Levels of Trace Elements in the Aqueous Humor of Cataract Patients Measured by the Inductively Coupled Plasma Optical Emission Spectrometry
Abstract
:1. Introduction
2. Results
2.1. Element Content
2.2. Statistical Analysis
2.2.1. Division Based on Pairwise Comparisons
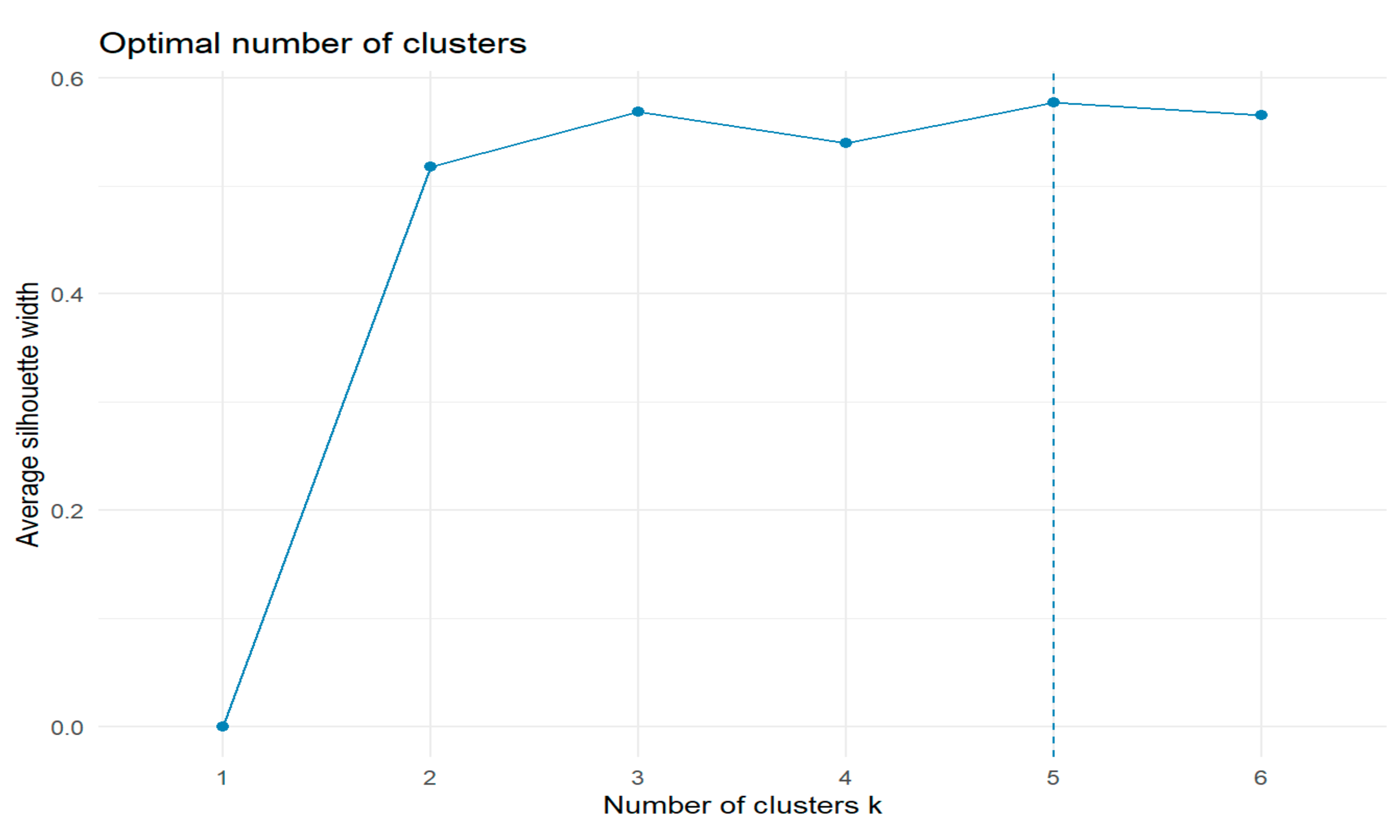
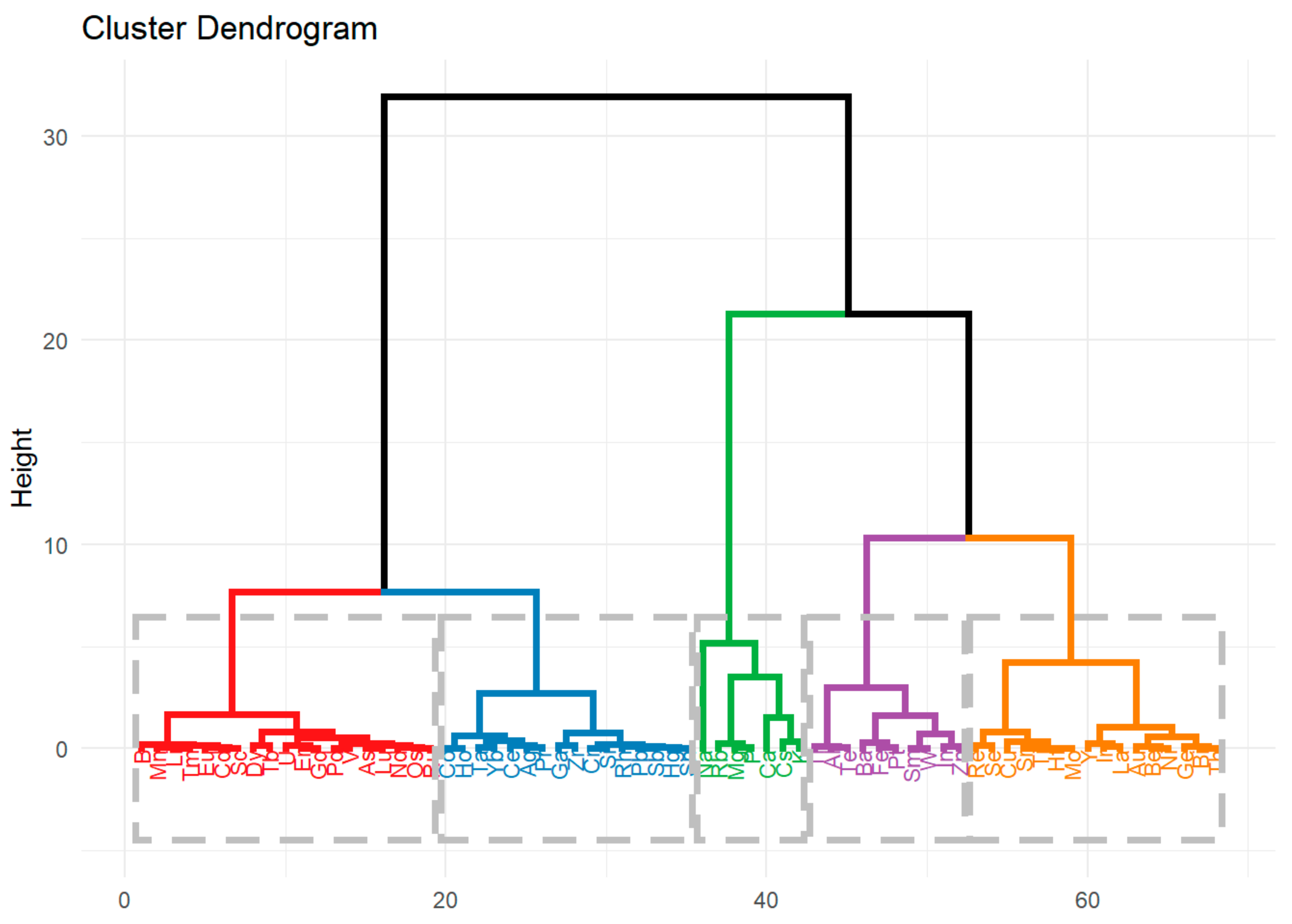
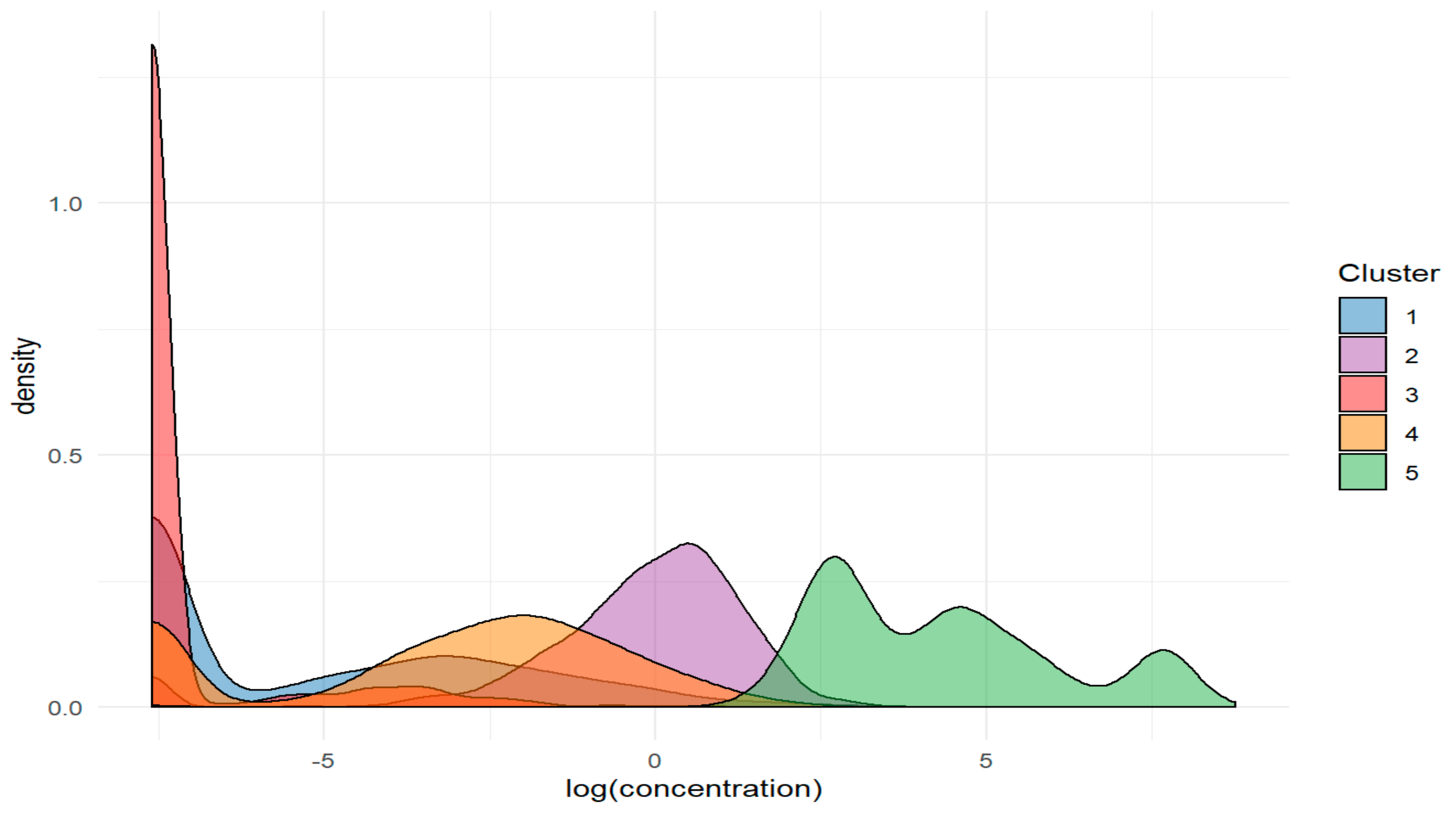
2.2.2. Division Based on Hierarchical Cluster Analysis
- Cluster 1. - Ag, Ce, Co, Cr, Ga, Hg, Ho, Pb, Pr, Rh, Sb, Si, Sn, Ta, Yb, Zr,
- Cluster 2. - Al, Ba, Fe, In, Pt, Sm, Te, Tl, W, Zn,
- Cluster 3. - As, B, Cd, Dy, Er, Eu, Gd, Li, Lu, Mn, Nd, Os, Pd, Ru, Sc, Tb, Tm, U, V,
- Cluster 4. - Au, Be, Bi, Cu, Ge, Hf, Ir, La, Mo, Ni, Re, Se, Sr, Th, Ti, Y,
- Cluster 5. - Ca, Cs, K, Mg, Na, P, Rb.
3. Materials and Methods
3.1. Subjects
3.2. Sample Preparation Procedure
3.3. Analytical Procedures
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pascolini, D.; Mariotti, S.P. Global estimates of visual impairment: 2010. Br. J. Ophthalmol. 2012, 96, 614–618. [Google Scholar]
- McCarty, C.A.; Keeffe, J.E.; Taylor, H.R. The need for cataract surgery: Projections based on lens opacity, visual acuity, and personal concern. Br. J. Ophthalmol. 1999, 83, 62–65. [Google Scholar]
- Chang, J.R.; Koo, E.; Agrón, E.; Hallak, J.; Clemons, T.; Azar, D.; Sperduto, R.D.; Ferris, F.L., 3rd; Chew, E.Y. Risk factors associated with incident cataract and cataract surgery in the Age-related Eye Disease Study (AREDS): AREDS report number 32. Ophthalmology 2011, 118, 2113–2119. [Google Scholar]
- Lydahl, E. Infrared radiation and cataract. Acta Ophthalmol. Suppl. 1984, 166, 1–63. [Google Scholar]
- Brian, G.; Taylor, H. Cataract blindness--challenges for the 21st century. Bull. World Health Organ. 2001, 79, 249–256. [Google Scholar]
- Ye, J.; He, J.; Wang, C.; Wu, H.; Shi, X.; Zhang, H.; Xie, J.; Lee, S.Y. Smoking and risk of age-related cataract: A meta-analysis. Invest. Ophthalmol. Vis. Sci. 2012, 53, 3885–3895. [Google Scholar]
- Langford-Smith, A.; Tilakaratna, V.; Lythgoe, P.R.; Clark, S.J.; Bishop, P.N.; Day, A.J. Age and Smoking Related Changes in Metal Ion Levels in Human Lens: Implications for Cataract Formation. PLoS ONE 2016, 11, e0147576. [Google Scholar] [CrossRef]
- Sigel, H. Metal Ions in Biological Systems: Inorganic Drugs in Deficiency and Disease; CRC Press: Boca Raton, FL, USA, 1982. [Google Scholar]
- Holm, R.H.; Kennepohl, P.; Solomon, E.I. Structural and Functional Aspects of Metal Sites in Biology. Chem. Rev. 1996, 96, 2239–2314. [Google Scholar]
- Lippard, S.J.; Berg, J.M. Principles of Bioinorganic Chemistry; University Science Books: Mill Valley, CA, USA, 1994. [Google Scholar]
- Cowan, J.A. Inorganic Biochemistry/An Introduction; Wiley-VCH Inc.: Hoboken, NJ, USA, 1994. [Google Scholar]
- Burtis, C.A.; Ashwood, R.E.; Bruns, D.E. Tietz Textbook of Clinical Chemistry, 2nd ed.; Saunders: Philadelphia, PA, USA, 1994. [Google Scholar]
- Gupta, S.P. Roles of metals in human health. MOJ Bioorg. Org. Chem. 2018, 2, 221–224. [Google Scholar]
- Sourkes, T.L. Influence of specific nutrients on catecholamine synthesis and metabolism. Pharmacol Rev. 1972, 24, 349–359. [Google Scholar]
- Paoletti, P.; Vergnano, A.M.; Barbour, B.; Casado, M. Zinc at glutamatergic synapses. Neuroscience 2009, 158, 126–136. [Google Scholar]
- Corona, C.; Pensalfini, A.; Frazzini, V.; Sensi, S.L. New therapeutic targets in Alzheimer’s disease: Brain deregulation of calcium and zinc. Cell Death Dis. 2011, 2, e176. [Google Scholar]
- Südhof, T.C. Calcium control of neurotransmitter release. Cold Spring Harb Perspect Biol. 2012, 4, a011353. [Google Scholar]
- Sigel, A.; Sigel, H.; Sigel, R.K.O. Neurodegenerative Diseases and Metal Ions: Metal Ions in the Life Sciences; Springer: West Sussex, UK, 2006; Volume 1. [Google Scholar]
- DeToma, A.S.; Salamekh, S.; Ramamoorthy, A.; Lim, M.H. Misfolded proteins in Alzheimer’s disease and type II diabetes. Chem Soc Rev. 2012, 41, 608–621. [Google Scholar]
- Pithadia, A.S.; Lim, M.H. Metal-associated amyloid-β species in Alzheimer’s disease. Curr. Opin. Chem. Biol. 2012, 16, 67–73. [Google Scholar]
- Kepp, K.P. Bioinorganic chemistry of Alzheimer’s disease. Chem. Rev. 2012, 112, 5193–5239. [Google Scholar]
- Savelieff, M.G.; Lee, S.; Liu, Y.; Lim, M.H. Untangling amyloid-β, tau, and metals in Alzheimer’s disease. ACS Chem. Biol. 2013, 8, 856–865. [Google Scholar]
- Bisaglia, M.; Tessari, I.; Mammi, S.; Bubacco, L. Interaction between alpha-synuclein and metal ions, still looking for a role in the pathogenesis of Parkinson’s disease. Neuromolecular Med. 2009, 11, 239–251. [Google Scholar]
- Dexter, D.T.; Jenner, P.; Schapira, A.H.; Marsden, C.D. Alterations in levels of iron, ferritin, and other trace metals in neurodegenerative diseases affecting the basal ganglia. The Royal Kings and Queens Parkinson’s Disease Research Group. Ann. Neurol. 1992, 32, S94–S100. [Google Scholar]
- Kass, D.K.; Heuer, E.J.; Higginbotham, C.A.; Johnson, J.L.; Keltner, J.P.; Parrish, R.K., 2nd; Wilson, M.R.; Gordon, M.O. The ocular hypertension treatment study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002, 120, 829–830. [Google Scholar] [CrossRef]
- Junemann, A.G.M.; Stopa, P.; Michalke, B.; Chaudhri, A.; Reulbach, U.; Cord Huchzermeyer, C.; Schlotzer-Schrehardt, U.; Kruse, F.E.; Zrenner, E.; Rejdak, R. Levels of Aqueous Humor Trace Elements in Patients with Non-Exsudative Age-related Macular Degeneration: A Case-control Study. PLoS ONE 2013, 8, e56734. [Google Scholar] [CrossRef]
- DeToma, A.S.; Dengler-Crish, C.M.; Deb, A.; Braymer, J.J.; Penner-Hahn, J.E.; van der Schyf, C.J.; Lim, M.H.; Crish, S.D. Abnormal metal levels in the primary visual pathway of the DBA/2J mouse model of glaucoma. Biometals 2014, 27, 1291–1301. [Google Scholar]
- Hohberger, B.; Chaudhri, M.A.; Michalke, B.; Lucio, M.; Nowomiejska, K.; Schlötzer-Schrehardt, U.; Grieb, P.; Rejdak, R.; Jünemann, A.G.M. Levels of aqueous humor trace elements in patients with open-angle Glaucoma. J. Trace Elem. Med. Biol. 2018, 45, 150–155. [Google Scholar]
- Akyol, N.; Deger, O.; Keha, E.E.; Kilic, S. Aqueous-humorand serum zinc and copper concentrations of patients with glaucoma and cataract. Br. J. Ophthalmol. 1990, 4, 661–662. [Google Scholar]
- Erie, J.C.; Butz, J.A.; Good, J.A.; Erie, E.A.; Burritt, M.F.; Cameron, J.D. Heavy Metal Concentrations in Human Eyes. Am. J. Ophthalmol. 2005, 139, 888–893. [Google Scholar]
- Schmeling, M.; Gaynes, B.I.; Tidow-Kebritchi, S. Heavy metal analysis in lens and aqueous humor of cataract patients by total reflection X-ray fluorescence spectrometry. Powder Diffr. 2014, 29, 155–158. [Google Scholar]
- Bauer, G.; Neouze, M.A.; Limbeck, A. Dispersed particle extraction — A new procedure for trace element enrichment from natural aqueous samples with subsequent ICP-OES analysis. Talanta 2013, 103, 145–152. [Google Scholar]
- Janski, R.; Neouze, M.A.; Limbeck, A. Determination of rare earth elements in saline matrices using dispersed particle extraction and inductively coupled plasma mass spectrometry. Rapid Commun. Mass Spectrom. 2014, 28, 1329–1337. [Google Scholar]
- Nischkauer, W.; Neouze, M.A.; Vanhaecke, F.; Limbeck, A. Extraction and pre-concentration of platinum and palladium from microwave-digested road dust via ion exchanging mesoporous silica microparticles prior to their quantification by quadrupole ICP-MS. Microchim. Acta 2015, 182, 2369–2376. [Google Scholar]
- Hosseinzadegan, S.; Nischkauer, W.; Bica, K.; Limbeck, A. Bioparticles coated with an ionic liquid for the pre-concentration of rare earth elements from microwave-digested tea samples and the subsequent quantification by ETV-ICP-OES. Anal. Methods 2016, 8, 7808–7815. [Google Scholar]
- Hosseinzadegan, S.; Nischkauer, W.; Bica, K.; Limbeck, A. FI-ICP-OES determination of Pb in drinking water after pre-concentration using magnetic nanoparticles coated with ionic liquid. Microchem. J. 2019, 146, 339–344. [Google Scholar]
- Yu, X.; Liu, C.; Guo, Y.; Deng, T. Speciation Analysis of Trace Arsenic, Mercury, Selenium and Antimony in Environmental and Biological Samples Based on Hyphenated Techniques. Molecules 2019, 24, 926–940. [Google Scholar]
- Marcinkowska, M.; Barałkiewicz, D. Multielemental speciation analysis by advanced hyphenated technique – HPLC/ICP-MS: A review. Talanta 2016, 161, 177–204. [Google Scholar]
- Sperling, M.; Kars, U. Advances in speciation techniques and methodology. Trends Anal. Chem. 2018, 104, 1–3. [Google Scholar]
- Donati, G.L.; Amais, R.S.; Williams, C.B. Recent advances in inductively coupled plasma optical emission spectrometry. J. Anal. At. Spectrom. 2017, 32, 1283–1296. [Google Scholar]
- McSheehy, S.; Sperling, M. Hyphenated ICP-MS Techniques for Speciation Analysis. Spectroscopy 2009, 24, 1–2. [Google Scholar]
- Sessions, D.; Heard, K.; Kosnett, M. Fatal Cesium Chloride Toxicity After Alternative Cancer Treatment. J. Altern. Complement. Med. 2013, 19, 973–975. [Google Scholar]
- Jaworowski, Z. Jak to z Czarnobylem było. Wiedza i Życie 1996, 5, 24–31. [Google Scholar]
- Gómez-Ayala, A.E.; Lisbona, F.; López-Aliaga, I.; Pallarés, I.; Barrionuevo, M.; Hartiti, S.; Rodríguez-Matas, M.C.; Campos, M.S. The absorption of iron, calcium, phosphorus, magnesium, copper and zinc in the jejunum-ileum of control and iron-deficient rats. Lab Anim. 1998, 32, 72–79. [Google Scholar]
- Chaussidon, M.; Netter, P.; Kessler, M.; Membre, H.; Fener, P.; Delons, S.; Albarède, F. Dialysis-associated arthropathy: Secondary ion mass spectrometry evidence of aluminum silicate in β-microglobulin amyloid synovial tissue and articular cartilage. Nephron 1993, 65, 559–563. [Google Scholar]
- Muma, N.A.; Singer, S.M. Aluminum-induced neuropathology: Transient changes in microtubule-associated proteins. Neurotoxicol. Teratol. 1996, 18, 679–690. [Google Scholar]
- Murayama, H.; Shin, R.W.; Higuchi, J.; Shibuya, S.; Muramoto, T.; Kitamoto, T. Interaction of aluminum with PHFtau in Alzheimer’s disease neurofibrillary degeneration evidenced by desferrioxamine-assisted chelating autoclave method. Am. J. Pathol. 1999, 155, 877–885. [Google Scholar]
- Uversky, V.N.; Fink, A.L. Metal-triggered structural transformations, aggregation, and fibrillation of human α-synuclein: A possible molecular link between parkinson’s disease and heavy metal exposure. J. Biol. Chem. 2001, 276, 44284–44296. [Google Scholar]
- Khan, A.; Ashcroft, A.E.; Korchazhkina, O.V.; Exley, C. Metal-mediated formation of fibrillar ABri amyloid. J. Inorg. Biochem. 2004, 98, 2006–2010. [Google Scholar]
- Rodella, L.F.; Ricci, F.; Borsani, E.; Stacchiotti, A.; Foglio, E.; Favero, G.; Rezzani, R.; Mariani, C.; Bianchi1, R. Aluminium exposure induces Alzheimer’s disease-like histopathological alterations in mouse brain. Histol. Histopathol. 2008, 23, 433–439. [Google Scholar]
- Grochowski, C.; Blicharska, E.; Bogucki, J.; Proch, J.; Mierzwińska, A.; Baj, J.; Litak, J.; Podkowiński, A.; Flieger, J.; Teresiński, G.; et al. Increased Aluminum Content in Certain Brain Structures is Correlated with Higher Silicon Concentration in Alcoholic Use Disorder. Molecules 2019, 24, 1721–1732. [Google Scholar]
- Exley, C.; Chappell, J.S.; Birchall, J.D. A mechanism for acute aluminium toxicity in fish. J. Theor. Biol. 1991, 151, 417–428. [Google Scholar]
- Edwardson, J.A.; Moore, P.B.; Ferrier, I.N.; Lilley, J.S.; Newton, G.W.A.; Barker, J.; Templar, J.; Day, J.P. Effect of silicon on gastrointestinal absorption of aluminium. Lancet 1993, 342, 211–212. [Google Scholar]
- Vanek, A.; Chrastny, V.; Komarek, V.; Penizek, V.; Teper, L.; Cabala, J.; Drabek, O. Geochemical position of thallium in soils from a smelter-impacted area. J. Geochem. Explor. 2013, 124, 176–182. [Google Scholar]
- Lukaszewski, Z.; Jakubowska, M.; Zembrzuski, W.; Pasieczna, A.; Karbowska, B. Flow-Injection Differential-Pulse Anodic Stripping Voltammetry as a Tool for Thallium Monitoring in the Environment. Electroanalysis 2010, 22, 1963–1966. [Google Scholar]
- John Peter, A.L.; Viraraghavan, T. Thallium: A review of public health and environmental concerns. Environ. Int. 2005, 31, 493–501. [Google Scholar]
- Leung, K.M.; Ooi, V.E.C. Studies on thallium toxicity, its tissue distribution and histopathological effects in rats. Chemosphere 2000, 41, 155–159. [Google Scholar]
- Spano, N.; Panzanelli, A.; Piu, P.C.; Pilo, M.I.; Sanna, G.; Seeber, R.; Tapparo, A. Anodic stripping voltammetric determination of traces and ultratraces of thallium at a graphite microelectrode Method development and application to environmental waters. Anal. Chim. Acta 2005, 553, 201–207. [Google Scholar]
- Sabbioni, E.; Minoia, C.; Ronchi, A.; Hansen, B.G.; Pietra, R.; Balducci, C. Trace Element Reference Vlues in Tissues from Inhabitants of The European Union. VIII Thallium in the Italian Population. Sci. Total Environ. 1994, 58, 227–236. [Google Scholar]
- Emsley, J. Thallium. In The Elements of Murder: A History of Poison; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Nordberg, G.F.; Fowler, B.A.; Nordberg, M.; Friberg, L. (Eds.) Handbook on the Toxicology of Metals, 3rd ed.; Elsevier: Oxford, UK, 2007. [Google Scholar]
- Gareth, J.; Witten, D.; Hastie, T.; Tibshirani, R. (Eds.) An Introduction to Statistical Learning: With Applications in R. Book; Springer: New York, NY, USA, 2015. [Google Scholar]
- Murray, L. Biostatistical Design and Analysis Using R: A Practical Guide; Wiley-Blackwell: Chichester, UK, 2010. [Google Scholar]
- Everitt, B.S. Cluster Analysis; Wiley Series in Probability and Statistics; John Wiley & Sons: Chichester, UK, 2011. [Google Scholar]
- Montgomery, D.C.; Runger, G.C. Applied Statistics and Probability for Engineers; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Scheffe, H. The Analysis of Variance; John Wiley & Sons: New York, NY, USA, 1999; Volume 72. [Google Scholar]
- Ward, J.H. Hierarchical Grouping to Optimize an Objective Function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Rousseeuw, P.J. Silhouettes: A Graphical Aid to the Interpretation and Validation of Cluster Analysis. J. Comput. Appl. Math. 1987, 20, 53–65. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses (1.0.4 ed.) R Package. CRAN. Available online: http://www.sthda.com/english/rpkgs/factoextra (accessed on 1 September 2019).
- Hadley, W. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Hadley, W. Tidyverse: Easily Install and Load the ‘Tidyverse’. 2017. Available online: https://CRAN.R-project.org/package=tidyverse (accessed on 1 September 2019).
- Xie, Y. Dynamic Documents with R and Knitr, 2nd ed.; Chapman; Hall/CRC: Boca Raton, FL, USA, 2015. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
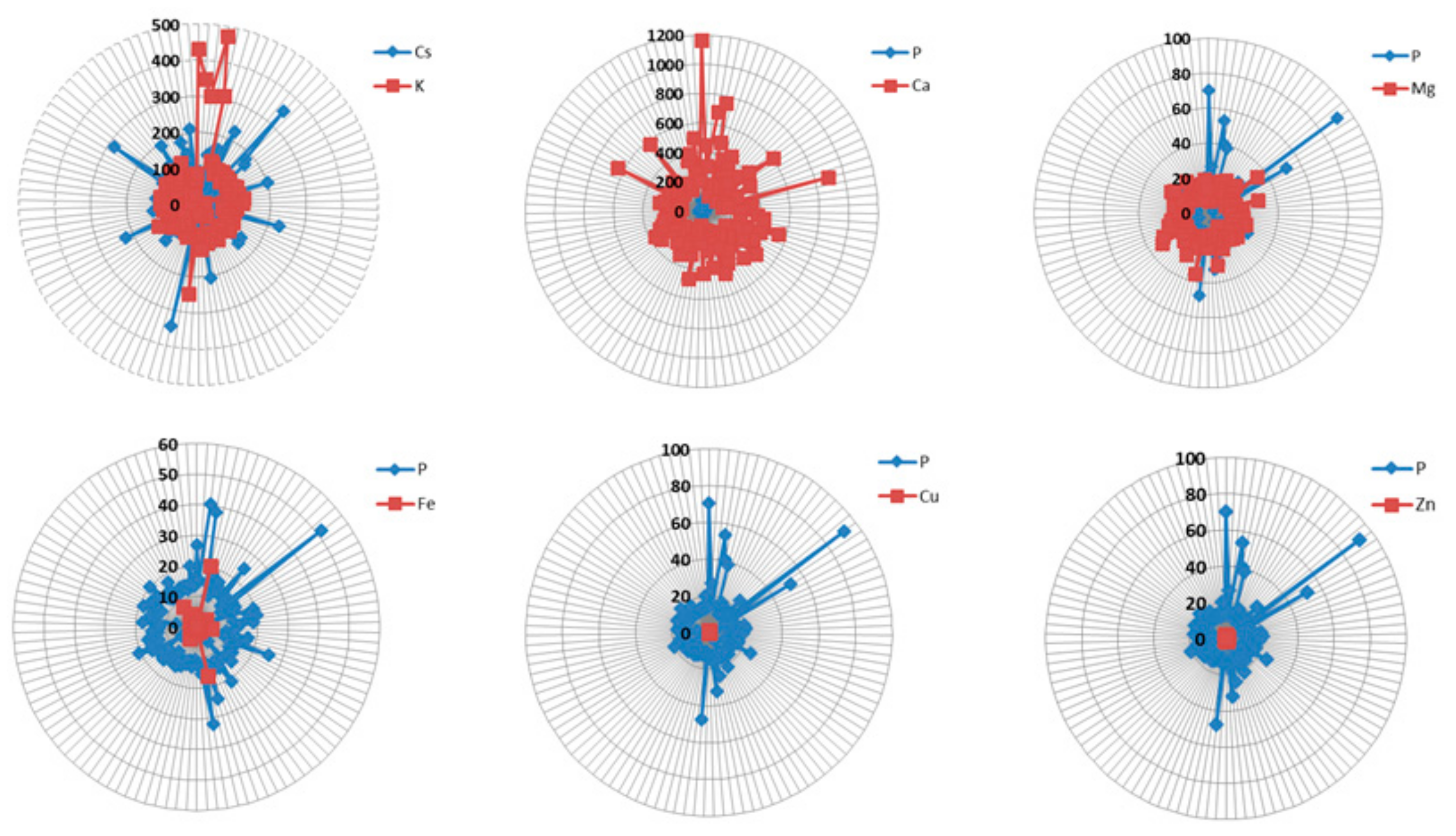
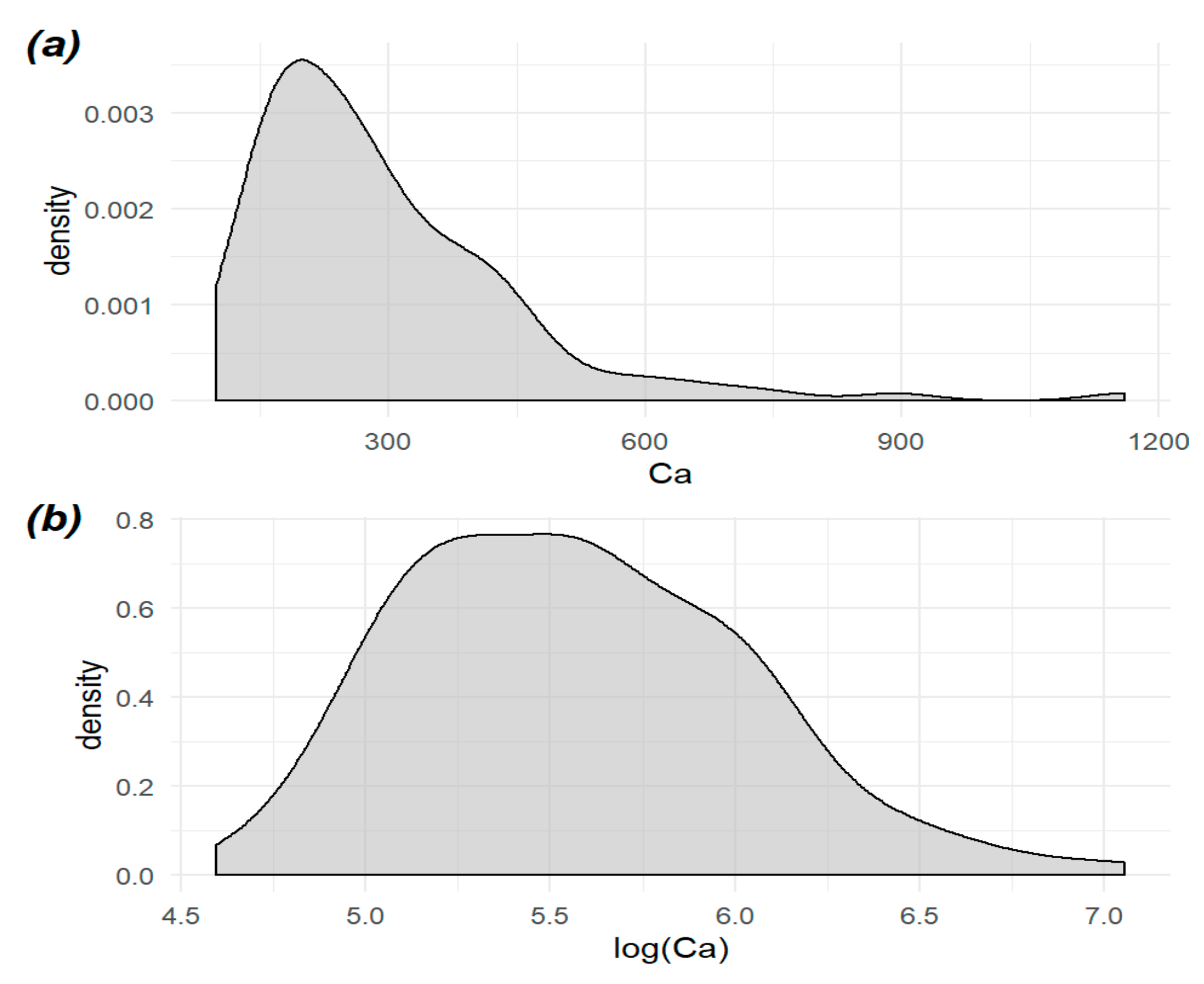
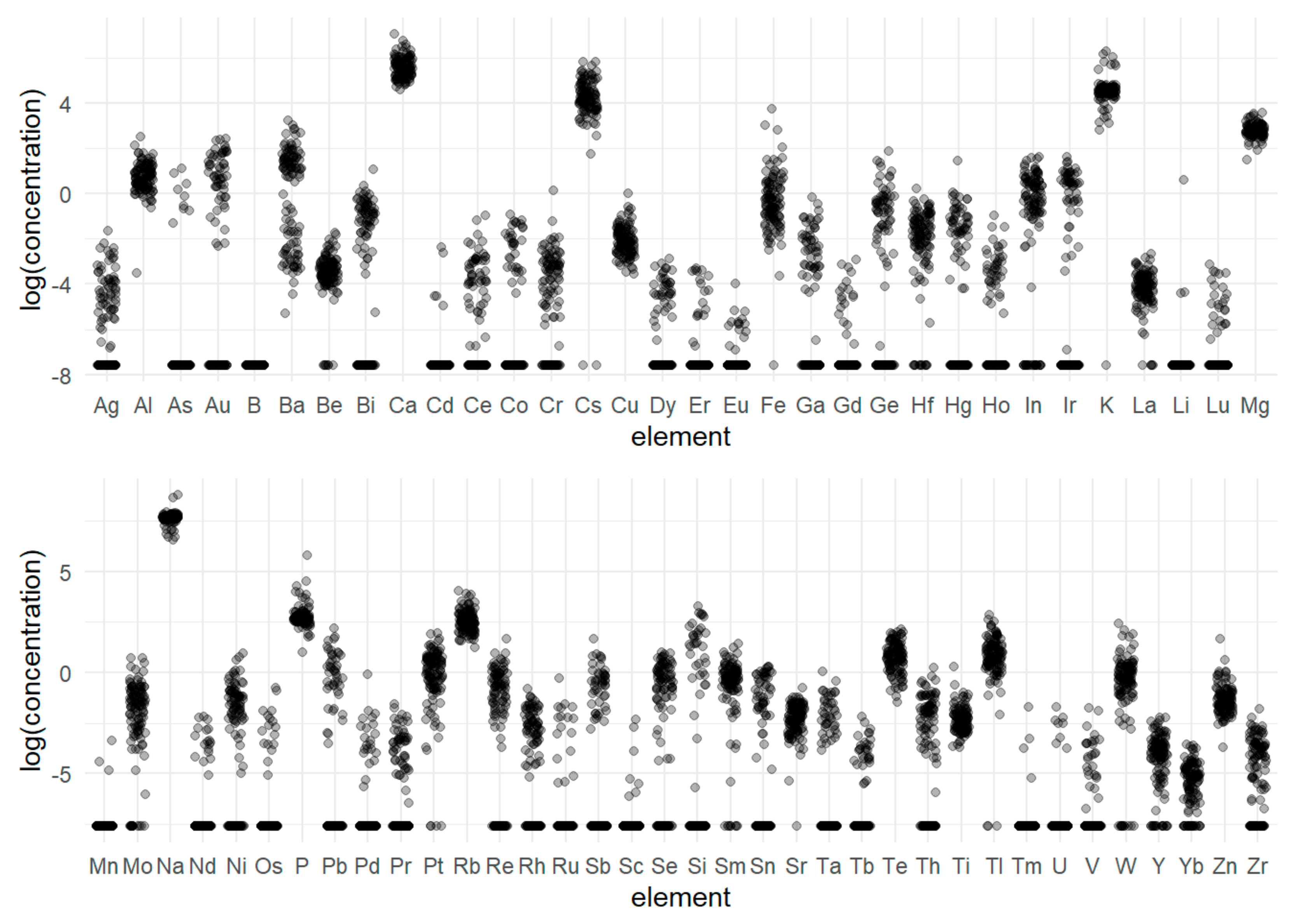
| Element/Wavelength [nm] | BDL (n) | ADL (n) | Mean [ppm] | SD | Median [ppm] | Min. [ppm] | Max [ppm] | Range [ppm] | CV |
|---|---|---|---|---|---|---|---|---|---|
| Ag/328.068 | 53 | 62 | 0.014 | 0.027 | 0.003 | 0.000 | 0.191 | 0.191 | 193.898 |
| Al/396.152 | 0 | 115 | 2.427 | 1.678 | 1.948 | 0.029 | 12.449 | 12.420 | 69.146 |
| As/188.980 | 106 | 9 | 0.097 | 0.419 | 0.000 | 0.000 | 3.071 | 3.071 | 434.053 |
| Au/197.742 | 59 | 56 | 1.658 | 2.640 | 0.000 | 0.000 | 11.284 | 11.284 | 159.214 |
| B/249.772 | 115 | 0 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Ba/455.403 | 0 | 115 | 3.547 | 4.551 | 2.869 | 0.005 | 25.461 | 25.456 | 128.288 |
| Be/313.042 | 7 | 108 | 0.038 | 0.029 | 0.033 | 0.000 | 0.176 | 0.175 | 76.811 |
| Bi/223.061 | 49 | 66 | 0.276 | 0.404 | 0.090 | 0.000 | 3.003 | 3.002 | 146.727 |
| Ca/422.673 | 0 | 115 | 294.508 | 162.330 | 259.183 | 98.981 | 1161.175 | 1062.194 | 55.119 |
| Cd/214.439 | 110 | 5 | 0.002 | 0.011 | 0.000 | 0.000 | 0.094 | 0.094 | 507.174 |
| Ce/446.021 | 60 | 55 | 0.023 | 0.052 | 0.000 | 0.000 | 0.382 | 0.381 | 224.203 |
| Co/238.892 | 82 | 33 | 0.039 | 0.082 | 0.000 | 0.000 | 0.397 | 0.397 | 210.387 |
| Cr/267.716 | 38 | 77 | 0.041 | 0.114 | 0.015 | 0.000 | 1.171 | 1.170 | 280.103 |
| Cs/697.327 | 2 | 113 | 89.742 | 65.695 | 71.684 | 0.000 | 350.000 | 350.000 | 73.205 |
| Cu/327.395 | 0 | 115 | 0.154 | 0.128 | 0.117 | 0.029 | 1.021 | 0.991 | 83.088 |
| Dy/364.540 | 75 | 40 | 0.006 | 0.011 | 0.000 | 0.000 | 0.057 | 0.057 | 171.710 |
| Er/349.910 | 97 | 18 | 0.003 | 0.008 | 0.000 | 0.000 | 0.037 | 0.037 | 256.549 |
| Eu/420.504 | 100 | 14 | 0.001 | 0.002 | 0.000 | 0.000 | 0.019 | 0.018 | 199.784 |
| Fe/238.204 | 1 | 114 | 1.692 | 4.649 | 0.593 | 0.000 | 42.727 | 42.726 | 274.763 |
| Ga/294.363 | 63 | 52 | 0.069 | 0.133 | 0.000 | 0.000 | 0.847 | 0.846 | 193.997 |
| Gd/342.246 | 96 | 19 | 0.003 | 0.008 | 0.000 | 0.000 | 0.055 | 0.054 | 274.318 |
| Ge/209.426 | 54 | 61 | 0.477 | 0.957 | 0.048 | 0.000 | 6.553 | 6.553 | 200.517 |
| Hf/264.141 | 12 | 103 | 0.223 | 0.204 | 0.166 | 0.000 | 1.274 | 1.274 | 91.585 |
| Hg/194.164 | 61 | 54 | 0.190 | 0.456 | 0.000 | 0.000 | 4.303 | 4.302 | 239.589 |
| Ho/348.484 | 74 | 41 | 0.021 | 0.051 | 0.000 | 0.000 | 0.382 | 0.381 | 238.256 |
| In/230.606 | 22 | 93 | 1.119 | 1.138 | 0.741 | 0.000 | 5.129 | 5.128 | 101.660 |
| Ir/205.116 | 63 | 51 | 0.727 | 1.100 | 0.000 | 0.000 | 5.098 | 5.098 | 151.379 |
| K/769.897 | 1 | 114 | 108.810 | 79.111 | 94.219 | 0.000 | 539.796 | 539.795 | 72.705 |
| La/398.852 | 5 | 110 | 0.018 | 0.012 | 0.016 | 0.000 | 0.070 | 0.069 | 66.576 |
| Li/670.783 | 112 | 3 | 0.017 | 0.171 | 0.000 | 0.000 | 1.837 | 1.836 | 1026.729 |
| Lu/307.760 | 90 | 25 | 0.003 | 0.007 | 0.000 | 0.000 | 0.044 | 0.044 | 234.920 |
| Mg/285.213 | 0 | 115 | 16.959 | 5.115 | 16.041 | 4.525 | 35.594 | 31.069 | 30.159 |
| Mn/257.610 | 112 | 3 | 0.001 | 0.003 | 0.000 | 0.000 | 0.034 | 0.034 | 351.520 |
| Mo/202.032 | 13 | 102 | 0.283 | 0.361 | 0.188 | 0.000 | 2.042 | 2.042 | 127.379 |
| Na/588.995 | 0 | 115 | 2176.450 | 643.624 | 2199.520 | 697.710 | 6430.328 | 5732.618 | 29.572 |
| Nd/406.108 | 97 | 18 | 0.007 | 0.020 | 0.000 | 0.000 | 0.113 | 0.113 | 278.466 |
| Ni/231.604 | 40 | 75 | 0.250 | 0.406 | 0.125 | 0.000 | 2.533 | 2.533 | 162.504 |
| Os/225.585 | 98 | 17 | 0.017 | 0.063 | 0.000 | 0.000 | 0.472 | 0.472 | 379.535 |
| P/213.618 | 0 | 115 | 19.734 | 31.336 | 14.025 | 2.742 | 329.010 | 326.269 | 158.792 |
| Pb/220.353 | 71 | 44 | 0.615 | 1.358 | 0.000 | 0.000 | 8.833 | 8.832 | 220.678 |
| Pd/340.458 | 93 | 22 | 0.017 | 0.086 | 0.000 | 0.000 | 0.893 | 0.892 | 507.677 |
| Pr/417.939 | 65 | 50 | 0.018 | 0.035 | 0.000 | 0.000 | 0.207 | 0.207 | 189.612 |
| Pt/203.646 | 4 | 111 | 1.418 | 1.293 | 1.083 | 0.000 | 7.185 | 7.185 | 91.241 |
| Rb/780.026 | 0 | 115 | 14.551 | 9.167 | 12.384 | 3.527 | 56.826 | 53.299 | 63.001 |
| Re/197.248 | 33 | 82 | 0.517 | 0.710 | 0.283 | 0.000 | 5.172 | 5.171 | 137.156 |
| Rh/343.488 | 46 | 69 | 0.062 | 0.083 | 0.029 | 0.000 | 0.438 | 0.438 | 134.481 |
| Ru/240.272 | 98 | 17 | 0.019 | 0.081 | 0.000 | 0.000 | 0.768 | 0.768 | 420.936 |
| Sb/206.834 | 68 | 47 | 0.341 | 0.719 | 0.000 | 0.000 | 5.407 | 5.407 | 211.004 |
| Sc/361.383 | 108 | 7 | 0.002 | 0.011 | 0.000 | 0.000 | 0.100 | 0.099 | 506.182 |
| Se/196.026 | 38 | 77 | 0.609 | 0.697 | 0.418 | 0.000 | 2.687 | 2.687 | 114.311 |
| Si/288.158 | 78 | 37 | 1.986 | 4.690 | 0.000 | 0.000 | 26.102 | 26.102 | 236.114 |
| Sm/442.434 | 8 | 107 | 0.840 | 0.685 | 0.746 | 0.000 | 4.253 | 4.252 | 81.588 |
| Sn/283.998 | 64 | 51 | 0.227 | 0.368 | 0.000 | 0.000 | 1.340 | 1.340 | 161.961 |
| Sr/460.733 | 1 | 114 | 0.122 | 0.076 | 0.112 | 0.000 | 0.478 | 0.478 | 62.245 |
| Ta/268.517 | 72 | 43 | 0.072 | 0.150 | 0.000 | 0.000 | 1.044 | 1.043 | 208.023 |
| Tb/350.914 | 85 | 30 | 0.008 | 0.017 | 0.000 | 0.000 | 0.114 | 0.114 | 225.447 |
| Te/214.282 | 0 | 115 | 2.672 | 1.826 | 2.159 | 0.236 | 8.404 | 8.168 | 68.316 |
| Th/283.730 | 38 | 77 | 0.137 | 0.198 | 0.061 | 0.000 | 1.294 | 1.294 | 144.333 |
| Ti/336.122 | 0 | 115 | 0.122 | 0.140 | 0.090 | 0.025 | 1.350 | 1.325 | 114.598 |
| Tl/190.794 | 3 | 112 | 3.101 | 2.810 | 2.081 | 0.000 | 16.943 | 16.943 | 90.629 |
| Tm/336.261 | 111 | 4 | 0.003 | 0.017 | 0.000 | 0.000 | 0.180 | 0.180 | 652.877 |
| U/385.957 | 107 | 8 | 0.006 | 0.024 | 0.000 | 0.000 | 0.184 | 0.183 | 399.184 |
| V/292.401 | 88 | 27 | 0.007 | 0.023 | 0.000 | 0.000 | 0.172 | 0.171 | 316.212 |
| W/207.912 | 11 | 104 | 1.148 | 1.602 | 0.768 | 0.000 | 11.296 | 11.296 | 139.611 |
| Y/361.104 | 15 | 100 | 0.024 | 0.023 | 0.018 | 0.000 | 0.109 | 0.108 | 97.013 |
| Yb/328.937 | 20 | 93 | 0.006 | 0.006 | 0.005 | 0.000 | 0.029 | 0.028 | 96.014 |
| Zn/213.857 | 0 | 115 | 0.357 | 0.520 | 0.234 | 0.025 | 5.163 | 5.138 | 145.568 |
| Zr/343.823 | 35 | 80 | 0.020 | 0.025 | 0.012 | 0.000 | 0.162 | 0.162 | 128.335 |
| Group | Min. | Median | Mean | Max. | SD |
|---|---|---|---|---|---|
| 1 2 3 4 5 | 0 0 0 0 0 | 0.000 1.071 0.000 0.072 62.918 | 0.234 1.832 0.012 0.352 388.679 | 26.102 42.727 3.071 11.284 6430.328 | 1.329 2.685 0.111 0.904 778.327 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolar-Szczasny, J.; Święch, A.; Flieger, J.; Tatarczak-Michalewska, M.; Niedzielski, P.; Proch, J.; Majerek, D.; Kawka, J.; Mackiewicz, J. Levels of Trace Elements in the Aqueous Humor of Cataract Patients Measured by the Inductively Coupled Plasma Optical Emission Spectrometry. Molecules 2019, 24, 4127. https://doi.org/10.3390/molecules24224127
Dolar-Szczasny J, Święch A, Flieger J, Tatarczak-Michalewska M, Niedzielski P, Proch J, Majerek D, Kawka J, Mackiewicz J. Levels of Trace Elements in the Aqueous Humor of Cataract Patients Measured by the Inductively Coupled Plasma Optical Emission Spectrometry. Molecules. 2019; 24(22):4127. https://doi.org/10.3390/molecules24224127
Chicago/Turabian StyleDolar-Szczasny, Joanna, Anna Święch, Jolanta Flieger, Małgorzata Tatarczak-Michalewska, Przemysław Niedzielski, Jędrzej Proch, Dariusz Majerek, Justyna Kawka, and Jerzy Mackiewicz. 2019. "Levels of Trace Elements in the Aqueous Humor of Cataract Patients Measured by the Inductively Coupled Plasma Optical Emission Spectrometry" Molecules 24, no. 22: 4127. https://doi.org/10.3390/molecules24224127
APA StyleDolar-Szczasny, J., Święch, A., Flieger, J., Tatarczak-Michalewska, M., Niedzielski, P., Proch, J., Majerek, D., Kawka, J., & Mackiewicz, J. (2019). Levels of Trace Elements in the Aqueous Humor of Cataract Patients Measured by the Inductively Coupled Plasma Optical Emission Spectrometry. Molecules, 24(22), 4127. https://doi.org/10.3390/molecules24224127







