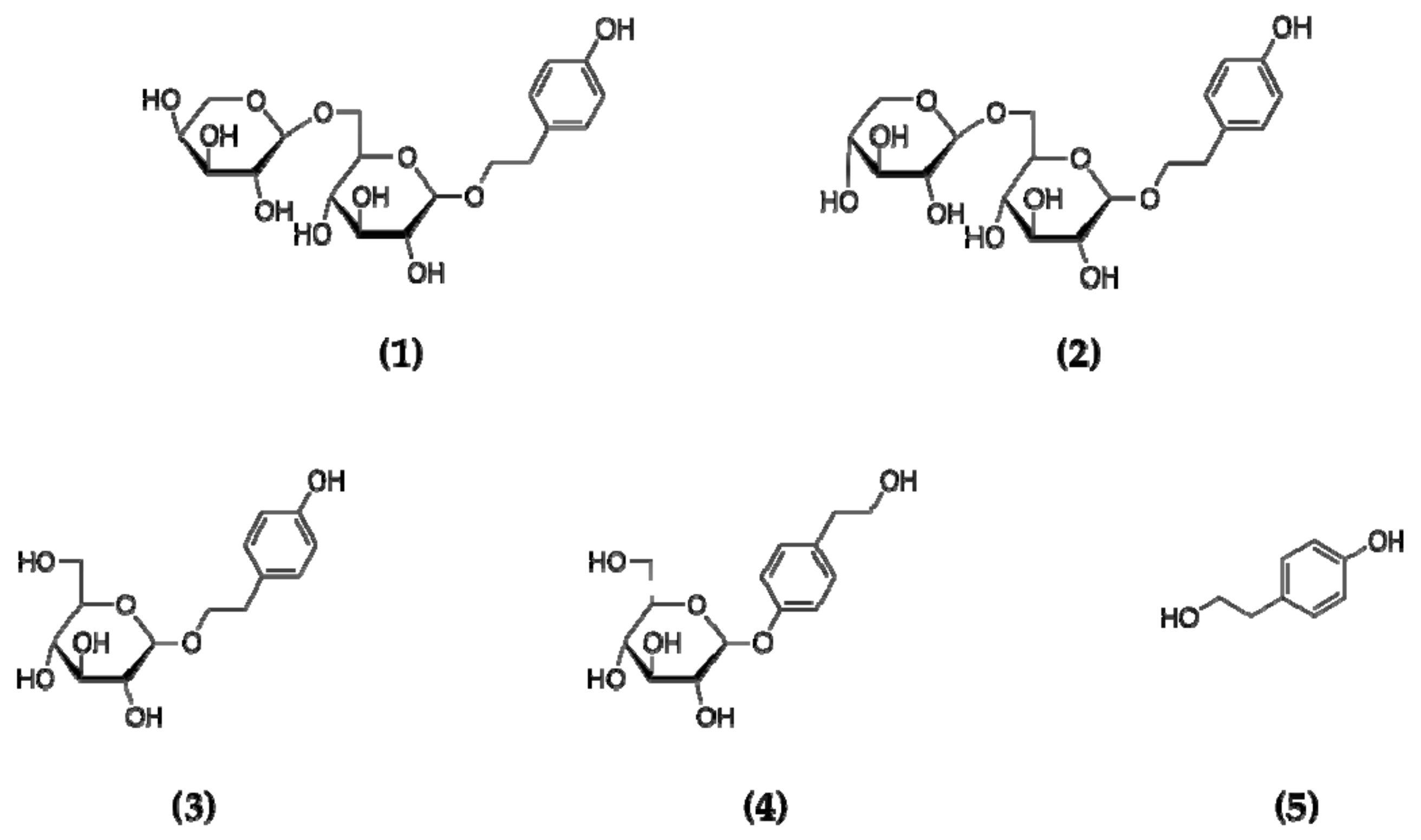
2.1. Structure Elucidation
2.2. Pharmacokinetic Properties
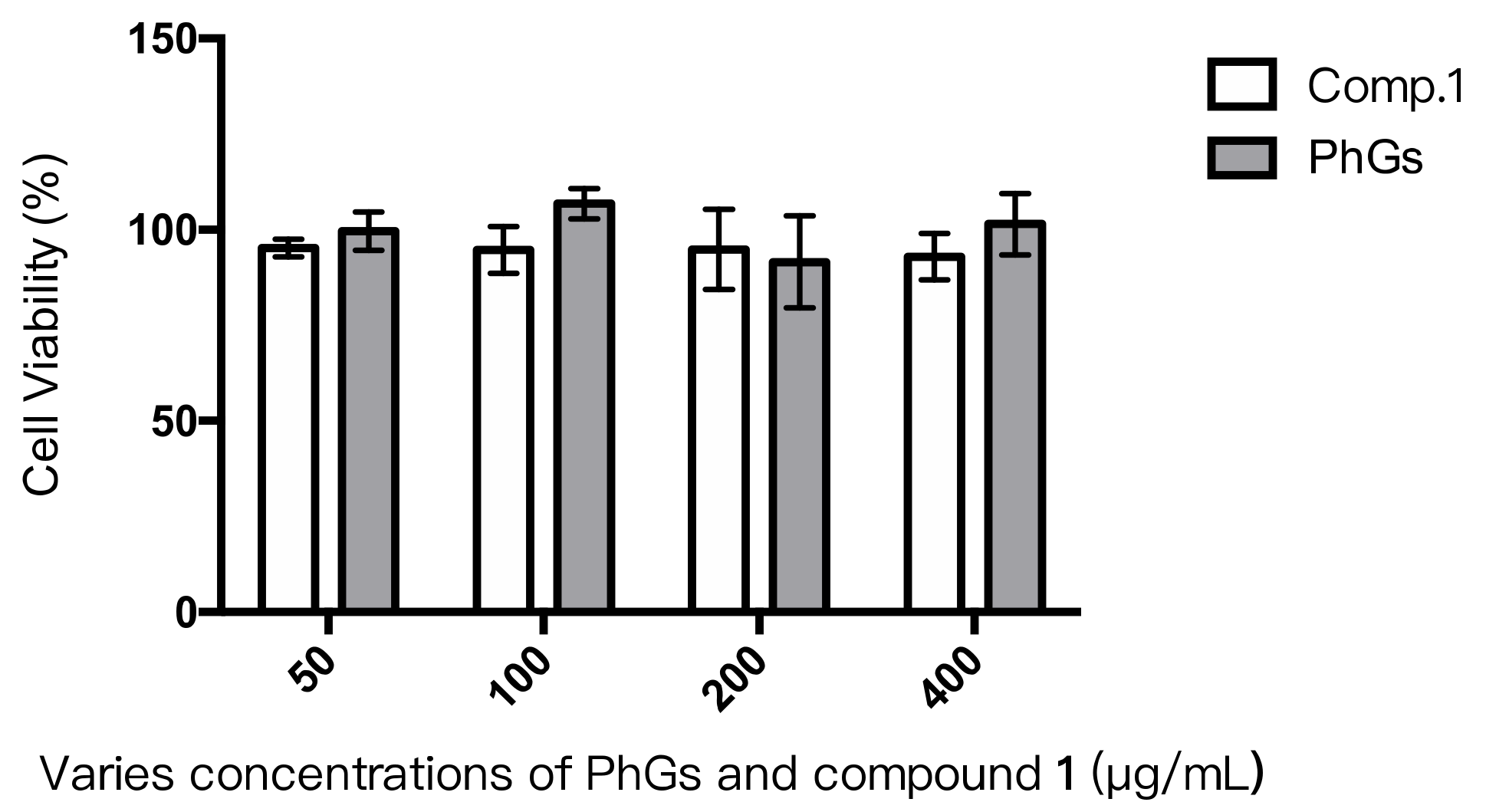
2.3. Cytotoxicity Assay
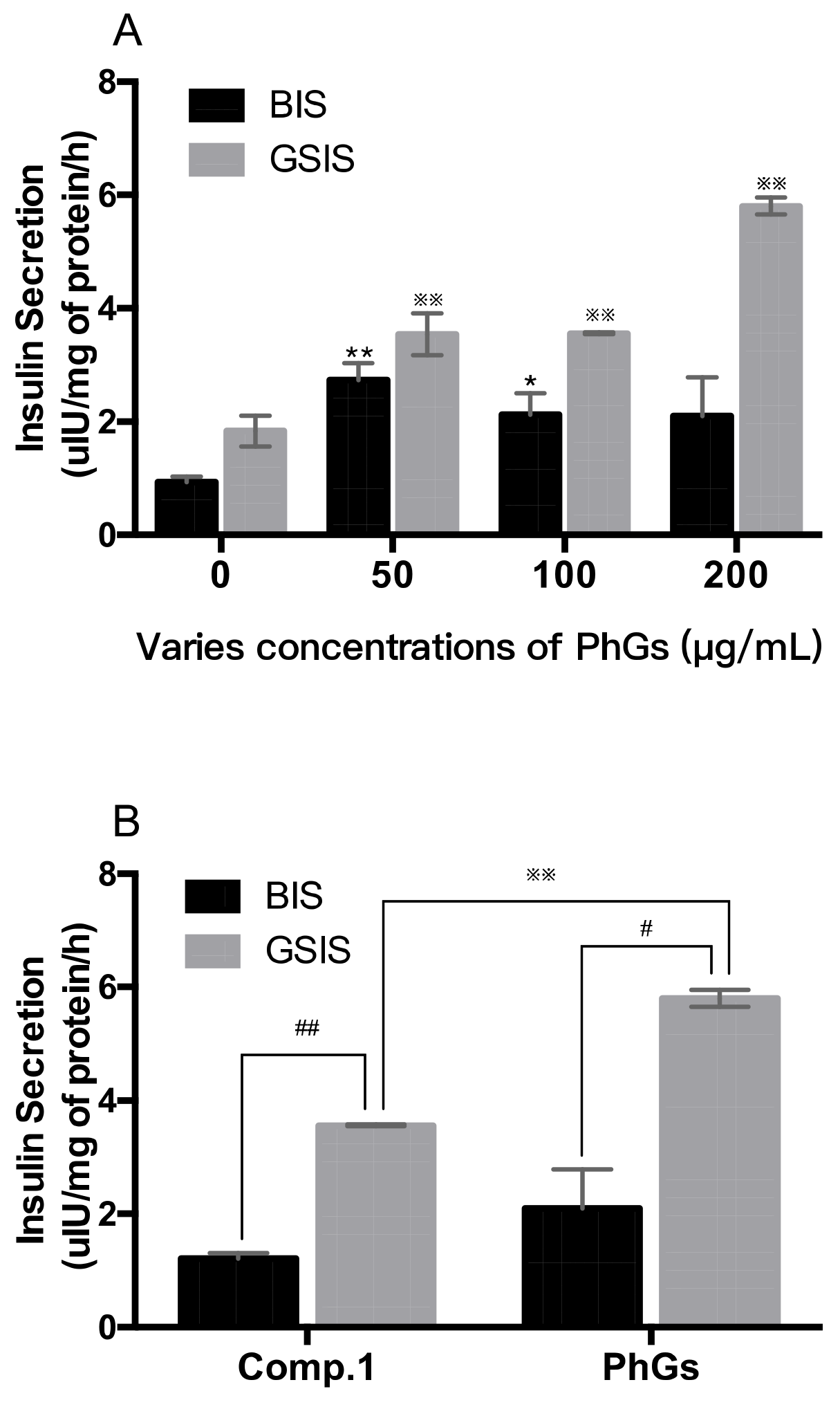
2.4. Insulin Secretion in Vitro
3. Materials and Methods
3.1. Material and Chemicals
3.2. Isolation and Purification of Compounds 1–5
3.3. Structure Validation and Property Prediction
3.3.1. Identification of the Isolated Compounds
3.3.2. Hydrolysis of Phenylethanoid Glycoside
3.3.3. ADMET Prediction
3.4. Cell Culture and Cytotoxicity Assay
3.5. Insulin Secretion Assay
3.6. Statistical Analysis
4. Conclusions
5. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Ferrazzano, G.F.; Cantile, T.; Alcidi, B.; Coda, M.; Ingenito, A.; Zarrelli, A.; Di Fabio, G.; Pollio, A. Is Stevia rebaudiana Bertoni a non cariogenic sweetener? A Review. Molecules 2015, 21, 38. [Google Scholar] [CrossRef] [PubMed]
- Pol, J.; Hohnova, B.; Hyotylainen, T. Characterisation of Stevia rebaudiana by comprehensive two-dimensional liquid chromatography time-of-flight mass spectrometry. J. Chromatogr. A 2007, 1150, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Tateo, F.; Escobar Sanchez, M.L.; Bononi, M.; Lubian, E. Stevioside content of Stevia rebaudiana (Bertoni) Bertoni grown in East Paraguay. Ital. J. Food Sci. 1999, 11, 265–269. [Google Scholar]
- Shivanna, N.; Naika, M.; Khanum, F.; Kaul, V.K. Antioxidant, anti-diabetic and renal protective properties of Stevia rebaudiana. J. Diabetes Complicat. 2013, 27, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.; Tomlinson, B.; Chen, Y.J.; Liu, J.C.; Hsieh, M.H.; Cheng, J.T. A double-blind placebo-controlled study of the effectiveness and tolerability of oral stevioside in human hypertension. Brit. J. Clin. Pharmacol. 2000, 50, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Kujur, R.S.; Singh, V.; Ram, M.; Yadava, H.N.; Singh, K.K.; Kumari, S.; Roy, B.K. Antidiabetic activity and phytochemical screening of crude extract of Stevia rebaudiana in alloxan-induced diabetic rats. Pharm. Res. 2010, 2, 258–263. [Google Scholar]
- Suanarunsawat, T.; Klongpanichapak, S.; Rungseesantivanon, S.; Chaiyabutr, N. Glycemic effect of stevioside and Stevia rebaudiana in streptozotocin- induced diabetic rats. East. J. Med. 2004, 9, 51–56. [Google Scholar]
- Curi, R.; Alvarez, M.; Bazotte, R.B.; Botion, L.M.; Godoy, J.L.; Bracht, A. Effect of Stevia rebaudiana on glucose tolerance in normal adult humans. Braz. J. Med. Boil. Res. 1986, 19, 771–774. [Google Scholar]
- Soejarto, D.D.; Kinghorn, A.D.; Farnsworth, N.R. Potential sweetening agents of plant origin. III. Organoleptic evaluation of Stevia leaf herbarium samples for sweetness. J. Nat. Prod. 1982, 45, 590. [Google Scholar] [CrossRef]
- Peng, P. Study on Preparation Technology and Quality Standard of Effective Parts of Stevia and Its Hypoglycemic Effect. Master’s Thesis, Beijing University of Chinese Medicine, Beijing, China, 2012. [Google Scholar]
- Zhu, N.L. The Drug System Characterization of stevia Based on Antidiabetic. Ph.D. Thesis, Beijing University of Chinese Medicine, Beijing, China, 2014. [Google Scholar]
- Li, J.; Hua, J.; Shi, R. A new acylated quercetin glycoside from the leaves of Stevia rebaudiana Bertoni. Nat. Prod. Res. 2009, 23, 1378–1383. [Google Scholar] [CrossRef]
- Martin, F.; Hay, A.E.; Corno, L.; Gupta, M.P.; Hostettmann, K. Iridoid glycosides from the stems of Pithecoctenium crucigerum (Bignoniaceae). Phytochemistry 2007, 68, 1307–1311. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, M.; Alipieva, K.; Orhan, I.; Abrashev, R.; Denev, P.; Angelova, M. Antioxidant and cholinesterases inhibitory activities of Verbascum xanthophoeniceum Griseb. and its phenylethanoid glycosides. Food Chem. 2011, 128, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Harput, U.S.; Genc, Y.; Saracoglu, I. Cytotoxic and antioxidative activities of Plantago lagopus L. and characterization of its bioactive compounds. Food Chem. Toxicol. 2012, 50, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Lee, Y.S.; Kim, S.H.; Bae, Y.S.; Lim, S.S. Inhibition of aldose reductase by phenylethanoid glycoside isolated from the seeds of Paulownia coreana. Biol. Pharm. Bull. 2011, 34, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Hashemi, Z.; Han, W.; Jin, A.; Yang, H.; Ozga, J.; Li, L.; Chan, C.B. Hydrolysis enhances bioavailability of proanthocyanidin-derived metabolites and improves beta-cell function in glucose intolerant rats. J. Nutr. Biochem. 2015, 26, 850–859. [Google Scholar] [CrossRef]
- Yang, K.; Chan, C.B. Epicatechin potentiation of glucose-stimulated insulin secretion in INS-1 cells is not dependent on its antioxidant activity. Acta Pharmacol. Sin. 2018, 39, 893–902. [Google Scholar] [CrossRef]
- Badolato, M.; Carullo, G.; Perri, M.; Cione, E.; Manetti, F.; Di Gioia, M.L.; Brizzi, A.; Caroleo, M.C.; Aiello, F. Quercetin/oleic acid-based G-protein-coupled receptor 40 ligands as new insulin secretion modulators. Future Med. Chem. 2017, 9, 1873–1885. [Google Scholar] [CrossRef]
- Rayasam, G.V.; Tulasi, V.K.; Sundaram, S.; Singh, W.; Kant, R.; Davis, J.A.; Saini, K.S.; Ray, A. Identification of berberine as a novel agonist of fatty acid receptor GPR40. Phytother. Res. 2010, 24, 1260–1263. [Google Scholar] [CrossRef]
- Carullo, G.; Perri, M.; Manetti, F.; Aiello, F.; Caroleo, M.C.; Cione, E. Quercetin-3-oleoyl derivatives as new GPR40 agonists: Molecular docking studies and functional evaluation. Bioorg. Med. Chem. Lett. 2019, 29, 1761–1764. [Google Scholar] [CrossRef]
- Clark, S.A.; Quaade, C.; Constandy, H.; Hansen, P.; Halban, P.; Ferber, S.; Newgard, C.B.; Normington, K. Novel insulinoma cell lines produced by iterative engineering of GLUT2, glucokinase, and human insulin expression. Diabetes 1997, 46, 958. [Google Scholar] [CrossRef]
- Miyase, T.; Ueno, A.; Takizawa, N.; Kobayashi, H.; Oguchi, H. Ionone and lignan glycosides from Epimedium diphyllum. Phytochemistry 1989, 28, 3483–3485. [Google Scholar] [CrossRef]
- Ma, S.J.; Mizutani, M.; Hiratake, J.; Sakata, K. Substrate specificity of β-primeverosidase, A key enzyme in aroma formation during oolong tea and black tea manufacturing. Biosci. Biotech. Bioch. 2001, 65, 2719–2729. [Google Scholar] [CrossRef] [PubMed]
- Bisset, N.G.; Choudhury, A.K.; Houghton, P.J. Phenolic glycosides from the fruit of strychnos nux-vomica. Phytochemistry 1989, 28, 1553–1554. [Google Scholar] [CrossRef]
- Saimaru, H.; Orihara, Y. Biosynthesis of acteoside in cultured cells of olea europaea. J. Nat. Med. 2010, 64, 139–145. [Google Scholar] [CrossRef]
- Owen, R.W.; Mier, W.; Giacosa, A.; Hull, W.E.; Spiegelhalder, B.; Bartsch, H. Identification of lignans as major components in the phenolic fraction of olive oil. Clin. Chem. 2000, 46, 976. [Google Scholar]
- Severina, P.; Brigida, D.A.; Monica, S.; Grazia, D.A.; Marialuisa, G.; Silvia, G.; Pietro, M.; Antonio, F. NMR-based metabolic profiling and in vitro antioxidant and hepatotoxic assessment of partially purified fractions from Golden germander (Teucrium polium L.) methanolic extract. J. Food Chem. 2012, 135, 1957–1967. [Google Scholar]
- Smith, D.A.; Di, L.; Kerns, E.H. The effect of plasma protein binding on in vivo efficacy: Misconceptions in drug discovery. Nat. Rev. Drug Discov. 2010, 9, 929–939. [Google Scholar] [CrossRef]
- Kujawski, J.; Popielarska, H.; Myka, A.; Drabińska, B.; Bernard, M.K. The log P parameter as a molecular descriptor in the computer-aided drug design—an overview. CMST 2012, 18, 81–88. [Google Scholar] [CrossRef]
- Szumilak, M.; Galdyszynska, M.; Dominska, K.; Bak, S., II; Merecz-Sadowska, A.; Stanczak, A.; Karwowski, B.T.; Piastowska-Ciesielska, A.W. Synthesis, biological activity and preliminary in Silico ADMET screening of polyamine conjugates with bicyclic systems. Molecules 2017, 22, 794. [Google Scholar] [CrossRef]
- Xiong, W.T.; Gu, L.; Wang, C.; Sun, H.X.; Liu, X. Anti-hyperglycemic and hypolipidemic effects of Cistanche tubulosa in type 2 diabetic db/db mice. J. Ethnopharmacol. 2013, 150, 935–945. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Gregersen, S.; Poulsen, C.R.; Hermansen, K. Stevioside acts directly on pancreatic beta cells to secrete insulin: Actions independent of cyclic adenosine monophosphate and adenosine triphosphate-sensitive K+-channel activity. Metabolism 2000, 49, 208–214. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Gregersen, S.; Alstrup, K.K.; Hermansen, K. Stevioside induces antihyperglycaemic, insulinotropic and glucagonostatic effects in vivo: Studies in the diabetic Goto-Kakizaki (GK) rats. Phytomedicine 2002, 9, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Babujanarthanam, R.; Kavitha, P.; Pandian, M.R. Quercitrin, a bioflavonoid improves glucose homeostasis in streptozotocin-induced diabetic tissues by altering glycolytic and gluconeogenic enzymes. Fund Clin. Pharmacol. 2010, 24, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H. Research on Manufacture Process, Chemical Constitude and Quality Control Method of Stevia rebaudianum Bertoni Effective Fraction. Master’s Thesis, Beijing University of Chinese Medicine, Beijing, China, 2007. [Google Scholar]
- Bhhatarai, B.; Wilson, D.M.; Parks, A.K.; Carney, E.W.; Spencer, P.J. Evaluation of TOPKAT, toxtree, and derek nexus in silico models for ocular irritation and development of a knowledge-based framework to improve the prediction of severe irritation. Chem. Res. Toxicol. 2016, 29, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Karunagaran, S.; Subhashchandrabose, S.; Lee, K.W.; Meganathan, C. Investigation on the isoform selectivity of novel kinesin-like protein 1 (KIF11) inhibitor using chemical feature based pharmacophore, molecular docking, and quantum mechanical studies. Comput. Biol. Chem. 2016, 61, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Thiyagarajan, V.; Lin, S.H.; Chang, Y.C.; Weng, C.F. Identification of novel FAK and S6K1 dual inhibitors from natural compounds via ADMET screening and molecular docking. Biomed. Pharm. 2016, 80, 52–62. [Google Scholar] [CrossRef]
- Zhu, S.; Larkin, D.; Lu, S.; Inouye, C.; Haataja, L.; Anjum, A.; Kennedy, R.; Castle, D.; Arvan, P. Monitoring C-peptide storage and secretion in islet beta-cells in vitro and in vivo. Diabetes 2016, 65, 699–709. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–5 are available from the authors. |
| Predicted Item | Compound 1 |
|---|---|
| Absorption level (2–4) | 3 |
| BBB level (2–4) | 4 |
| Solubility level | 4 |
| CYP2D6 prediction (False-non inhibitor) | FALSE |
| Hepatotoxic prediction (False-non toxic) | FALSE |
| PPB Prediction (False-poorly bound) | FALSE |
| Mutagen | Non-Mutagen |
| Rodent Carcinogenicity | Non-Carcinogen |
| Skin irritancy | Non-Irritant |
| Skin sensitization | 0.765 |
| Rat oral LD50 (g/kg) | 5.61 |
| Ocular Irritancy | 0.84 |
| DTP (Developmental Toxicity Potential) | 0.861 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Zhu, N.-L.; Kong, J.; Peng, P.; Li, L.-F.; Wei, X.-L.; Jiang, Y.-Y.; Zhang, Y.-L.; Bian, B.-L.; She, G.-M.; et al. A Newly Discovered Phenylethanoid Glycoside from Stevia rebaudiana Bertoni Affects Insulin Secretion in Rat INS-1 Islet β Cells. Molecules 2019, 24, 4178. https://doi.org/10.3390/molecules24224178
He J, Zhu N-L, Kong J, Peng P, Li L-F, Wei X-L, Jiang Y-Y, Zhang Y-L, Bian B-L, She G-M, et al. A Newly Discovered Phenylethanoid Glycoside from Stevia rebaudiana Bertoni Affects Insulin Secretion in Rat INS-1 Islet β Cells. Molecules. 2019; 24(22):4178. https://doi.org/10.3390/molecules24224178
Chicago/Turabian StyleHe, Jing, Nai-Liang Zhu, Jing Kong, Ping Peng, Lin-Fu Li, Xiao-Lu Wei, Yan-Yan Jiang, Yan-Ling Zhang, Bao-Lin Bian, Gai-Mei She, and et al. 2019. "A Newly Discovered Phenylethanoid Glycoside from Stevia rebaudiana Bertoni Affects Insulin Secretion in Rat INS-1 Islet β Cells" Molecules 24, no. 22: 4178. https://doi.org/10.3390/molecules24224178
APA StyleHe, J., Zhu, N.-L., Kong, J., Peng, P., Li, L.-F., Wei, X.-L., Jiang, Y.-Y., Zhang, Y.-L., Bian, B.-L., She, G.-M., & Shi, R.-B. (2019). A Newly Discovered Phenylethanoid Glycoside from Stevia rebaudiana Bertoni Affects Insulin Secretion in Rat INS-1 Islet β Cells. Molecules, 24(22), 4178. https://doi.org/10.3390/molecules24224178





