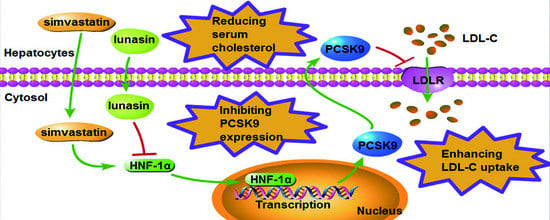
Lunasin Improves the LDL-C Lowering Efficacy of Simvastatin via Inhibiting PCSK9 Expression in Hepatocytes and ApoE−/− Mice
Abstract
1. Introduction
2. Results
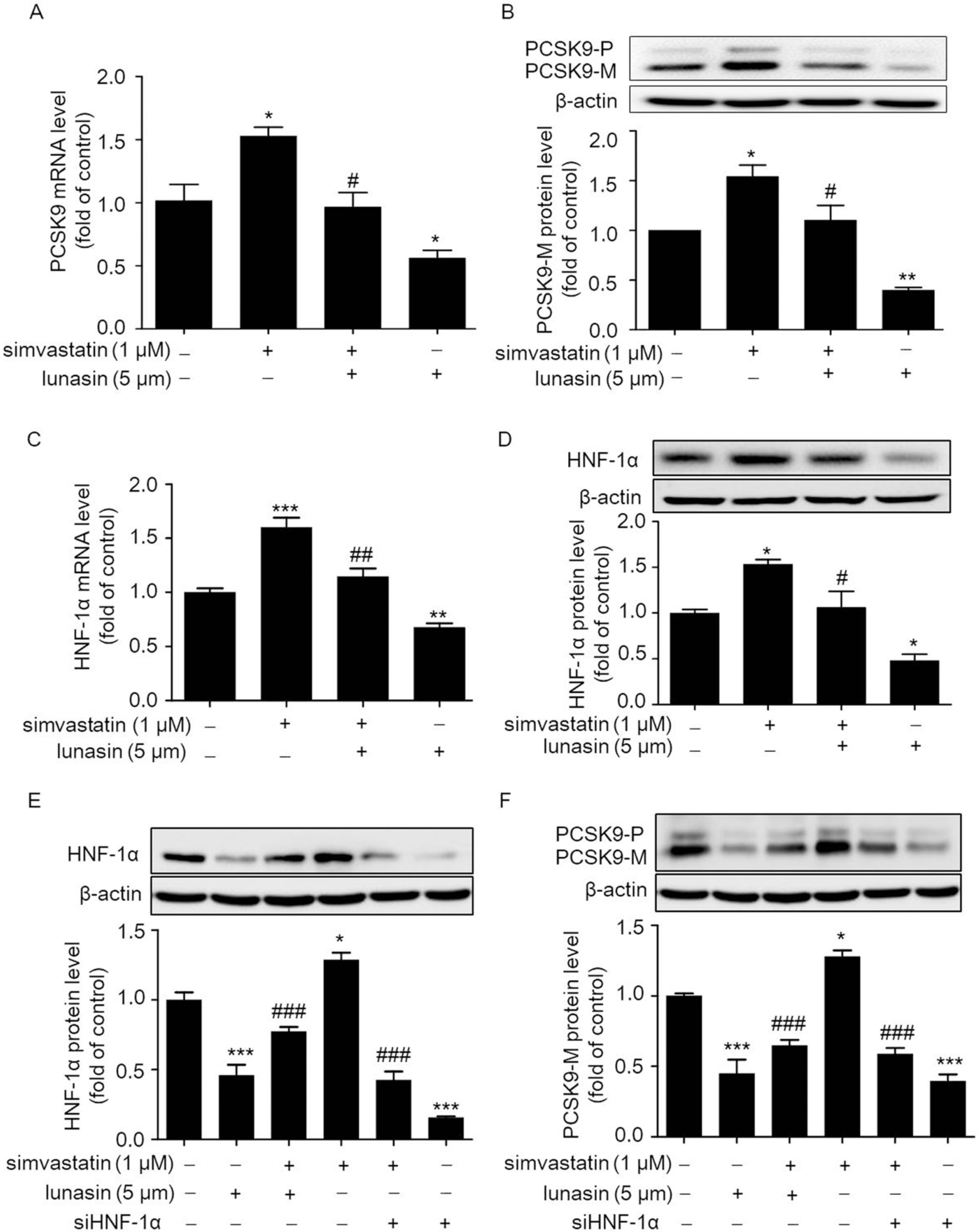
2.1. Lunasin Suppresses Simvastatin Induced Elevation of PCSK9 Levels via Down-Regulating HNF-1α in HepG2 Cells
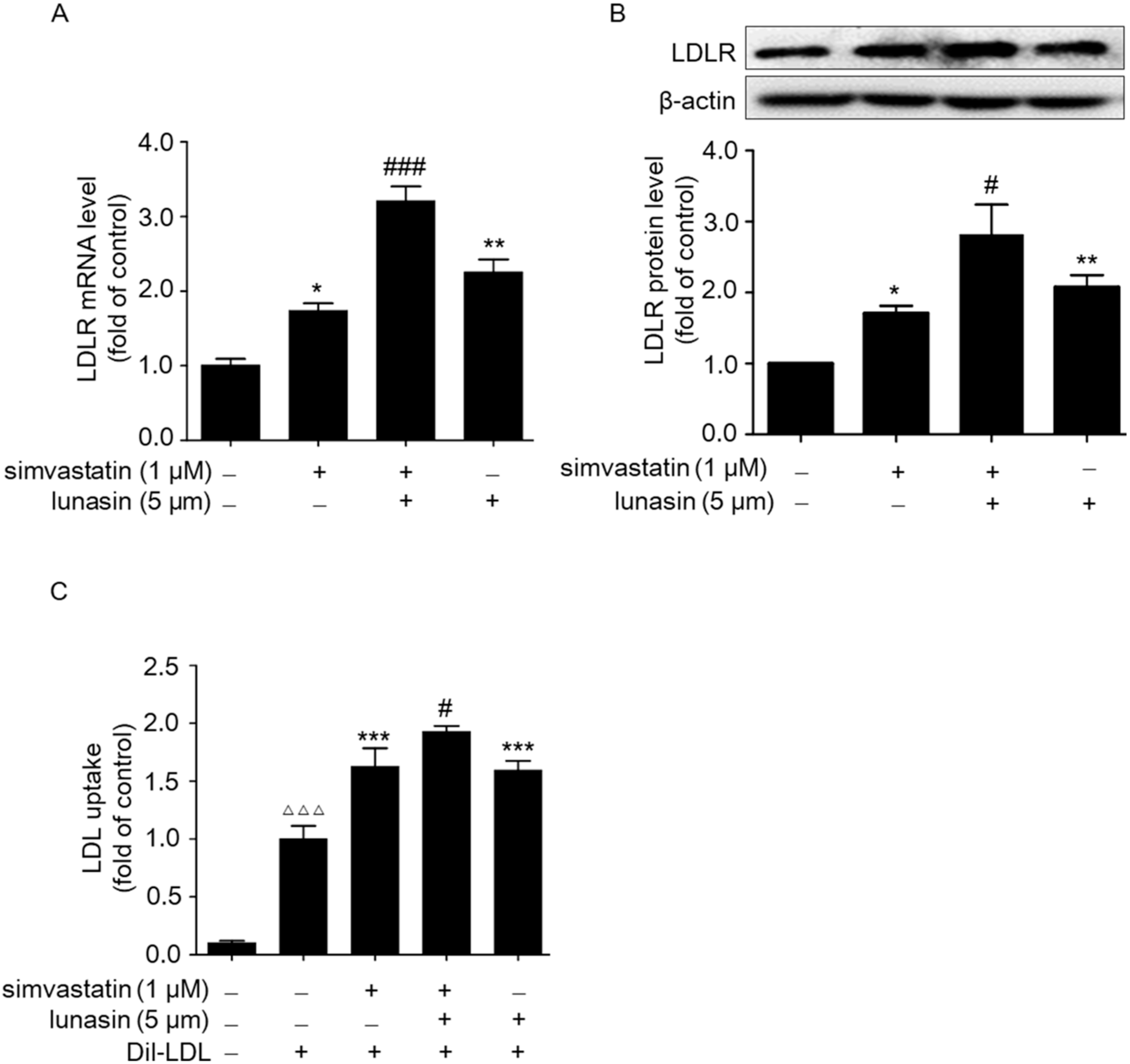
2.2. Simvastatin Combined with Lunasin Synergistically Increases LDLR Level and Functionally Enhances LDL Uptake in HepG2 Cells
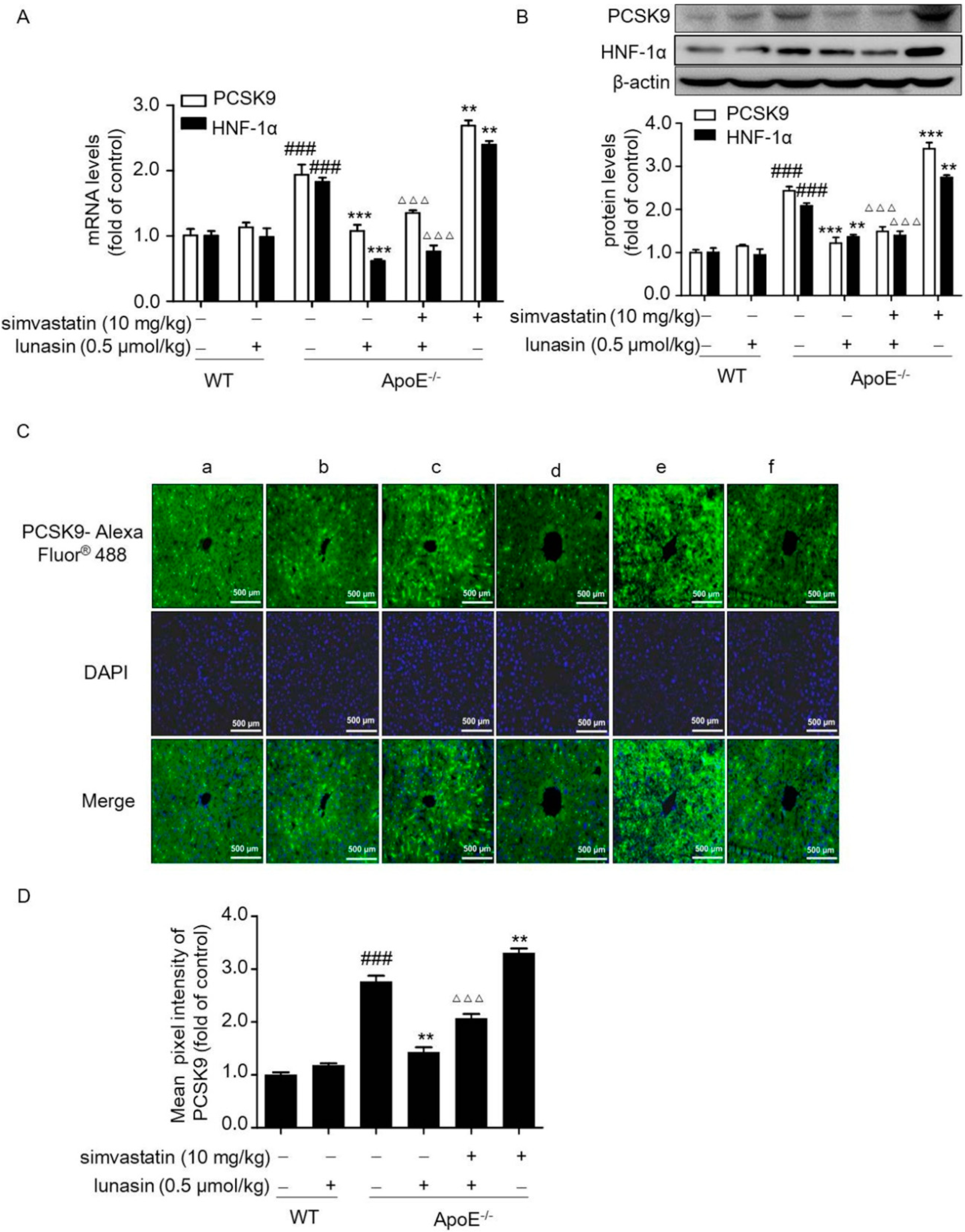
2.3. Lunasin Reduces LDLR Degradation by Counteracting Simvastatin-Induced Up-Regulation of PCSK9 in ApoE−/− Mice
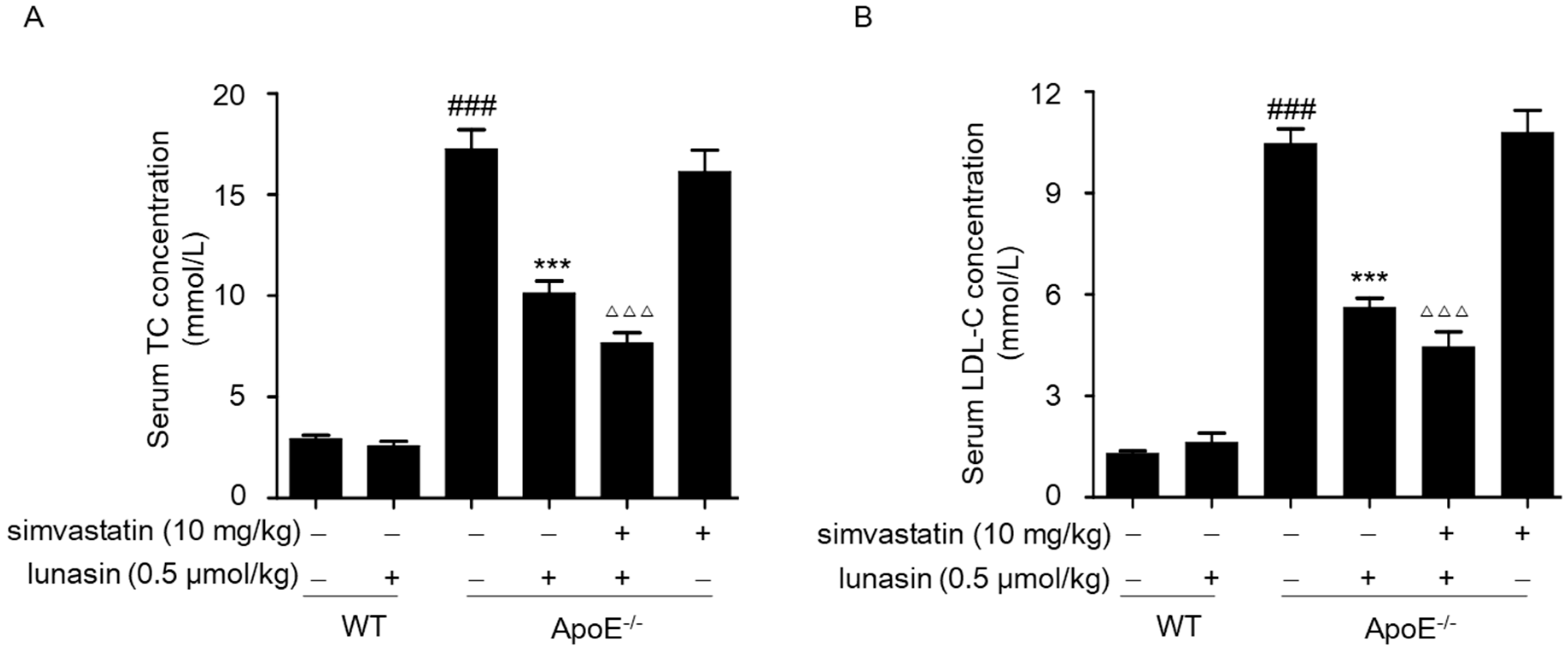
2.4. Simvastatin Combined with Lunasin Improves Its Serum Cholesterol Lowering Efficacy in ApoE−/− Mice
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. In Vivo Study
4.3. Cell Culture and Treatments
4.4. LDL Uptake Assay
4.5. qRT-PCR
4.6. Western Blot
4.7. Small Interference RNA Transfection
4.8. Serum Cholesterol Level Test
4.9. Immunohistochemistry Staining
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PCSK9 | proprotein convertase subtilisin/kexin type 9 |
| PCSK9-M | mature PCSK9 |
| PCSK9-P | precursor PCSK9 |
| PCSK9-S | secreted PCSK9 |
| LDL | low density lipoprotein |
| LDL-C | low density lipoprotein cholesterol |
| LDLR | low density lipoprotein receptor |
| VLDL | very low density lipoprotein |
| CVD | cardiovascular disease |
| CM | chylomicron |
| HMG-CoA reductase | 3-hydroxy-3-methylglutaryl coenzyme A reductase |
| PBS | phosphate-buffered saline |
| MEM | minimum essential medium |
| FBS | fetal bovine serum |
| TC | total-cholesterol |
| HNF-1α | hepatocyte nuclear factor-1α |
| PI3K | phosphatidylinositol 3 kinase |
| NS | normal saline |
| DMSO | dimethyl sulfoxide |
| HFD | high fat diet |
| i.p. | intraperitoneally |
| SREBP | sterol regulatory element binding protein |
| SREBP-2-P | precursor SREBP-2 |
| SREBP-2-N | nuclear SREBP-2 |
References
- Jacobson, T.A.; Ito, M.K.; Maki, K.C.; Orringer, C.E.; Bays, H.E.; Jones, P.H.; McKenney, J.M.; Grundy, S.M.; Gill, E.A.; Wild, R.A.; et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: Part 1 - executive summary. J. Clin. Lipidol. 2014, 8, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J. 2018 Cholesterol Clinical Practice Guidelines: Synopsis of the 2018 American Heart Association/American College of Cardiology/Multisociety Cholesterol Guideline. Ann. Intern. Med. 2019, 170, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Goldstein, J.L. A Receptor-Mediated Pathway for Cholesterol Homeostasis (Nobel Lecture). Science 1986, 232, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Endo, A.; Tsujita, Y.; Kuroda, M.; Tanzawa, K. Inhibition of Cholesterol Synthesis in vitro and in vivo by ML-236A and ML-236B, Competitive Inhibitors of 3-Hydroxy-3-methylglutaryl-Coenzyme A Reductase. Eur. J. Biochem. 1977, 77, 31–36. [Google Scholar] [CrossRef]
- Rudling, M.; Angelin, B.; Stahle, L.; Reihner, E.; Sahlin, S.; Olivecrona, H.; Bjorkhem, I.; Einarsson, C. Regulation of hepatic low density lipoprotein receptor, 3-hydroxy-3-methylglutaryl coenzyme A reductase, and cholesterol 7alpha-hydroxylase mRNAs in human liver. J. Clin. Endocrinol. Metab. 2002, 87, 4307–4313. [Google Scholar] [CrossRef]
- Dong, B.; Wu, M.; Li, H.; Kraemer, F.B.; Adeli, K.; Seidah, N.G.; Park, S.W.; Liu, J. Strong induction of PCSK9 gene expression through HNF1alpha and SREBP2: Mechanism for the resistance to LDL-cholesterol lowering effect of statins in dyslipidemic hamsters. J. Lipid Res. 2010, 51, 1486–1495. [Google Scholar] [CrossRef]
- Dong, B.; Singh, A.B.; Shende, V.R.; Liu, J. Hepatic HNF1 transcription factors control the induction of PCSK9 mediated by rosuvastatin in normolipidemic hamsters. Int. J. Mol. Med. 2017, 39, 749–756. [Google Scholar] [CrossRef]
- Seidah, N.G.; Awan, Z.; Chretien, M.; Mbikay, M. PCSK9: A key modulator of cardiovascular health. Circ. Res. 2014, 114, 1022–1036. [Google Scholar] [CrossRef]
- Toth, P.P.; Farnier, M.; Tomassini, J.E.; Foody, J.M.; Tershakovec, A.M. Statin combination therapy and cardiovascular risk reduction. Future Cardiol. 2016, 12, 289–315. [Google Scholar] [CrossRef]
- Mandeville, W.H.; Arbeeny, C. Bile acid sequestrants: Their use in combination with other lipid-lowering agents. IDrugs 1999, 2, 237–242. [Google Scholar]
- Hou, R.; Goldberg, A.C. Lowering low density lipoprotein cholesterol: Statins, ezetimibe, bile acid sequestrants, and combinations: Comparative efficacy and safety. Endocrinol Metab. Clin. North Am. 2009, 38, 79–97. [Google Scholar] [CrossRef]
- Toth, P.P.; Catapano, A.; Tomassini, J.E.; Tershakovec, A.M. Update on the efficacy and safety of combination ezetimibe plus statin therapy. Clin. Lipidol. 2010, 5, 655–684. [Google Scholar] [CrossRef]
- Khera, A.V.; Qamar, A.; Reilly, M.P.; Dunbar, R.L.; Rader, D.J. Effects of niacin, statin, and fenofibrate on circulating proprotein convertase subtilisin/kexin type 9 levels in patients with dyslipidemia. Am. J. Cardiol. 2015, 115, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Abd El Aal, H.A.; Ahmed, L.A.; Hassan, W.A.; Fawzy, H.M.; Moawad, H. Combination of carvacrol with simvastatin improves the lipid-lowering efficacy and alleviates simvastatin side effects. J. Biochem. Mol. Toxicol. 2017, 31, e21981. [Google Scholar] [CrossRef]
- Kong, W.J.; Wei, J.; Zuo, Z.Y.; Wang, Y.M.; Song, D.Q.; You, X.F.; Zhao, L.X.; Pan, H.N.; Jiang, J.D. Combination of simvastatin with berberine improves the lipid-lowering efficacy. Metabolism 2008, 57, 1029–1037. [Google Scholar] [CrossRef]
- Dia, V.P.; Wang, W.; Oh, V.L.; De Lumen, B.O.; de Mejia, E.G. Isolation, purification and characterisation of lunasin from defatted soybean flour and in vitro evaluation of its anti-inflammatory activity. Food Chem. 2009, 114, 108–115. [Google Scholar] [CrossRef]
- Hernándezledesma, B.; Hsieh, C.C.; De Lumen, B.O. Chemopreventive properties of Peptide Lunasin: A review. Protein Pept. Lett. 2013, 20, 424–432. [Google Scholar]
- de Mejia, E.G.; Dia, V.P. Lunasin and lunasin-like peptides inhibit inflammation through suppression of NF-kappaB pathway in the macrophage. Peptides 2009, 30, 2388–2398. [Google Scholar] [CrossRef]
- Cam, A.; Sivaguru, M.; De Mejia, E.G. Endocytic Mechanism of Internalization of Dietary Peptide Lunasin into Macrophages in Inflammatory Condition Associated with Cardiovascular Disease. PLoS ONE 2013, 8, e72115. [Google Scholar] [CrossRef]
- Gu, L.; Wang, Y.; Xu, Y.; Tian, Q.; Lei, G.; Zhao, C.; Gao, Z.; Pan, Q.; Zhao, W.; Nong, L.; et al. Lunasin functionally enhances LDL uptake via inhibiting PCSK9 and enhancing LDLR expression in vitro and in vivo. Oncotarget 2017, 8, 80826–80840. [Google Scholar] [CrossRef]
- Dubuc, G.; Chamberland, A.; Wassef, H.; Davignon, J.; Seidah, N.G.; Bernier, L.; Prat, A. Statins Upregulate PCSK9, the Gene Encoding the Proprotein Convertase Neural Apoptosis-Regulated Convertase-1 Implicated in Familial Hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1454. [Google Scholar] [CrossRef] [PubMed]
- Abifadel, M.; Varret, M.; Rabès, J.P.; Allard, D.; Ouguerram, K.; Devillers, M.; Cruaud, C.; Benjannet, S.; Wickham, L.; Erlich, D. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 2003, 34, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Welder, G.; Zineh, I.; Pacanowski, M.A.; Troutt, J.S.; Cao, G.; Konrad, R.J. High-dose atorvastatin causes a rapid sustained increase in human serum PCSK9 and disrupts its correlation with LDL cholesterol. J. Lipid Res. 2010, 51, 2714–2721. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, J.P.; Major, A.S. How Oxidized Low-Density Lipoprotein Activates Inflammatory Responses. Crit. Rev. Immunol. 2018, 38, 333–342. [Google Scholar] [CrossRef]
- Walenbergh, S.M.; Koek, G.H.; Bieghs, V.; Shiri-Sverdlov, R. Non-alcoholic steatohepatitis: The role of oxidized low density lipoproteins. J. Hepatol. 2013, 58, 801–810. [Google Scholar] [CrossRef]
- Diakowska, D.; Grabowski, K.; Nienartowicz, M.; Zarębski, P.; Fudalej, K.; Markocka-Mączka, K. Circulating Oxidized Low-Density Lipoproteins and Antibodies against Oxidized Low-Density Lipoproteins as Potential Biomarkers of Colorectal Cancer. Gastroenterol. Res. Pract. 2015, 2015. [Google Scholar] [CrossRef]
- Scoles, D.R.; Xu, X.; Wang, H.; Tran, H.; Taylor-Harding, B.; Li, A.; Karlan, B.Y. Liver X receptor agonist inhibits proliferation of ovarian carcinoma cells stimulated by oxidized low density lipoprotein. Gynecol Oncol. 2010, 116, 109–116. [Google Scholar] [CrossRef]
- Pandya, V.; Rao, A.; Chaudhary, K. Lipid abnormalities in kidney disease and management strategies. World J. Nephrol. 2015, 4, 83. [Google Scholar] [CrossRef]
- Baragetti, A.; Norata, G.; Sarcina, C.; Rastelli, F.; Catapano, A. High density lipoprotein cholesterol levels are an independent predictor of the progression of chronic kidney disease. J. Intern. Med. 2013, 274, 252–262. [Google Scholar] [CrossRef]
- Balla, S.; Nusair, M.B.; Alpert, M.A. Risk factors for atherosclerosis in patients with chronic kidney disease: Recognition and management. Curr. Opin. Pharmacol. 2013, 13, 192–199. [Google Scholar] [CrossRef]
- Sucajtys-Szulc, E.; Szolkiewicz, M.; Swierczynski, J.; Rutkowski, B. Up-regulation of liver Pcsk9 gene expression as a possible cause of hypercholesterolemia in experimental chronic renal failure. Mol. Cell. Biochem. 2016, 411, 281–287. [Google Scholar] [CrossRef]
- Hai, L.; Bin, D.; Sahng Wook, P.; Hyun-Sook, L.; Wei, C.; Jingwen, L. Hepatocyte nuclear factor 1alpha plays a critical role in PCSK9 gene transcription and regulation by the natural hypocholesterolemic compound berberine. J. Biol. Chem. 2009, 284, 28885–28895. [Google Scholar]
- Ding, A.; Chiyuan, C.; Seongah, H.; Anjali, G.; Murphy, A.J.; Rebecca, H.; Edward, T.; Domenico, A.; Horton, J.D.; Tall, A.R. Regulation of hepatic LDL receptors by mTORC1 and PCSK9 in mice. J. Clin. Investig. 2012, 122, 1262–1270. [Google Scholar]
- Breslow, J.L. Mouse models of atherosclerosis. Science 1996, 272, 685–688. [Google Scholar] [CrossRef]
- Wang, Y.X.; Martin-McNulty, B.; Huw, L.Y.; da Cunha, V.; Post, J.; Hinchman, J.; Vergona, R.; Sullivan, M.E.; Dole, W.; Kauser, K. Anti-atherosclerotic effect of simvastatin depends on the presence of apolipoprotein E. Atherosclerosis 2002, 162, 23–31. [Google Scholar] [CrossRef]
- Donnelly, L.A.; Palmer, C.N.; Whitley, A.L.; Lang, C.C.; Doney, A.S.; Morris, A.D.; Donnan, P.T. Apolipoprotein E genotypes are associated with lipid-lowering responses to statin treatment in diabetes: A Go-DARTS study. Pharm. Genom. 2008, 18, 279–287. [Google Scholar] [CrossRef]
- Postmus, I.; Trompet, S.; Deshmukh, H.A.; Barnes, M.R.; Li, X.; Warren, H.R.; Chasman, D.I.; Zhou, K.; Arsenault, B.J.; Donnelly, L.A.; et al. Pharmacogenetic meta-analysis of genome-wide association studies of LDL cholesterol response to statins. Nat. Commun. 2014, 5, 5068. [Google Scholar] [CrossRef]
- Reiner, Z. Resistance and intolerance to statins. Nutr. Metab. Cardiovasc Dis. 2014, 24, 1057–1106. [Google Scholar] [CrossRef]
- Tian, Q.; Zhang, P.; Gao, Z.; Li, H.; Bai, Z.; Tan, S. Hirudin as a novel fusion tag for efficient production of lunasin in Escherichia coli. Prep. Biochem. Biotechnol. 2017, 47, 619–626. [Google Scholar] [CrossRef]
- Ly, K.; Saavedra, Y.G.; Canuel, M.; Routhier, S.; Desjardins, R.; Hamelin, J.; Mayne, J.; Lazure, C.; Seidah, N.G.; Day, R. Annexin A2 reduces PCSK9 protein levels via a translational mechanism and interacts with the M1 and M2 domains of PCSK9. J. Biol. Chem. 2014, 289, 17732–17746. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Li, Y.; Wang, H.; Fleming, J.; Li, M.; Kang, Y.; Zhang, R.; Li, D. Hepatocyte nuclear factor 1A (HNF1A) as a possible tumor suppressor in pancreatic cancer. PLoS ONE 2015, 10, e0121082. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


| Gene | Forward Primer (5ʹ→3ʹ) | Reverse Primer (5ʹ→3ʹ) |
|---|---|---|
| Homo-PCSK9 | AGGGGAGGACATCATTGGTG | CAGGTTGGGGGTCAGTACC |
| Homo-HNF-1α | AGGACGAGACGGACGACGAT | AGTGCCCTTGTTGAGGTGTT |
| Homo-LDLR | GAACCCATCAAAGAGTGCG | TCTTCCTGACCTCGTGCC |
| Homo-β-actin | CTCTTCCAGCCTTCCTTCCT | CAGGGCAGTGATCTCCTTCT |
| Mus-PCSK9 | CAGAGGTCATCACAGTCGGG | GGGGCAAAGAGATCCACACA |
| Mus-HNF-1α | GAGCCTGAATCGAGCAGAAC | AGCCTTCTCTGGACACCTGA |
| Mus-LDLR | GTATGAGGTTCCTGTCCATC | CCTCTGTGGTCTTCTGGTAG |
| Mus-β-actin | GTGACGTTGACATCCGTAAAGA | GCCGGACTCATCGTACTCC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, L.; Gong, Y.; Zhao, C.; Wang, Y.; Tian, Q.; Lei, G.; Liang, Y.; Zhao, W.; Tan, S. Lunasin Improves the LDL-C Lowering Efficacy of Simvastatin via Inhibiting PCSK9 Expression in Hepatocytes and ApoE−/− Mice. Molecules 2019, 24, 4140. https://doi.org/10.3390/molecules24224140
Gu L, Gong Y, Zhao C, Wang Y, Tian Q, Lei G, Liang Y, Zhao W, Tan S. Lunasin Improves the LDL-C Lowering Efficacy of Simvastatin via Inhibiting PCSK9 Expression in Hepatocytes and ApoE−/− Mice. Molecules. 2019; 24(22):4140. https://doi.org/10.3390/molecules24224140
Chicago/Turabian StyleGu, Lili, Yaqin Gong, Cheng Zhao, Yue Wang, Qinghua Tian, Gaoxin Lei, Yalin Liang, Wenfeng Zhao, and Shuhua Tan. 2019. "Lunasin Improves the LDL-C Lowering Efficacy of Simvastatin via Inhibiting PCSK9 Expression in Hepatocytes and ApoE−/− Mice" Molecules 24, no. 22: 4140. https://doi.org/10.3390/molecules24224140
APA StyleGu, L., Gong, Y., Zhao, C., Wang, Y., Tian, Q., Lei, G., Liang, Y., Zhao, W., & Tan, S. (2019). Lunasin Improves the LDL-C Lowering Efficacy of Simvastatin via Inhibiting PCSK9 Expression in Hepatocytes and ApoE−/− Mice. Molecules, 24(22), 4140. https://doi.org/10.3390/molecules24224140