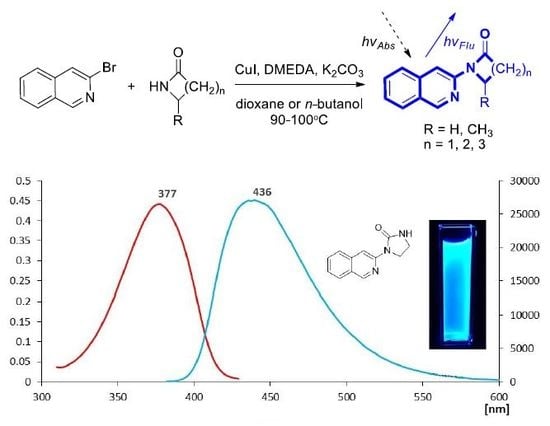
Synthesis and Fluorescent Properties of Novel Isoquinoline Derivatives
Abstract
:1. Introduction
2. Results and Discussion
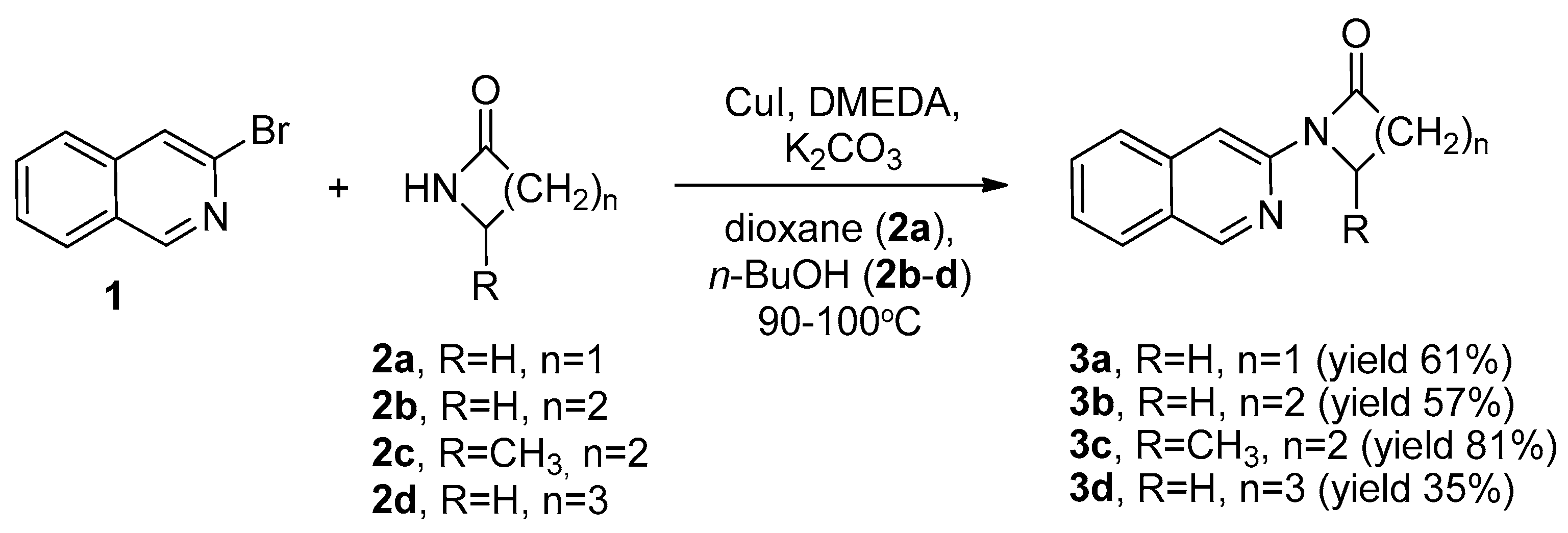
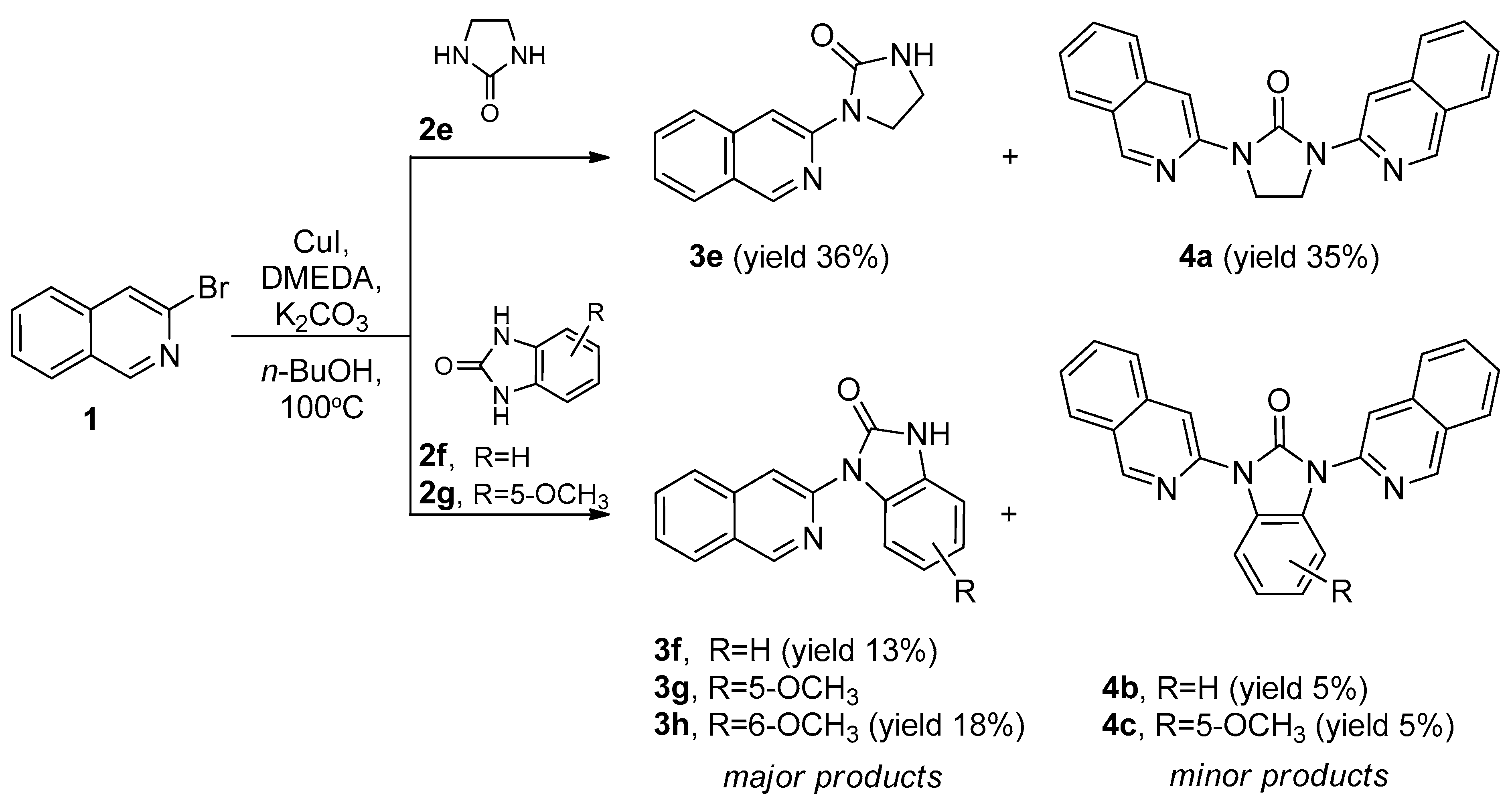
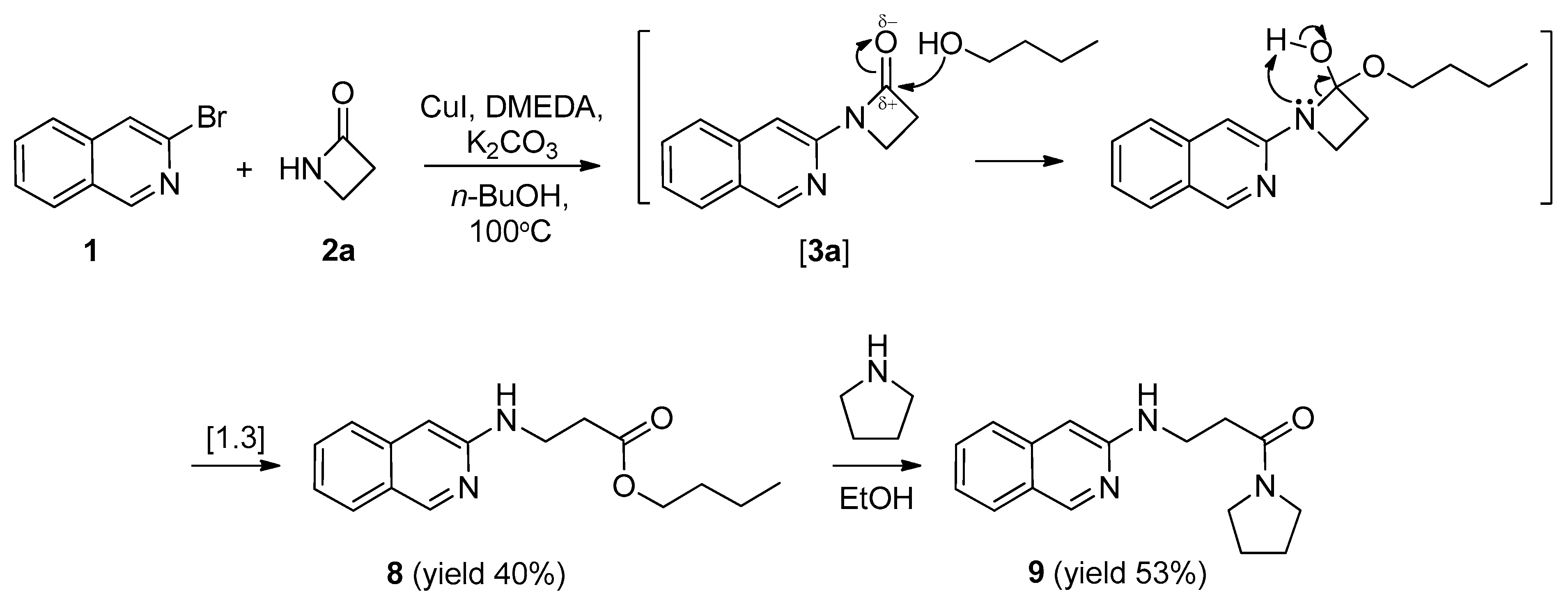
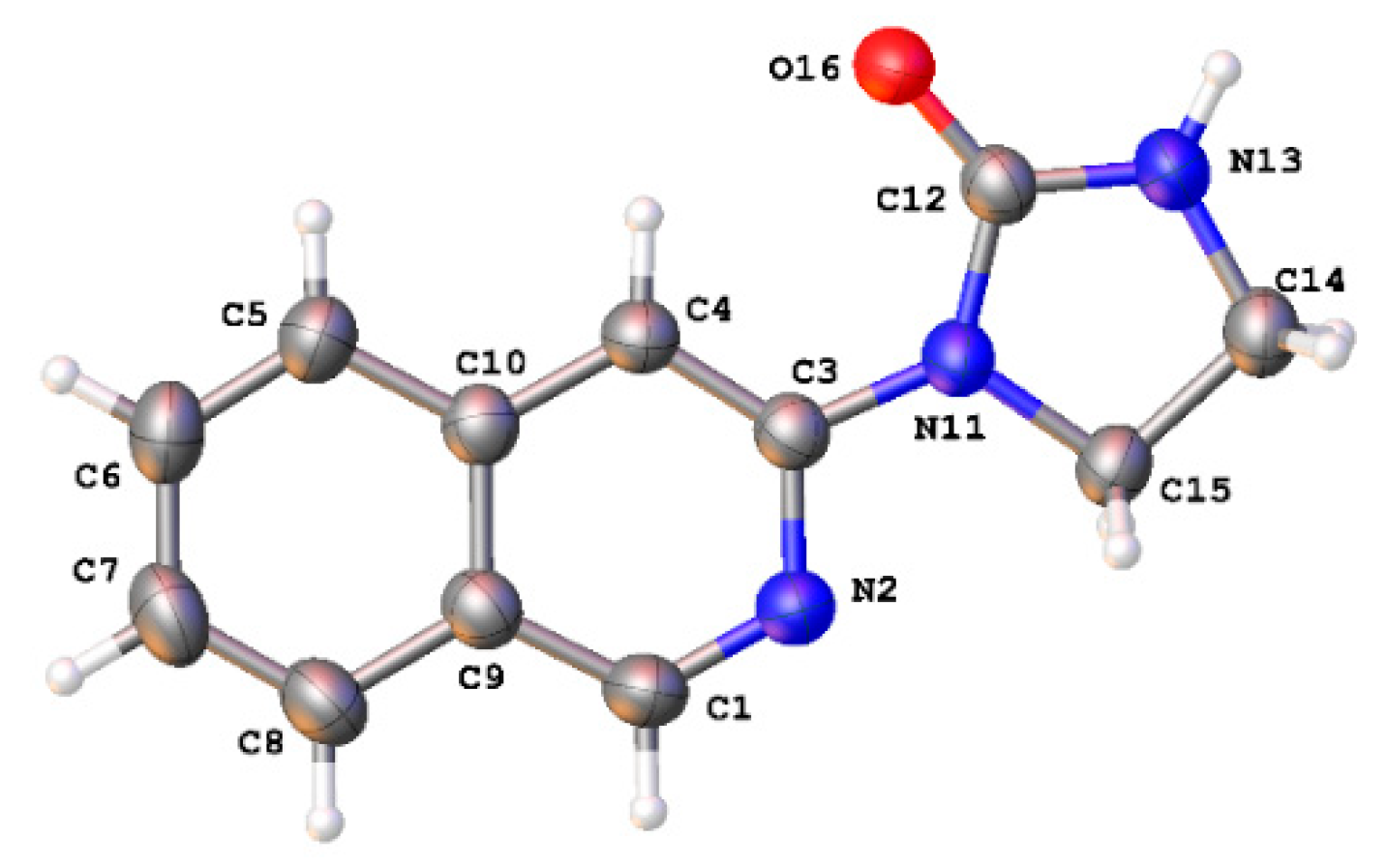
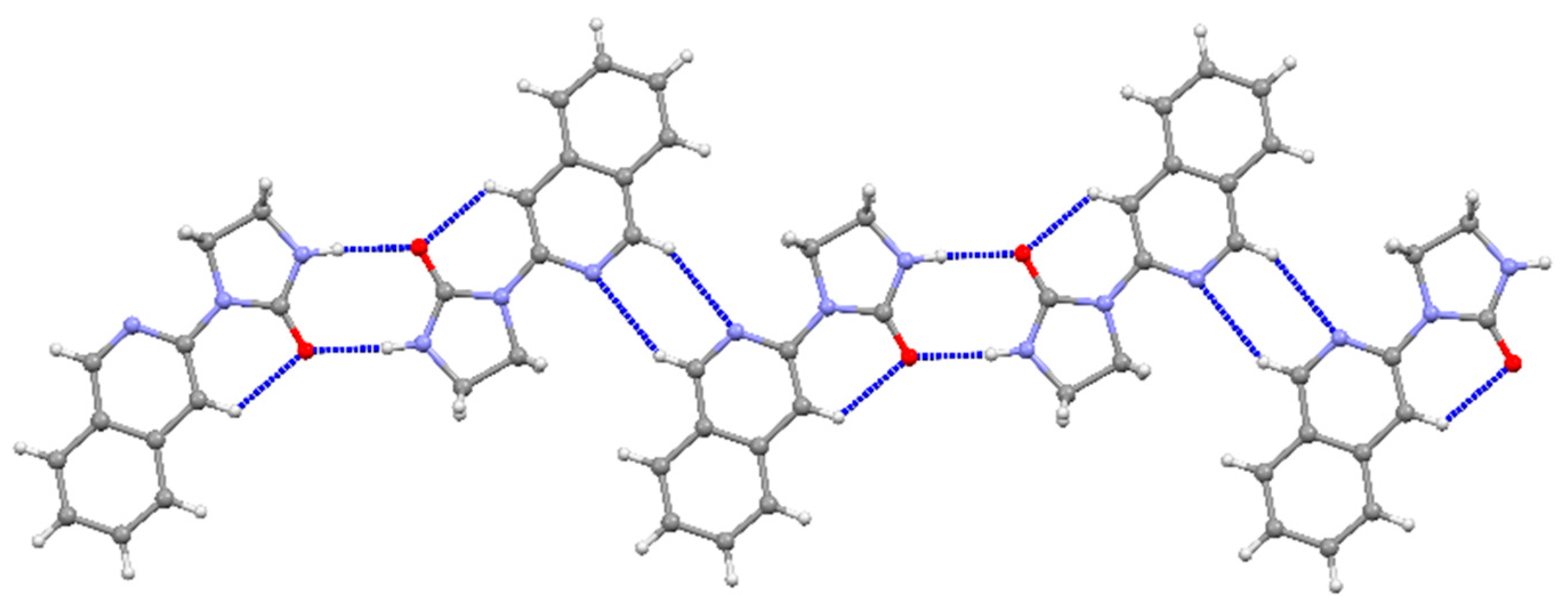
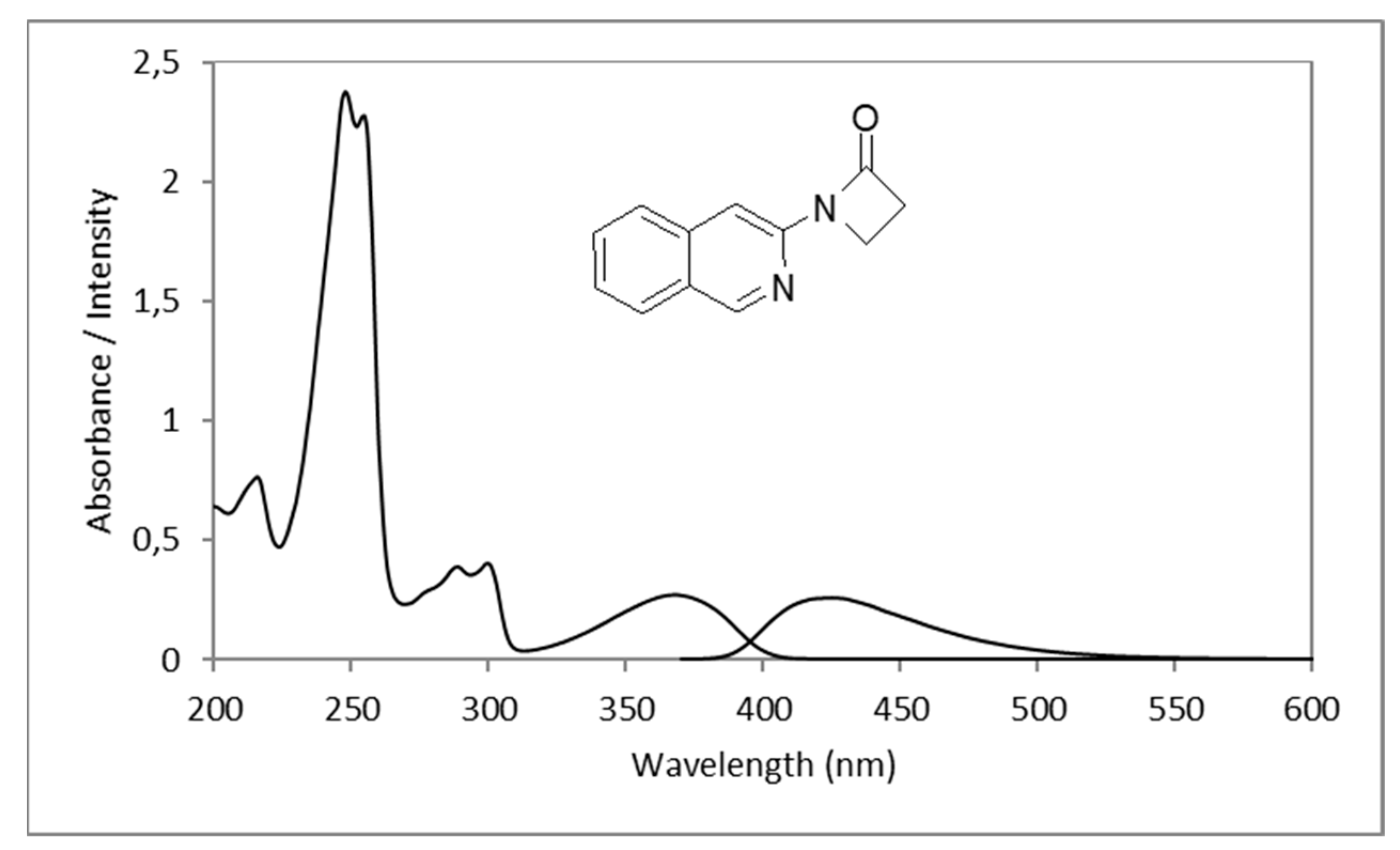
2.1. Synthesis of 1-(Isoquinolin-3-yl)heteroalkyl(aryl)-2-ones 3a-d and 1,3-di(Isoquinolin-3-yl)heteroalkyl(aryl)-2-ones 4a-c
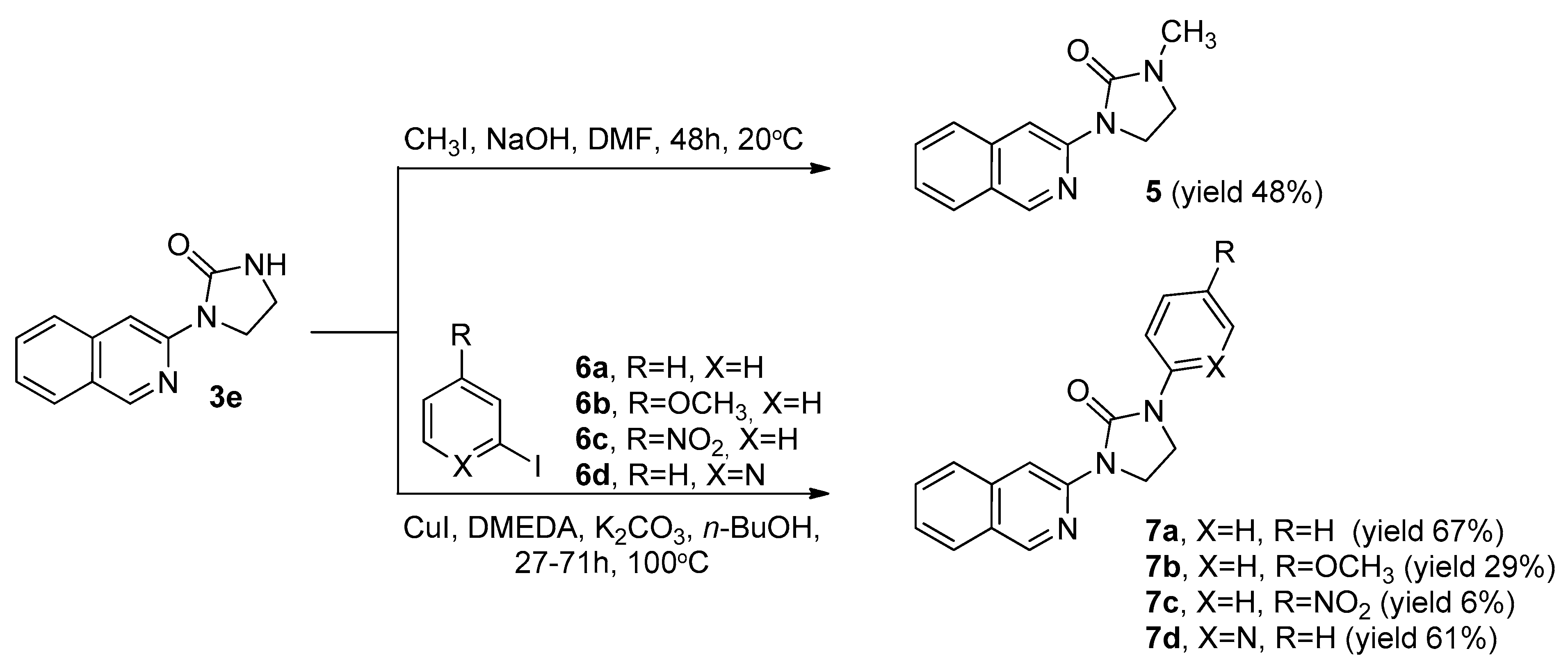
2.2. Synthesis of 1-(Isoquinolin-3-yl)-3-methylimidazolidin-2-one (5) and 1-(Isoquinolin-3-yl)-3-(hetero)aryl-2-ones 7a-d
2.3. Synthesis of n-Butyl 3-(isoquinolin-3-ylamino)propanoate (8) and 3-(Isoquinolin-3-ylamino)-1-(pyrrolidin-1-yl)propan-1-one (9)
3. Experimental Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Menachery, M.D.; Lavanier, G.L.; Wetherly, M.L.; Guinaudeau, H.; Shamma, M. Simple isoquinoline alkaloids. J. Nat. Prod. 1986, 49, 745–778. [Google Scholar] [CrossRef]
- Li, G.-Y.; Li, B.-G.; Yang, T.; Liu, G.-Y.; Zhang, G.-L. Chaetoindicins A-C, three isoquinoline alkaloids from the fungus Chaetomium indicum. Org. Lett. 2006, 8, 3613–3615. [Google Scholar] [CrossRef] [PubMed]
- Demerson, C.A.; Philipp, A.H.; Humber, L.G.; Kraml, M.J.; Charest, M.P.; Tom, H.; Vavra, I. Pyrrolo [4,3,2-de]isoquinolines with central nervous system and antihypertensive activities. J. Med. Chem. 1974, 17, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Alagumuthu, M.; Sathiyanarayanan, K.I.; Arumugam, S. Molecular docking, design, synthesis, in vitro antioxidant and anti-inflammatory evaluations of new isoquinoline derivatives. Int. J Pharm. Pharm. Sci. 2015, 7, 200–208. [Google Scholar]
- Manikandan, A.; Sivakumar, A. Analgesic, anti-inflammatory and antipyretic evaluations of new isoquinoline derivatives. J. Pharm. Sci. 2016, 8, 339–343. [Google Scholar]
- Galán, A.; Moreno, L.; Párraga, J.; Serrano, A.; Sanz, M.J.; Cortes, D.; Cabedo, N. Novel isoquinoline derivatives as antimicrobial agents. Bioorg. Med. Chem. 2013, 21, 3221–3230. [Google Scholar] [CrossRef]
- Pingaew, R.; Prachayasittikul, S.; Ruchirawat, S. Synthesis, cytotoxic and antimalarial activities of benzoyl thiosemicarbazone analogs of isoquinoline and related compounds. Molecules 2010, 15, 988–996. [Google Scholar] [CrossRef]
- Zajdel, P.; Marciniec, K.; Maślankiewicz, A.; Grychowska, K.; Satała, G.; Duszyńska, B.; Lenda, T.; Siwek, A.; Nowak, G.; Partyka, A.; et al. Antidepressant and antipsychotic activity of new quinoline- and isoquinoline-sulfonamide analogs of aripiprazole targeting serotonin 5-HT1A/5-HT2A/5-HT7 and dopamine D2/D3 receptors. Eur. J. Med. Chem. 2013, 60, 42–50. [Google Scholar] [CrossRef]
- Hassaneen, H.M.; Wardkhan, W.W.; Mohammed, Y.S. A novel route to isoquinoline[2,1-g][1,6]naphthyridine, pyrazolo[5,1-a] isoquinoline and pyridazino[4’,5´:3,4]pyrazolo[5,1-a]isoquinoline derivatives with evaluation of antitumor activities. Z. Naturforsch. 2013, 68, 895–904. [Google Scholar] [CrossRef]
- Kakhki, S.; Shahosseini, S.; Zarghi, A. Design, synthesis and cytotoxicity evaluation of new 2-aryl-5, 6-dihydropyrrolo[2,1-a]isoquinoline derivatives as topoisomerase inhibitors. Iran J. Pharm. Res. 2014, 13, 71–77. [Google Scholar]
- Wu, H.L.; Hsu, C.Y.; Liu, W.H.; Yung, B.Y.M. Berberine-induced apoptosis of human leukemia HL-60 cells is associated with down-regulation of nucleophosmin/B23 and telomerase activity. Int. J. Cancer 1999, 81, 923–929. [Google Scholar] [CrossRef]
- Cushman, M.; Jayaraman, M.; Vroman, J.A.; Fukunaga, A.K.; Fox, B.M.; Kohlhagen, G.; Strumberg, D.; Pommier, Y. Synthesis of new indeno[1,2-c]isoquinolines: cytotoxic non-camptothecin topoisomerase I inhibitors. J. Med. Chem. 2000, 43, 3688–3698. [Google Scholar] [CrossRef] [PubMed]
- Balog, J.; Riedl, Z.; Hajós, G.; Miskolczy, Z.; Biczók, L. Novel fluorescent isoquinoline derivatives obtained via Buchwald-Hartwig coupling of isoquinolin-3-amines. ARKIVOC 2012, 5, 109–119. [Google Scholar]
- Balog, J.; Riedl, Z.; Hajós, G.; Miskolczy, Z.; Biczók, L. New fluorescent isoquinoline derivatives. Tetrahedron Lett. 2011, 52, 5264–5266. [Google Scholar] [CrossRef]
- Wan, Y.; Niu, W.; Behof, W.J.; Wang, Y. Aminoisoquinolines as colorimetric Hg2+ sensors: The importance of molecular structure and sacrificial base. Tetrahedron 2009, 65, 4293–4297. [Google Scholar] [CrossRef]
- Evans, D.A.; Smith, G.F.; Wahid, M.A. The tautomerism of 3-hydroxyisoquinolines. J. Chem. Soc. (B) 1967, 590–595. [Google Scholar] [CrossRef]
- Huber, J.R.; Mahaney, M.; Morris, J.V. Temperature dependence of radiationless processes. Isoquinoline in solution. Chem. Phys. 1976, 16, 329–335. [Google Scholar] [CrossRef]
- Moomaw, W.R.; Anton, M.F. Luminescence studies of proton transfer in the excited electronic states of hydrogen bonded quinoline and isoquinoline. J. Phys. Chem. 1976, 80, 2243–2247. [Google Scholar] [CrossRef]
- Rollinson, A.M.; Schuster, G.B.; Drickamer, H.G. High pressure studies of isoquinoline luminescence in polymeric media. Chem. Phys. Lett. 1978, 59, 559–561. [Google Scholar] [CrossRef]
- Lai, T.; Lim, E.C. Temperature effects on S1-S0/S1-T1 branching ratio in radiationless transitions of N-heterocyclic compounds. Chem. Phys. Lett. 1979, 62, 507–510. [Google Scholar] [CrossRef]
- Wei, C.-Y.; Yu, W.-S.; Chou, P.-T.; Hung, F.-T.; Chang, C.-P.; Lin, T.-C. Conjugated dual hydrogen-bond mediating proton-transfer reaction in 3-hydroxyisoquinoline. J. Phys. Chem. B 1998, 102, 1053–1064. [Google Scholar] [CrossRef]
- Krishnakumar, V.; Ramasamy, R. DFT studies and vibrational spectra of isoquinoline and 8-hydroxyquinoline. Spectrochim. Acta Part A 2005, 61, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Megyesi, M.; Biczók, L. Effect of ion pairing on the fluorescence of berberine, a natural isoquinoline alkaloid. Chem. Phys. Lett. 2007, 447, 247–251. [Google Scholar] [CrossRef]
- Singh, D.; Ghosh, S.K.; Baruah, J.B. Solvent-dependent fluorescence emission in heterocyclic compounds having isoquinoline backbone. J. Heterocyclic Chem. 2010, 47, 199–206. [Google Scholar] [CrossRef]
- Joshi, N.K.; Joshi, H.C.; Gahlaut, R.; Tewari, N.; Rautela, R.; Pant, S. Steady state and time-resolved fluorescence study of isoquinoline: Reinvestigation of excited state proton transfer. J. Phys. Chem. A 2012, 116, 7272–7278. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ogawa, M.; Alford, R.; Choyke, P.L.; Urano, Y. New strategies for fluorescent probe design in medical diagnostic imaging. Chem. Rev. 2010, 110, 2620–2640. [Google Scholar] [CrossRef]
- Stabile, P.; Lamonica, A.; Ribecai, A.; Guercio, G.; Castoldi, D.; Curcuruto, O. Mild, convenient and versatile Cu-mediated synthesis of N-aryl-2-imidazolidinones. Tetrahedron Lett. 2010, 51, 3232–3235. [Google Scholar] [CrossRef]
- Klapars, A.; Huang, X.; Buchwald, S.L. A general and efficient copper catalyst for the amidation of aryl halides. J. Am. Chem. Soc. 2002, 124, 7421–7428. [Google Scholar] [CrossRef]
- Balewski, Ł.; Sączewski, F.; Gdaniec, M.; Bednarski, P.J.; Jara, I. Synthesis of N-(2-pyridyl)imidazolidin-2-ones and 1-(2-pyridyl)-2,3,7,8-tetrahydro-1H-imidazo[2,1-b][1,3,5]triazepin-5(6H)-ones with potential biological activities. Heterocycl. Commun. 2013, 19, 331–341. [Google Scholar] [CrossRef]
- German, E.A.; Ross, J.E.; Knipe, P.C.; Don, M.F.; Thompson, S.; Hamilton, A.D. β--Strand Mimetic Foldamers Rigidified through Dipolar Repulsion. Angew. Chem. Int. Ed. 2015, 54, 2649–2652. [Google Scholar] [CrossRef]
- Knipe, P.C.; Thompson, S.; Hamilton, A.D. Acid-mediated topological control in a functionalized foldamer. Chem. Commun. 2016, 52, 6521–6524. [Google Scholar] [CrossRef] [PubMed]
- Young, I.S.; Glass, A.-L.; Cravillion, T.; Han, C.; Zhang, H.; Gosselin, F. Palladium-catalyzed site-selective amidation of dichloroazines. Org. Lett. 2018, 20, 3902–3906. [Google Scholar] [CrossRef] [PubMed]
- Balewski, Ł.; Sączewski, F.; Bednarski, P.J.; Gdaniec, M.; Borys, E.; Makowska, A. Structural diversity of copper(II) complexes with N-(2-pyridyl)imidazolidin-2-ones(thiones) and their in vitro antitumor activity. Molecules 2014, 19, 17026–17051. [Google Scholar] [CrossRef]
- Dey, J.; Haynes, J.L.; Warner, I.M. Fluorescence spectral study of 9-acridinecarboxylic acid and its methyl ester. Understanding the unusual fluorescence behavior of 9-anthroic acid. J. Phys. Chem. 1997, 101, 2271–2278. [Google Scholar] [CrossRef]
- Motoyoshiya, J.; Fengqiang, Z.; Nishii, Y.; Aoyama, H. Fluorescence quenching of versatile fluorescent probes based on strongly electron-donating distyrylbenzenes responsive to aromatic chlorinated and nitro compounds, boronic acid and Ca2+. Spectrochim. Acta Part A 2008, 69, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Al-Kindy, S.M.Z.; Miller, J.N. Fluorescence quenching studies of nitrated polycyclic aromatic hydrocarbons. Luminescence 2011, 26, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Eastman, J.W. Quantitative spectrofluorimetry - the fluorescence quantum yield of quinine sulfate. Photochem. Photobiol. 1967, 6, 55–72. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlisPro Software system, version 1.171.38.46; Rigaku Corporation: Oxford, UK, 2015. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT - Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
Sample Availability: None. |

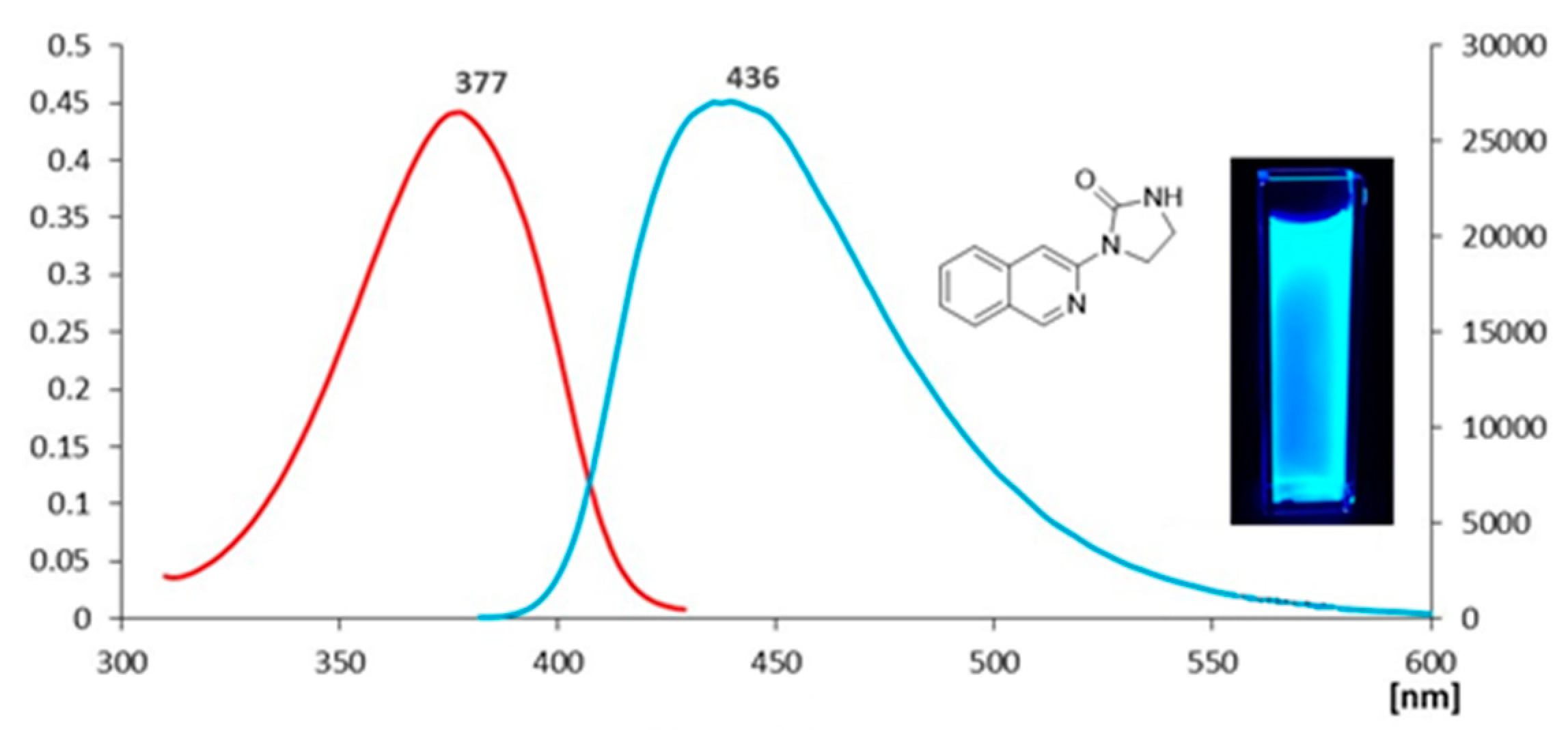
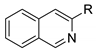
| Comp. | R | ε (M−1cm−1) | λmax abs. (nm) | λmax em. (nm) | Δυ (nm) | Φfl |
|---|---|---|---|---|---|---|
| 3a | 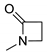 | 5066 | 368 | 425 | 57 | 0.963 |
| 3b | 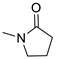 | 4744 | 363 | 417 | 54 | 0.559 |
| 3c | 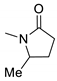 | 2955 | 356 | 417 | 61 | 0.954 |
| 3d | 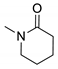 | 2541 | 340 | 416 | 76 | 0.389 |
| 3e | 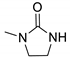 | 5083 | 377 | 436 | 59 | 0.639 |
| 3f* |  | 2032 | 328 | 383 | 55 | 0.101 |
| 5 | 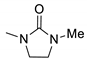 | 5271 | 380 | 448 | 68 | 0.479 |
| 7a* | 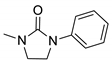 | 34,537 | 302 | 391 | 89 | 0.251 |
| 7b* | 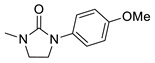 | 2251 | 344 | 391 | 47 | 0.443 |
| 7c* | 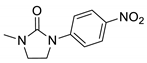 | 11,072 | 350 | nd | nd | nd |
| 7d* | 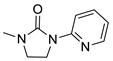 | 15,581 | 304 | 385 | 81 | 0.570 |
| 8 |  | 3826 | 406 | 495 | 89 | 2.23·10−14 |
| 9 | 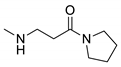 | 3705 | 406 | 494 | 88 | 0.053 |
| I-3-A** | nd | 363 | 431 | 68 | 0.28 | |
| QS*** | nd | (exc. at 350) | nd | nd | 0.577 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balewski, Ł.; Sączewski, F.; Gdaniec, M.; Kornicka, A.; Cicha, K.; Jalińska, A. Synthesis and Fluorescent Properties of Novel Isoquinoline Derivatives. Molecules 2019, 24, 4070. https://doi.org/10.3390/molecules24224070
Balewski Ł, Sączewski F, Gdaniec M, Kornicka A, Cicha K, Jalińska A. Synthesis and Fluorescent Properties of Novel Isoquinoline Derivatives. Molecules. 2019; 24(22):4070. https://doi.org/10.3390/molecules24224070
Chicago/Turabian StyleBalewski, Łukasz, Franciszek Sączewski, Maria Gdaniec, Anita Kornicka, Karolina Cicha, and Aleksandra Jalińska. 2019. "Synthesis and Fluorescent Properties of Novel Isoquinoline Derivatives" Molecules 24, no. 22: 4070. https://doi.org/10.3390/molecules24224070
APA StyleBalewski, Ł., Sączewski, F., Gdaniec, M., Kornicka, A., Cicha, K., & Jalińska, A. (2019). Synthesis and Fluorescent Properties of Novel Isoquinoline Derivatives. Molecules, 24(22), 4070. https://doi.org/10.3390/molecules24224070