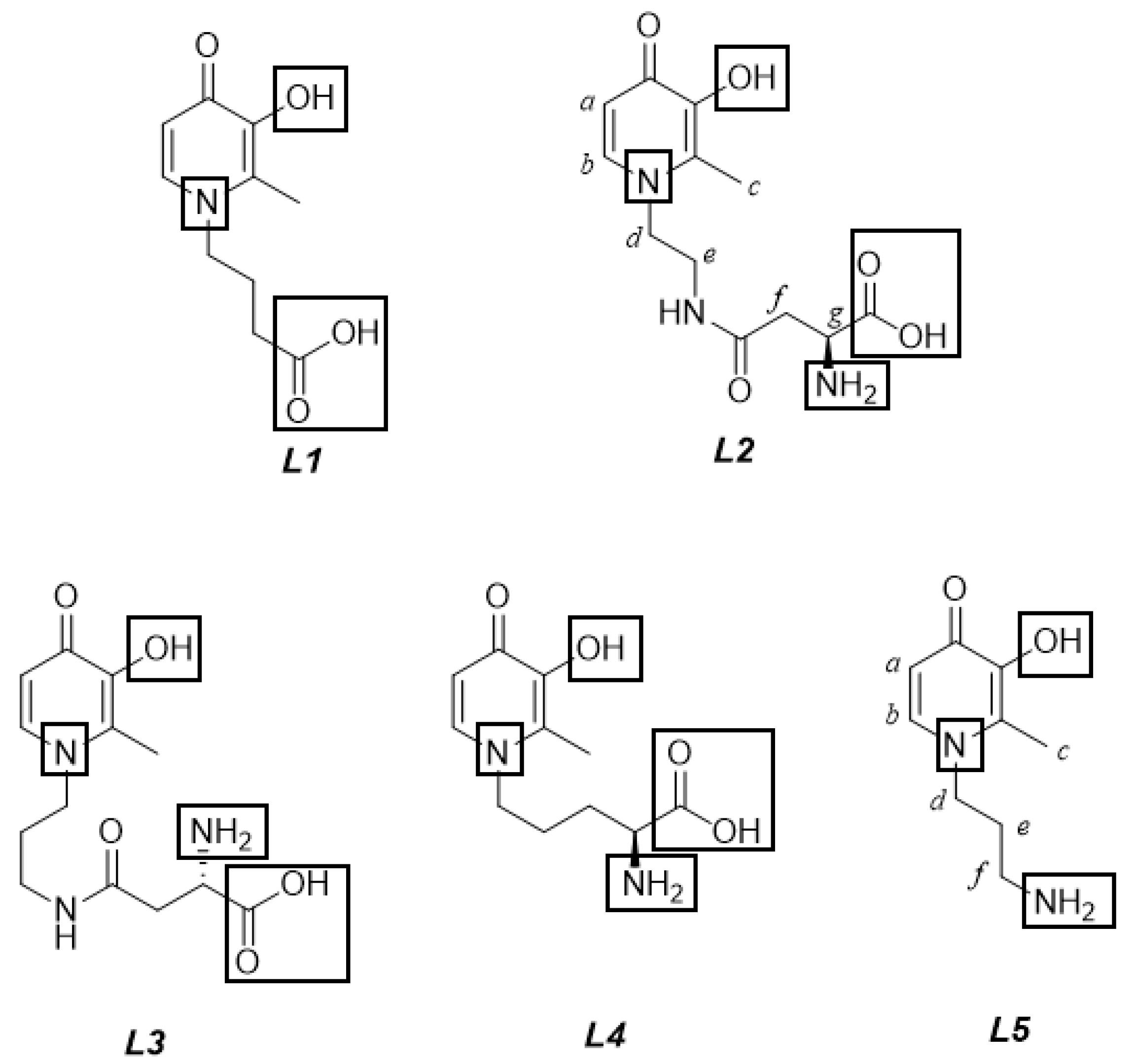
Speciation Studies of Bifunctional 3-Hydroxy-4-Pyridinone Ligands in the Presence of Zn2+ at Different Ionic Strengths and Temperatures
Abstract
1. Introduction
2. Results and Discussion
2.1. Equilibria and Thermodynamic Models
2.2. Protonation Constants of the Ligands
- the hydroxyl group of the aromatoid ring;
- the –NH2 and –COOH groups, potentially present in the alkyl moiety;
- the pyridinone nitrogen in the N-heterocyclic ring, with the proton supplied by excess of inorganic acid [8].
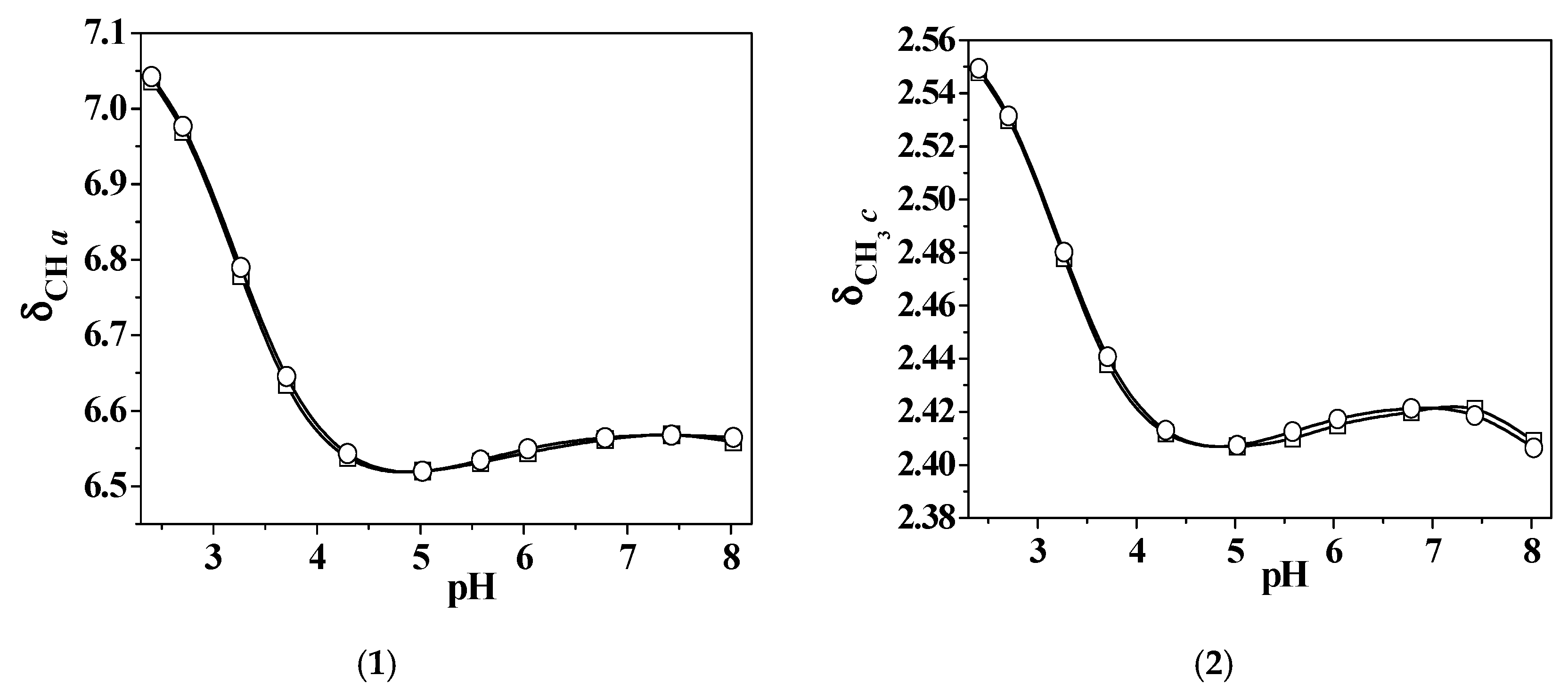
2.2.1. L2 Behavior in Aqueous Solution
- εmax(H4(L2)2+) = 5221 and 5742 at λmax = 278 nm with increasing ionic increasing;
- εmax(H3(L2)+) = 8756 and 9400 at λmax = 291 nm, I = 0.506 mol L−1 and 1.012 mol L−1, respectively;
- εmax(H2(L2)0(aq)) = 7468 and 9681 at λmax = 284 nm and 281 nm, respectively;
- εmax(H(L2)−) = 5016 at λmax = 296 nm and both the variable conditions;
- εmax((L2)2−) = 7913 and 8276 at λmax = 311 nm, with increasing ionic strength.
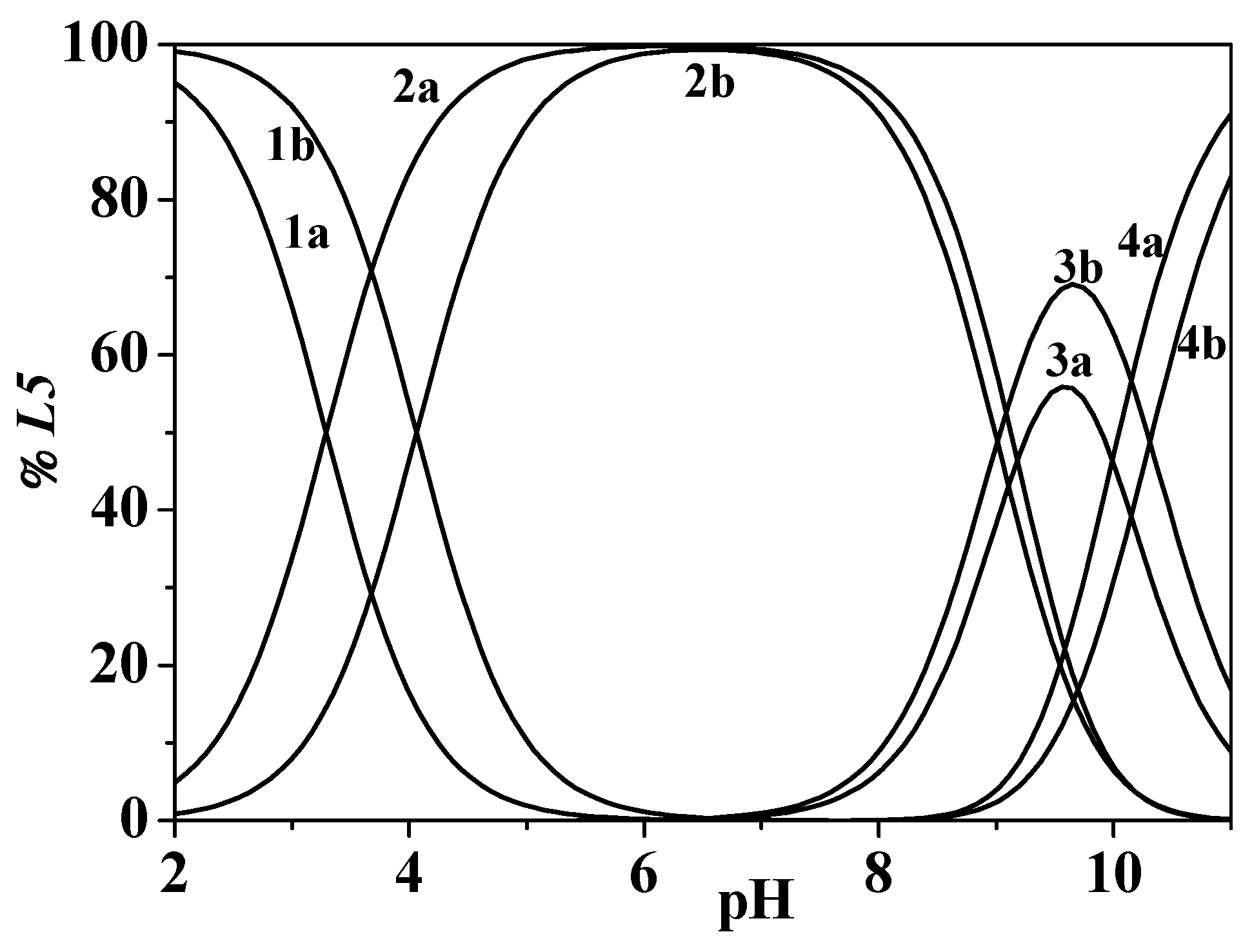
2.2.2. L5 Ligand Protonation
2.3. Hydrolysis of the Metal Cation
2.4. Binding Ability Towards Zn2+
- simplicity and probability of the model;
- formation percentages of the species across the pH range under investigation;
- statistical parameters (standard deviation on logβpqr values and on the fitting values of the systems);
- values of corresponding ratios with single variances in comparison with those from the accepted model.
2.4.1. Zn2+/L1, L3 and L4 Systems
2.4.2. Zn2+/L2 Investigation
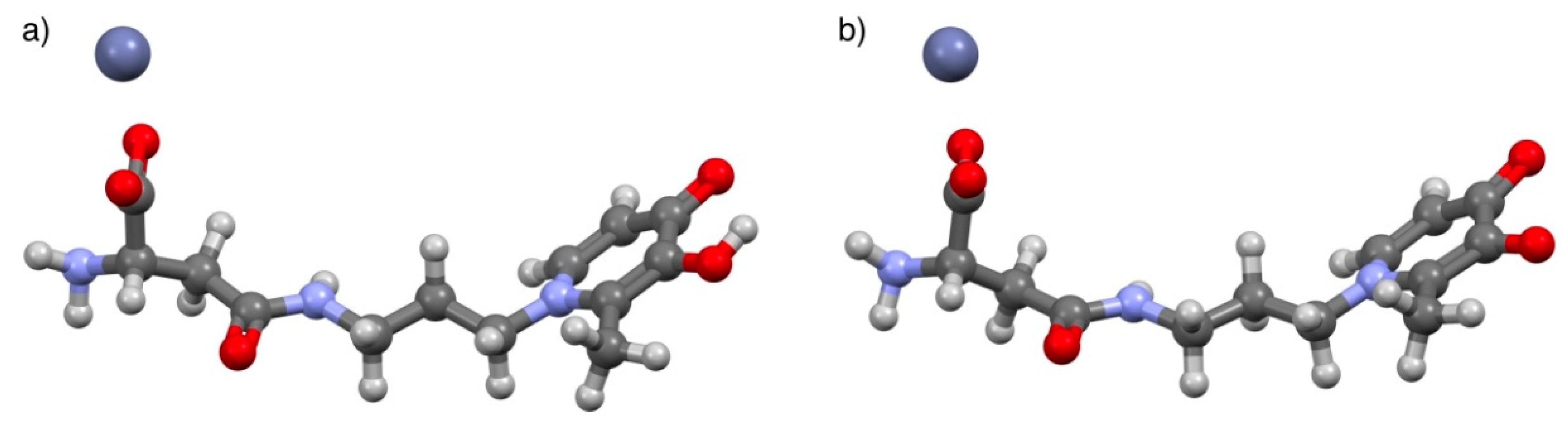
2.4.3. Zn2+/L5 System
Confirmation of Zn2+/L5 Species Formation by ESI-MS
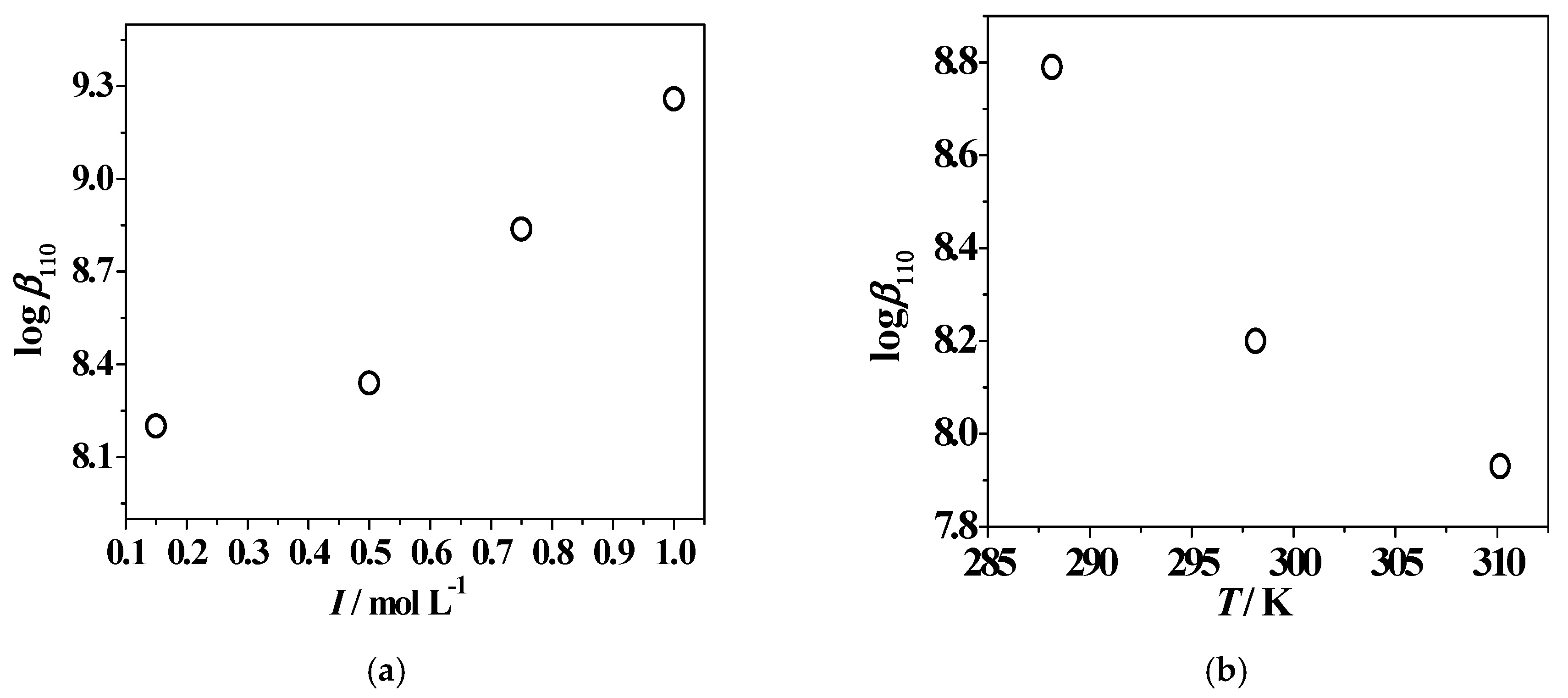
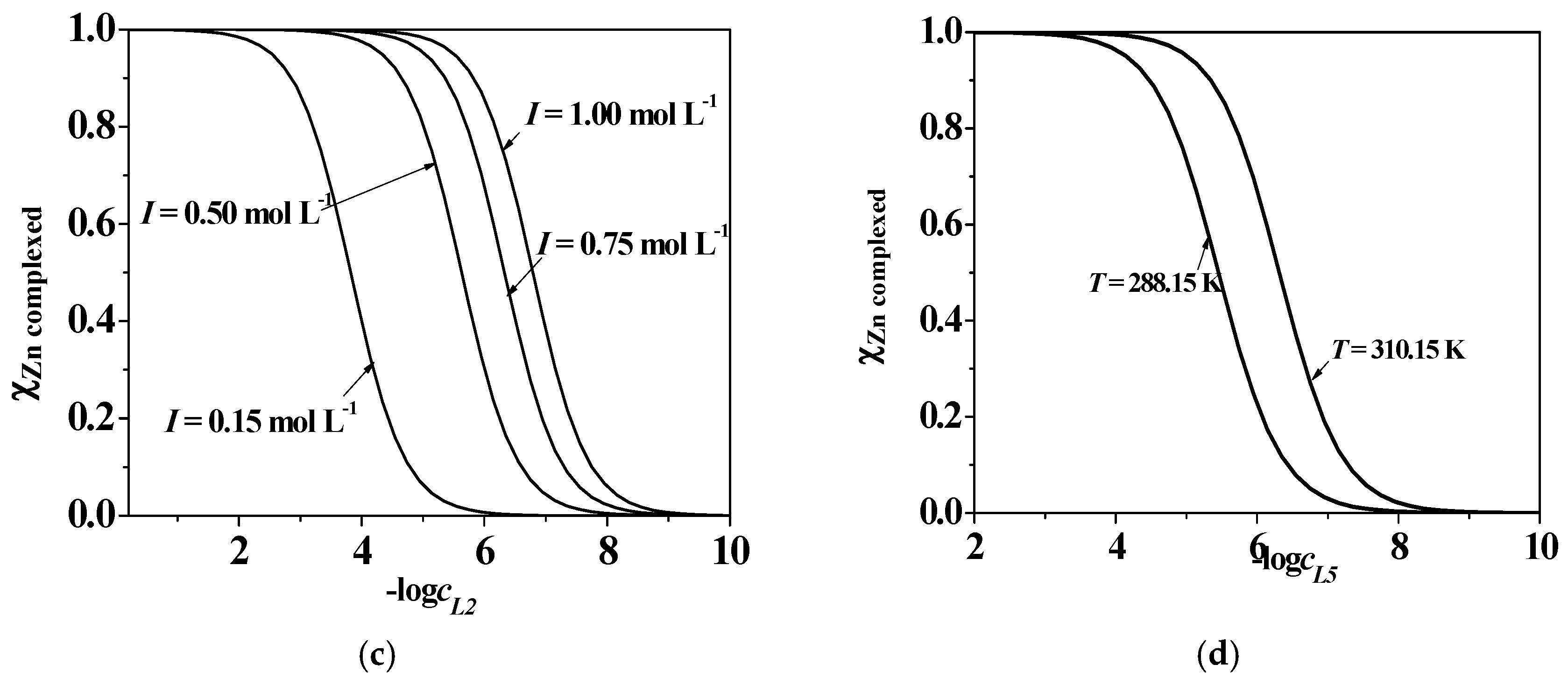
2.5. Dependence on Ionic Strength and Temperature
2.6. Literature Data Comparison
2.6.1. Protonation of the Ligands
2.6.2. Zn2+/Ligands Systems
| Ligand | I/mol L−1 | T/K | Ionic Medium | logK111 | logK110 | logK120 | logK130 | Ref. |
|---|---|---|---|---|---|---|---|---|
| DFP | 0.100 | 298.15 | KCl | - | 7.19 | 6.34 | - | [30] |
| Pyridine | 0.100 | 298.15 | NaClO4 | 5.50 | 1.10 | 0.60 | 0.38 | [44] |
| 0.500 | 298.15 | NaNO3 | - | 1.15 | - | - | [43] | |
| Dopamine | 0.500 | 293.15 | NaNO3 | 7.28 | - | - | - | [31] |
| Aspartic acid | 0.100 | 298.15 | NaNO3 | - | 5.35 | - | - | [45] |
| 0.100 | Na+ | - | 5.87 | - | - | [6] | ||
| 0.500 | Na+ | - | 5.60 | 9.93 | - | [6] | ||
| 1.000 | Na+ | - | 5.64 | - | - | [6] | ||
| 0.100 | 303.15 | Na+ | - | - | 10.16 | - | [6] | |
| 0.150 | 310.15 | Na+ | 1.55 | 5.82 | 10.13 | - | [6] |
2.7. Sequestering Ability
2.8. Zn2+ Depletion vs. Al3+ Sequestration
3. Materials and Methods
3.1. Chemicals
3.2. Analytical Equipment and Procedures
3.2.1. Potentiometric Tools and Procedure
3.2.2. UV-Vis Spectrophotometric Equipment and Procedure
3.2.3. 1H NMR Apparatus and Procedure
3.2.4. MS Spectroscopy Apparatus and Procedure
3.3. Computer Programs
3.4. Computational Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Santos, M.A. Recent developments on 3-hydroxy-4-pyridinones with respect to their clinical applications: Mono and combined ligand approaches. Coordin. Chem. Rev. 2008, 252, 1213–1224. [Google Scholar] [CrossRef]
- Santos, M.A.; Chaves, S. 3-hydroxypyridinone derivatives as metal sequestering agents for therapeutic use. Future Med. Chem. 2015, 7, 383–410. [Google Scholar] [CrossRef] [PubMed]
- Queiros, C.; Amorim, M.J.; Leite, A.; Ferreira, M.; Gameiro, P.; de Castro, B.; Biernacki, K.; Magalhães, A.; Burgess, J.; Rangel, M. Nickel(II) and Cobalt(II) 3-Hydroxy-4-pyridinone Complexes: Synthesis, Characterization and Speciation Studies in Aqueous Solution. Eur. J. Inorg. Chem. Wiley Online Libr. 2011, 131–140. [Google Scholar] [CrossRef]
- Irto, A.; Cardiano, P.; Chand, K.; Cigala, R.M.; Crea, F.; De Stefano, C.; Gano, L.; Gattuso, G.; Sammartano, S.; Santos, M.A. New bis-(3-hydroxy-4-pyridinone)-NTA-derivative: Synthesis, binding ability towards Ca2+, Cu2+, Zn2+, Al3+, Fe3+ and biological assays. J. Mol. Liq. 2018, 272, 609–624. [Google Scholar] [CrossRef]
- Clevette, D.J.; Nelson, W.O.; Nordin, A.; Orvig, C.; Sjoeberg, S. The complexation of aluminum with N-substituted 3-hydroxy-4-pyridinones. Inorg. Chem. 1989, 28, 2079–2081. [Google Scholar] [CrossRef]
- Martell, A.E.; Smith, R.M.; Motekaitis, R.J. NIST Critically Selected Stability Constants of Metal Complexes Database, 8.0; National Institute of Standard and Technology: Garthersburg, MD, USA, 2004.
- Neilands, J.B. Microbial Iron Compounds. Annu. Rev. Biochem. 1981, 50, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Irto, A.; Cardiano, P.; Chand, K.; Cigala, R.M.; Crea, F.; De Stefano, C.; Gano, L.; Sammartano, S.; Santos, M.A. Bifunctional 3-hydroxy-4-pyridinones as effective aluminium chelators: Synthesis, solution equilibrium studies and in vivo evaluation. J. Inorg. Biochem. 2018, 186, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.A.; Gil, M.; Marques, S.; Gano, L.; Cantinho, G.; Chaves, S. N-Carboxyalkyl derivatives of 3-hydroxy-4-pyridinones: Synthesis, complexation with Fe(III), Al(III) and Ga(III) and in vivo evaluation. J. Inorg. Biochem. 2002, 92, 43–54. [Google Scholar] [CrossRef]
- Buffle, J. Complexation Reactions in Aquatic Systems: An Analytical Approach; Ellis Horwood: Chichester, UK, 1988. [Google Scholar]
- Lentner, C. Geigy Scientific Tables, 8th ed.; CIBA-Geigy: Basilea, Switzerland, 1981. [Google Scholar]
- Millero, F.J. Physical Chemistry of Natural Waters; John Wiley & Sons, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Kot, A.; Namiesnik, J. The role of speciation in analytical chemistry. Trends Analyt. Chem. 2000, 19, 69–79. [Google Scholar] [CrossRef]
- Templeton, D.M.; Ariese, F.; Cornelis, R.; Danielsson, L.G.; Muntau, H.; Van Leeuwen, H.P.; Łobinsky, R. Guidelines for terms related to chemical speciation and fractionation of elements. Definitions, structural aspects, and methological approaches. Pure App. Chem. 2000, 72, 1453–1470. [Google Scholar] [CrossRef]
- Deshpande, J.D.; Joshi, M.M.; Giri, P.A. Zinc: The trace element of major importance in human nutrition and health. Int. J. Med. Sci. Public Health 2013, 2, 1–6. [Google Scholar] [CrossRef]
- Powell, S.R. The antioxidant properties of zinc. J. Nutr. 2000, 130, 1447S–1454S. [Google Scholar] [CrossRef] [PubMed]
- Kaltenberg, J.; Plum, L.M.; Ober-Blöbaum, J.L.; Hönscheid, A.; Rink, L.; Haase, H. Zinc signals promote IL-2-dependent proliferation of T cells. Eur. J. Immunol. 2010, 40, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; De Stefano, C.; Foti, C.; Milea, D.; Sammartano, S. Chelating agents for the sequestration of mercury(II) and monomethyl mercury(II). Curr. Med. Chem. 2014, 21, 3819–3836. [Google Scholar] [CrossRef] [PubMed]
- Foti, C.; Sammartano, S. Ionic Strength Dependence of Protonation Constants of Carboxylate Ions in NaClaq (0 ≤ I ≤ 5.6 mol·kg−1) and KClaq (0 ≤ I ≤ 4.5 mol·kg−1): Specific Ion Interaction Theory and Pitzer Parameters and the Correlation between Them. J. Chem. Eng. Data 2010, 55, 904–911. [Google Scholar] [CrossRef]
- Biederman, G. Ionic Media. In Dahlem Workshop on the Nature of Seawater; Dahlem Konferenzen: Berlin, Germany, 1975. [Google Scholar]
- Biederman, G. Introduction to the specific interaction theory with emphasis on chemical equilibria. In Metal Complexes in Solution; Jenne, E.A., Rizzarelli, E., Romano, V., Sammartano, S., Eds.; Piccin: Padua, Italy, 1986; pp. 303–314. [Google Scholar]
- Grenthe, I.; Puigdomenech, I. Modelling in Aquatic Chemistry; OECD: Paris, France, 1997. [Google Scholar]
- Stunzi, H.; Perrin, D.D.; Teitei, T.; Harris, R.L.N. Stability Constants of Some Metal Complexes Formed by Mimosine and Related Compounds. Aust. J. Chem. 1979, 32, 21–30. [Google Scholar] [CrossRef]
- Bretti, C.; Crea, F.; De Stefano, C.; Foti, C.; Materazzi, S.; Vianelli, G. Thermodynamic Properties of Dopamine in Aqueous Solution. Acid–Base Properties, Distribution, and Activity Coefficients in NaCl Aqueous Solutions at Different Ionic Strengths and Temperatures. J. Chem. Eng. Data 2013, 58, 2835–2847. [Google Scholar] [CrossRef]
- Baes, C.F.; Mesmer, R.E. The Hydrolysis of Cations; John Wyley & Sons: New York, NY, USA, 1976. [Google Scholar]
- Pettit, D.; Powell, K.K. Stability Constants Database, Academic Software; IUPAC: Otley, UK, 1997. [Google Scholar]
- Irto, A.; Cardiano, P.; Chand, K.; Cigala, R.M.; Crea, F.; De Stefano, C.; Gano, L.; Gattuso, G.; Sammartano, S.; Santos, M.A. A new bis-(3-hydroxy-4-pyridinone)-DTPA-derivative: Synthesis, complexation of di-/tri-valent metal cations and in vivo M3+ sequestering ability. J. Mol. Liq. 2019, 281, 280–294. [Google Scholar] [CrossRef]
- Manganaro, N.; Lando, G.; Pisagatti, I.; Notti, A.; Pappalardo, S.; Parisi, M.F.; Gattuso, G. Hydrophobic interactions in the formation of a complex between a polycationic water-soluble oxacalix [4] arene and a neutral aromatic guest. Supramol. Chem. 2016, 28, 493–498. [Google Scholar] [CrossRef]
- Colombo, S.; Coluccini, C.; Caricato, M.; Gargiulli, C.; Gattuso, G.; Pasini, D. Shape selectivity in the synthesis of chiral macrocyclic amides. Tetrahedron 2010, 66, 4206–4211. [Google Scholar] [CrossRef]
- Clarke, E.T.; Martell, A.E. Stabilities of 1,2-dimethyl-3-hydroxy-4-pyridinone chelates of divalent and trivalent metal ions. Inorg. Chim. Acta 1992, 191, 57–63. [Google Scholar] [CrossRef]
- Grgas-Kužnar, B.; Simeon, V.; Weber, O.A. Complexes of adrenaline and related compounds with Ni2+, Cu2+, Zn2+, Cd2+ and Pb2+. J. Inorg. Nucl. Chem. 1974, 36, 2151–2154. [Google Scholar] [CrossRef]
- Huang, N.; Siegel, M.M.; Kruppa, G.H.; Laukien, F.H. Automation of a Fourier transform ion cyclotron resonance mass spectrometer for acquisition, analysis, and e-mailing of high-resolution exact-mass electrospray ionization mass spectral data. J. Am. Soc. Mass Spectrom. 1999, 10, 1166–1173. [Google Scholar] [CrossRef]
- Annesley, T.M. Ion Suppression in Mass Spectrometry. Clin. Chem. 2003, 49, 1041–1044. [Google Scholar] [CrossRef] [PubMed]
- Constantopoulos, T.L.; Jackson, G.S.; Enke, C.G. Effects of salt concentration on analyte response using electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 1999, 10, 625–634. [Google Scholar] [CrossRef]
- Karki, S.; Shi, F.; Archer, J.J.; Sistani, H.; Levis, R.J. Direct Analysis of Proteins from Solutions with High Salt Concentration Using Laser Electrospray Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2018, 29, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.L.; Siu, K.W.M.; Guevremont, R.; Berman, S.S. Electrospray mass spectrometry: A study on some aqueous solutions of metal salts. J. Am. Soc. Mass Spectrom. 1992, 3, 281–288. [Google Scholar] [CrossRef]
- Crisponi, G.; Nurchi, V.M.; Zoroddu, M.A. Iron chelating agents for iron overload diseases. Thalass. Rep. 2014, 4, 2046. [Google Scholar]
- Crisponi, G.; Remelli, M. Iron chelating agents for the treatment of iron overload. Coordin. Chem. Rev. 2008, 252, 1225–1240. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Crisponi, G.; Pivetta, T.; Donatoni, M.; Remelli, M. Potentiometric, spectrophotometric and calorimetric study on iron(III) and copper(II) complexes with 1,2-dimethyl-3-hydroxy-4-pyridinone. J. Inorg. Biochem. 2008, 102, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Bretti, C.; Cigala, R.M.; Crea, F.; Lando, G.; Sammartano, S. Thermodynamics of proton binding and weak (Cl-, Na+ and K+) species formation, and activity coefficients of 1,2-dimethyl-3-hydroxypyridin-4-one (deferiprone). J. Chem. Thermodyn. 2014, 77, 98–106. [Google Scholar] [CrossRef]
- Bretti, C.; Cigala, R.M.; De Stefano, C.; Lando, G.; Sammartano, S. Thermodynamics for Proton Binding of Pyridine in Different Ionic Media at Different Temperatures. J. Chem. Eng. Data 2014, 59, 143–156. [Google Scholar] [CrossRef]
- Desai, A.G.; Kabadi, M.B. Stepwise stability constants of complexes of pyridine and substituted pyridines with zinc (II). J. Inorg. Nucl. Chem. 1966, 28, 1279–1282. [Google Scholar] [CrossRef]
- Kapinos, L.E.; Sigel, H. Acid–base and metal ion binding properties of pyridine-type ligands in aqueous solution.: Effect of ortho substituents and interrelation between complex stability and ligand basicity. Inorg. Chim. Acta 2002, 337, 131–142. [Google Scholar] [CrossRef]
- Desai, A.G.; Kabadi, M.B. On the ratio of successive stepwise complex formation constants: Zinc and cadmium complexes of pyridine and substituted pyridines. Recueil des Travaux Chimiques des Pays-Bas 1965, 84, 1066–1070. [Google Scholar] [CrossRef]
- Sajadi, S.A.A. Metal ion-binding properties of L-glutamic acid and L-aspartic acid, a comparative investigation. Nat. Sci. 2010, 2, 85–90. [Google Scholar] [CrossRef]
- Öhman, L.O. Equilibrium Studies of Ternary Aluminium (III) Hydroxo Complexes with Ligands Related to Conditions in Natural Waters. Ph.D. Thesis, University of Umeà, Umeà, Sweden, 1983. [Google Scholar]
- Flaschka, H.A. EDTA Titration; Pergamon Press: London, UK, 1959. [Google Scholar]
- De Stefano, C.; Princi, P.; Rigano, C.; Sammartano, S. Computer Analysis of Equilibrium Data in Solution. ESAB2M: An Improved Version of the ESAB Program. Ann. Chim. (Rome) 1987, 7, 643–675. [Google Scholar]
- De Stefano, C.; Foti, C.; Giuffrè, O.; Mineo, P.; Rigano, C.; Sammartano, S. Binding of Tripolyphosphate by Aliphatic Amines: Formation, Stability and Calculation Problems. Ann. Chim. (Rome) 1996, 86, 257–280. [Google Scholar]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Frassineti, C.; Ghelli, S.; Gans, P.; Sabatini, A.; Moruzzi, M.S.; Vacca, A. Nuclear Magnetic Resonance as a Tool for Determining Protonation Constants of Natural Polyprotic Bases in Solution. Anal. Biochem. 1995, 231, 374–382. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, C.; Sammartano, S.; Mineo, P.; Rigano, C. Computer Tools for the Speciation of Natural Fluids. In Marine Chemistry—An Environmental Analytical Chemistry Approach; Gianguzza, A., Pelizzetti, E., Sammartano, S., Eds.; Kluwer Academic Publishers: Amsterdam, The Netherlands, 1997; pp. 71–83. [Google Scholar]
- Alderighi, L.; Gans, P.; Ienco, A.; Peters, D.; Sabatini, A.; Vacca, A. Hyperquad simulation and speciation (HySS): A utility program for the investigation of equilibria involving soluble and partially soluble species. Coord. Chem. Rev. 1999, 184, 311–318. [Google Scholar] [CrossRef]
- Shao, Y.; Molnar, L.F.; Jung, Y.; Kussmann, J.; Ochsenfeld, C.; Brown, S.T.; Gilbert, A.T.B.; Slipchenko, L.V.; Levchenko, S.V.; O’Neill, D.P.; et al. Advances in methods and algorithms in a modern quantum chemistry program package. Phys. Chem. Chem. Phys. 2006, 8, 3172–3191. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

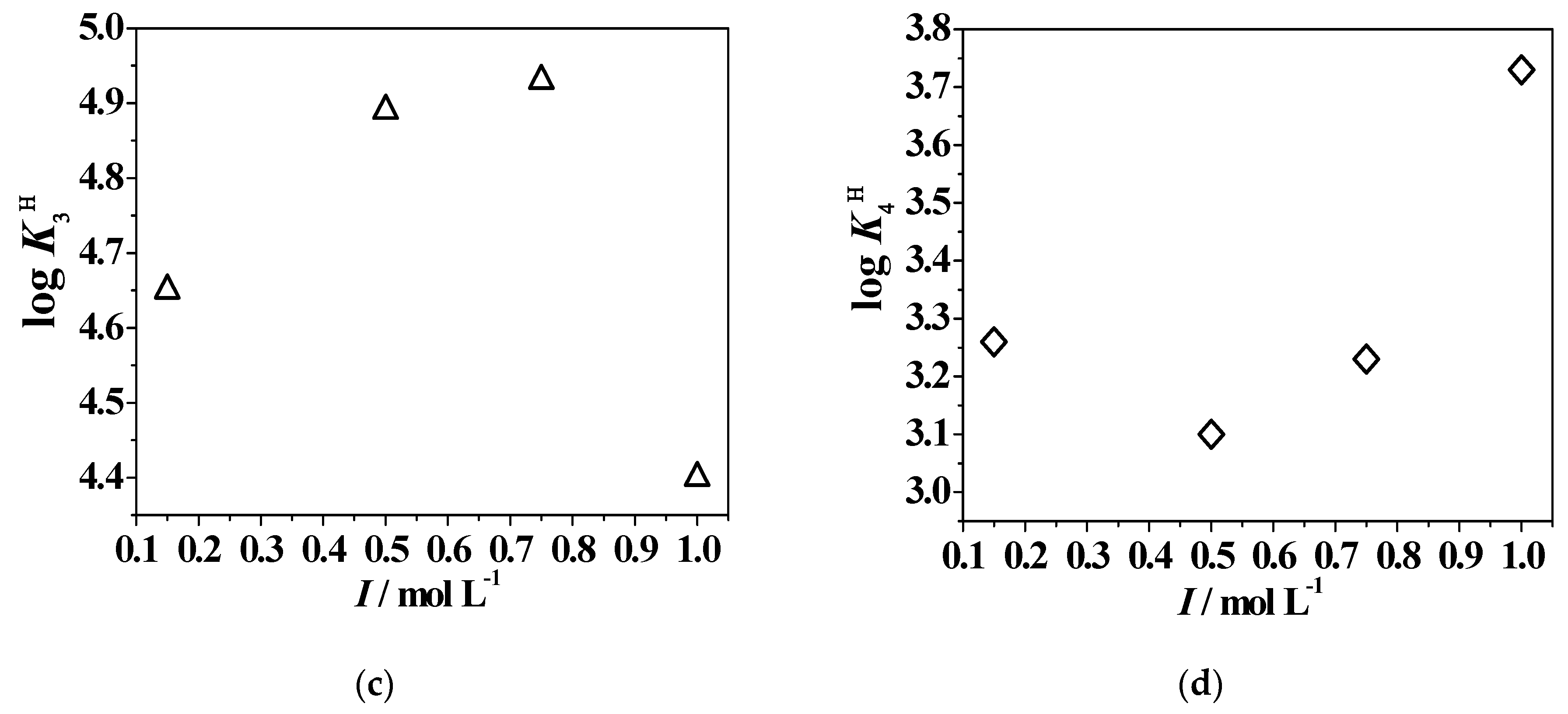





| Ligand | I/mol L−1 | T/K | logβrH 1 (logKrH) 2 | |||
|---|---|---|---|---|---|---|
| HL(1−z) | H2L(2−z) | H3L(3−z) | H4L(4−z) | |||
| L23 | 0.149 | 288.15 | 10.28 ± 0.014 | 19.56 ± 0.01 4 (9.28) | 24.29 ± 0.08 4 (4.73) | 27.46 ± 0.08 4 (3.17) |
| 0.506 | 298.15 | 9.962 ± 0.005 | 17.63 ± 0.03 (7.668) | 22.52 ± 0.02 (4.89) | 25.62 ± 0.02 (3.10) | |
| 0.744 | 298.15 | 9.882 ± 0.008 | 17.25 ± 0.07 (7.365) | 22.18 ± 0.10 (4.93) | 25.41 ± 0.10 (3.23) | |
| 1.012 | 298.15 | 10.059 ± 0.001 | 16.78 ± 0.02 (6.721) | 21.18 ± 0.03 (4.40) | 24.91 ± 0.12 (3.73) | |
| L55 | 0.166 | 288.15 | 10.53 ± 0.03 4 | 19.92 ± 0.02 4 (9.39) | 23.58 ± 0.03 4 (3.66) | - |
| 0.165 | 298.15 | 10.82 ± 0.07 | 20.44 ± 0.06 (9.62) | 24.02 ± 0.07 (3.58) | - | |
| 0.140 | 298.15 | 11.20 6 ± 0.02 | 20.466 6 ± 0.006 (9.266) | 23.679 6 ± 0.007 (3.213) | - | |
| 0.473 | 298.15 | 9.99 ± 0.01 | 19.17 ± 0.01 (9.18) | 22.46 ± 0.01 (3.29) | - | |
| 0.723 | 298.15 | 9.79 ± 0.03 | 18.86 ± 0.03 (9.07) | 22.02 ± 0.04 (3.16) | - | |
| 1.008 | 298.15 | 10.31 ± 0.02 | 19.32 ± 0.03 (9.01) | 23.38 ± 0.06 (4.06) | - | |
| logβpqr 1 (logKpqr) 2 | logβpqr 1 (logKpqr) 2 | logβpqr 1 (logKpqr) 2 | |||||
| I/mol L−1 | 0.146 | 0.147 | 0.150 | 0.145 | 0.150 | 0.148 | |
| Species | L1 | L2 | L3 | ||||
| ZnLH(3−z) | - | 15.50 4 ± 0.06 7 (4.77) | 15.51 5 ± 0.08 7(4.78) | 15.79 6 ± 0.31 7 (5.05) | 15.56 3 ± 0.08 8 (4.83) | 17.06 4 ± 0.09 6 (6.10) | |
| ZnL(2−z) | 7.27 4 ± 0.03 7 | 8.12 ± 0.06 | 8.11 ± 0.04 | 8.54 ± 0.48 | 8.20 ± 0.10 | 9.52 ± 0.06 | |
| ZnL(OH)(1−z) | 0.25 ± 0.07 (9.45) 9 | −0.72 ± 0.09 (8.48) 9 | −0.55 ± 0.04 (8.64) 9 | −0.72 ± 0.09 (8.64) 9 | −0.68 ± 0.06 (8.56) 9 | 0.70 ± 0.08 (9.90) 9 | |
| logβpqr 1 (logKpqr) 2 | logβpqr 1 (logKpqr) 2 | ||||||
| I/mol L−1 | 0.145 | 0.150 | 0.147 | 0.149 | 0.150 | 0.145 | 0.150 |
| Species | L4 | L5 | |||||
| ZnLH(3−z) | 16.69 4 ± 0.05 7 (5.59) | 16.68 5 ± 0.20 7 (5.58) | 16.68 3 ± 0.02 8 (5.58) | 17.21 4 ± 0.06 7 (6.13) | 17.41 5 ± 0.07 7 (6.33) | 17.64 6 ± 0.08 7 (6.56) | 17.46 3 ± 0.09 8 (6.38) |
| ZnL(2−z) | 9.68 ± 0.04 | 9.68 ± 0.10 | 9.68 ± 0.08 | 9.89 ± 0.08 | 9.08 ± 0.02 | 9.04 ± 0.04 | 9.22 ± 0.20 |
| ZnL(OH)(1−z) | 0.60 ± 0.09 (9.80) 9 | 0.33 ± 0.20 (9.53) 9 | 0.46 ± 0.08 (9.66) 9 | - | - | - | - |
| System | I/mol L−1 | T/K | logβ111 1 (logK111) 2 | logβ110 1,2 | logβ11−1 1 (logK11−1) 3 |
|---|---|---|---|---|---|
| Zn2+/L2 | 0.147 | 288.15 | 15.90 1 ± 0.03 4 (5.62) | 8.791 ± 0.104 | −1.50 1 ± 0.12 4 (8.18)3 |
| 0.501 | 298.15 | 14.95 ± 0.05 (4.99) | 8.34 ± 0.03 | −1.26 ± 0.05 (7.82) | |
| 0.759 | 298.15 | 14.97 ± 0.10 (5.085) | 8.839 ± 0.009 | −1.055 ± 0.009 (8.067) | |
| 1.005 | 298.15 | 15.47 ± 0.08 (5.44) | 9.26 ± 0.01 | −0.804 ± 0.003 (8.357) | |
| 0.152 | 310.15 | 15.50 ± 0.04 (4.51) | 7.93 ± 0.16 | 0.47 ± 0.04 (9.25) | |
| Zn2+/L5 | 0.161 | 288.15 | 17.51 1 ± 0.10 4 (6.97) | 9.671 ± 0.104 | - |
| 0.472 | 298.15 | 16.45 ± 0.02 (6.46) | 9.03 ± 0.04 | - | |
| 0.725 | 298.15 | 16.09 ± 0.04 (6.29) | 8.91 ± 0.06 | - | |
| 0.951 | 298.15 | 16.75 ± 0.09 (6.44) | 8.49 ± 0.11 | - | |
| 0.155 | 310.15 | 16.65 ± 0.10 (6.08) | 8.08 ± 0.11 | - |
| Species | Theoretical m/z | Experimental m/z | Formula |
|---|---|---|---|
| [H(L5) + H]+ | 183.11 | 183.1 | C9H15N2O2 |
| [H(L5) + Na]+ | 205.10 | 205.1 | C9H14N2NaO2 |
| [H(L5) + K]+ | 221.07 | 221.1 | C9H14N2KO2 |
| [Zn(H2O)3]2+ | 58.98 | 59.0 | ZnH6O3 |
| [Zn + NaCl]2+ | 60.94 | 61.0 | ZnClNa |
| [Zn(H2O)5]2+ | 76.99 | 76.9 | ZnH10O5 |
| [Zn(OH)]+ | 80.93 | 81.0 | ZnHO |
| [Zn(OH)(H2O)]+ | 98.94 | 98.9 | ZnH3O2 |
| [Zn(H2O)6(L5) + H + NaCl]2+ | 206.03 | 206.0 | ZnC9H26N2ClNaO8 |
| Species | logTβpqr 1 (logTKpqr) 2 | C 3 | Δε 4 | ΔH 5,6 (Stepwise ΔH) | ΔG 5,6 (Stepwise ΔG) | TΔS5,6 (Stepwise TΔS) |
|---|---|---|---|---|---|---|
| H(L2)− | 11.32 ± 0.08 7 | −0.71 ± 0.10 7 | −0.69 ± 0.15 7 | 55 ± 12 7 | −61.2 ± 0.2 7 | 116 ± 4 7 |
| H2(L2)0(aq) | 20.54 ± 0.02 (9.22) | −2.72 ± 0.07 | −2.74 ± 0.15 | −200 ± 3 (−255) | −111.4 ± 0.5 (−50.2) | −88 ± 3 (−204) |
| H3(L2)+ | 25.35 ± 0.03 (4.81) | −2.94 ± 0.02 | −2.88 ± 0.07 | −260 ± 4 (−60) | −137.9 ± 0.5 (−26.5) | −122 ± 6 (−34) |
| H4(L2)2+ | 28.34 ± 0.04 (2.99) | −2.88 ± 0.02 | −2.86 ± 0.10 | −269 ± 14 (−9) | −156.5 ± 0.5 (−18.6) | −112 ± 6 (10) |
| Zn(L2)H+ | 16.26 ± 0.12 (4.94) | 0.59 ± 0.10 | 0.64 ± 0.07 | −27 ± 9 (−80) | −88.5 ± 0.2 (−27.4) | 61 ± 8 (−53) |
| Zn(L2)0(aq) | 8.77 ± 0.05 | 2.12 ± 0.06 | 2.01 ± 0.01 | −62 ± 1 | −46.3 ± 0.2 | −16 ± 1 |
| Zn(L2)OH− | −0.10 ± 0.01 (8.86) | 0.25 ± 0.10 | 0.37 ± 0.05 | 153 ± 3 (96) | 3.0 ± 0.2 (−49.4) | 150 ± 3 (146) |
| H(L5)0(aq) | 11.12 ± 0.10 7 | −0.83 ± 0.10 7 | −0.80 ± 0.197 | 1 ± 4 7 | −63 ± 0.4 7 | 64 ± 4 7 |
| H2(L5)+ | 20.54 ± 0.10 (9.42) | −1.26 ± 0.20 | −1.24 ± 0.20 | −271 ± 2 (−272) | −112.6 ± 1.1 (−49.6) | −158 ± 7 (−222) |
| H3(L5)2+ | 23.86 ± 0.08 (3.32) | −1.42 ± 0.20 | −1.50 ± 0.32 | −322 ± 12 (−51) | −135.1 ± 1.1 (−22.5) | −187 ± 12 (−29) |
| Zn(L5)H2+ | 17.33 ± 0.18 (6.00) | −0.60 ± 0.09 | −0.49 ± 0.14 | −65 ± 8 (−66) | −97.7 ± 0.3 (−34.7) | 33 ± 8 (−31) |
| Zn(L5)+ | 10.57 ± 0.10 | −1.24 ± 0.11 | −1.38 ± 0.10 | −127 ± 15 | −52.1 ± 0.8 | −75 ± 15 |
| Ligand | I/molL−1 | T/K | Ionic Medium | logK1H | logK2H | logK3H | logK4H | Ref. |
|---|---|---|---|---|---|---|---|---|
| DFP | 0.099 | 283.15 | NaCl | 10.00 | 3.80 | - | - | [40] |
| 0.152 | 298.15 | NaCl | 9.82 | 3.67 | - | - | [40] | |
| 0.150 | NaCl | 9.86 | 3.70 | - | - | [5] | ||
| 0.495 | NaCl | 9.70 | 3.74 | - | - | [40] | ||
| 1.005 | NaCl | 9.66 | 3.83 | - | - | [40] | ||
| 0.100 | 310.15 | NaCl | 9.70 | 3.60 | - | - | [40] | |
| 0.100 | 298.15 | KCl | 9.82 | 3.66 | - | - | [39] | |
| 0.150 | KCl | 9.82 | 3.66 | - | - | [37,38] | ||
| 0.150 | KCl | 9.77 | 3.68 | - | - | [30] | ||
| 0.500 | KCl | 9.78 | 3.75 | - | - | [39] | ||
| 1.000 | KCl | 9.75 | 3.80 | - | - | [39] | ||
| 0.100 | 310.15 | KCl | 9.70 | 3.57 | - | - | [39] | |
| Pyridine | 0.294 | 283.15 | NaCl | 5.50 | - | - | - | [41] |
| 0.197 | 298.15 | NaCl | 5.28 | - | - | - | [41] | |
| 0.480 | NaCl | 5.37 | - | - | - | [41] | ||
| 0.960 | NaCl | 5.52 | - | - | - | [41] | ||
| 2.018 | 310.15 | NaCl | 5.66 | - | - | - | [41] | |
| Dopamine | 0.147 | 288.15 | NaCl | 10.61 | 9.41 | - | - | [24] |
| 0.162 | 298.15 | NaCl | 10.59 | 9.18 | - | - | [24] | |
| 0.504 | NaCl | 10.44 | 9.07 | - | - | [24] | ||
| 0.737 | NaCl | 10.278 | 8.99 | - | - | [24] | ||
| 0.982 | NaCl | 10.25 | 8.85 | - | - | [24] | ||
| 0.165 | 310.15 | NaCl | 10.02 | 8.69 | - | - | [24] | |
| 0.500 | 303.15 | NaNO3 | 12.05 | 10.60 | 9.06 | - | [31] | |
| Aspartic acid | 0.100 | 298.15 | Na+ | 9.66 | 3.71 | 1.95 | - | [6] |
| 0.500 | Na+ | 9.58 | 3.68 | 1.98 | - | [6] | ||
| 1.000 | NaCl | 9.61 | 3.64 | 2.00 | - | [6] | ||
| 0.150 | 310.15 | Na+ | 9.33 | 3.64 | 1.94 | - | [6] | |
| Mimosine | 0.150 | 310.15 | KNO3 | 8.86 | 7.00 | 2.62 | 1.10 | [23] |
| System | pH | pL0.5 | I/mol L−1 | T/K | System | pH | pL0.5 | I/mol L−1 | T/K |
|---|---|---|---|---|---|---|---|---|---|
| Zn2+/L1 | 2.5 | <1.0 | 0.15 | 298.15 | Zn2+/L2 | 7.4 | 6.3 | 0.75 | 298.15 |
| 3.0 | <1.0 | 0.15 | 298.15 | 7.4 | 6.8 | 1.00 | 298.15 | ||
| 4.0 | 1.0 | 0.15 | 298.15 | 7.4 | 5.2 | 0.15 | 310.15 | ||
| 5.0 | 2.5 | 0.15 | 298.15 | Zn2+/L3 | 7.4 | 4.2 | 0.15 | 298.15 | |
| 6.0 | 3.6 | 0.15 | 298.15 | Zn2+/L4 | 7.4 | 4.4 | 0.15 | 298.15 | |
| 7.4 | 5.5 | 0.15 | 298.15 | Zn2+/L5 | 7.4 | 5.4 | 0.15 | 288.15 | |
| 8.1 | 6.7 | 0.15 | 298.15 | 2.5 | <1.0 | 0.15 | 298.15 | ||
| 9.0 | 7.6 | 0.15 | 298.15 | 3.0 | <1.0 | 0.15 | 298.15 | ||
| 10.0 | 7.3 | 0.15 | 298.15 | 4.0 | <1.0 | 0.15 | 298.15 | ||
| Zn2+/L2 | 7.4 | 6.3 | 0.15 | 288.15 | 5.0 | 2.0 | 0.15 | 298.15 | |
| 2.5 | <1.0 | 0.15 | 298.15 | 6.0 | 3.0 | 0.15 | 298.15 | ||
| 3.0 | <1.0 | 0.15 | 298.15 | 7.4 | 4.7 | 0.15 | 298.15 | ||
| 4.0 | <1.0 | 0.15 | 298.15 | 8.1 | 5.8 | 0.15 | 298.15 | ||
| 5.0 | 1.0 | 0.15 | 298.15 | 9.0 | 6.6 | 0.15 | 298.15 | ||
| 6.0 | 2.0 | 0.15 | 298.15 | 10.0 | 4.7 | 0.15 | 298.15 | ||
| 7.4 | 3.8 | 0.15 | 298.15 | 7.4 | 5.2 | 0.50 | 298.15 | ||
| 8.1 | 5.0 | 0.15 | 298.15 | 7.4 | 5.3 | 0.75 | 298.15 | ||
| 9.0 | 5.7 | 0.15 | 298.15 | 7.4 | 5.1 | 1.00 | 298.15 | ||
| 10.0 | 5.5 | 0.15 | 298.15 | 7.4 | 6.3 | 0.15 | 310.15 | ||
| 7.4 | 5.6 | 0.50 | 298.15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irto, A.; Cardiano, P.; Cataldo, S.; Chand, K.; Maria Cigala, R.; Crea, F.; De Stefano, C.; Gattuso, G.; Muratore, N.; Pettignano, A.; et al. Speciation Studies of Bifunctional 3-Hydroxy-4-Pyridinone Ligands in the Presence of Zn2+ at Different Ionic Strengths and Temperatures. Molecules 2019, 24, 4084. https://doi.org/10.3390/molecules24224084
Irto A, Cardiano P, Cataldo S, Chand K, Maria Cigala R, Crea F, De Stefano C, Gattuso G, Muratore N, Pettignano A, et al. Speciation Studies of Bifunctional 3-Hydroxy-4-Pyridinone Ligands in the Presence of Zn2+ at Different Ionic Strengths and Temperatures. Molecules. 2019; 24(22):4084. https://doi.org/10.3390/molecules24224084
Chicago/Turabian StyleIrto, Anna, Paola Cardiano, Salvatore Cataldo, Karam Chand, Rosalia Maria Cigala, Francesco Crea, Concetta De Stefano, Giuseppe Gattuso, Nicola Muratore, Alberto Pettignano, and et al. 2019. "Speciation Studies of Bifunctional 3-Hydroxy-4-Pyridinone Ligands in the Presence of Zn2+ at Different Ionic Strengths and Temperatures" Molecules 24, no. 22: 4084. https://doi.org/10.3390/molecules24224084
APA StyleIrto, A., Cardiano, P., Cataldo, S., Chand, K., Maria Cigala, R., Crea, F., De Stefano, C., Gattuso, G., Muratore, N., Pettignano, A., Sammartano, S., & Amélia Santos, M. (2019). Speciation Studies of Bifunctional 3-Hydroxy-4-Pyridinone Ligands in the Presence of Zn2+ at Different Ionic Strengths and Temperatures. Molecules, 24(22), 4084. https://doi.org/10.3390/molecules24224084











