Oxidized Dextran as a Macromolecular Crosslinker Stabilizes the Zein/Caseinate Nanocomplex for the Potential Oral Delivery of Curcumin
Abstract
1. Introduction
2. Results and Discussion
2.1. Particulate Properties and Colloidal Stability
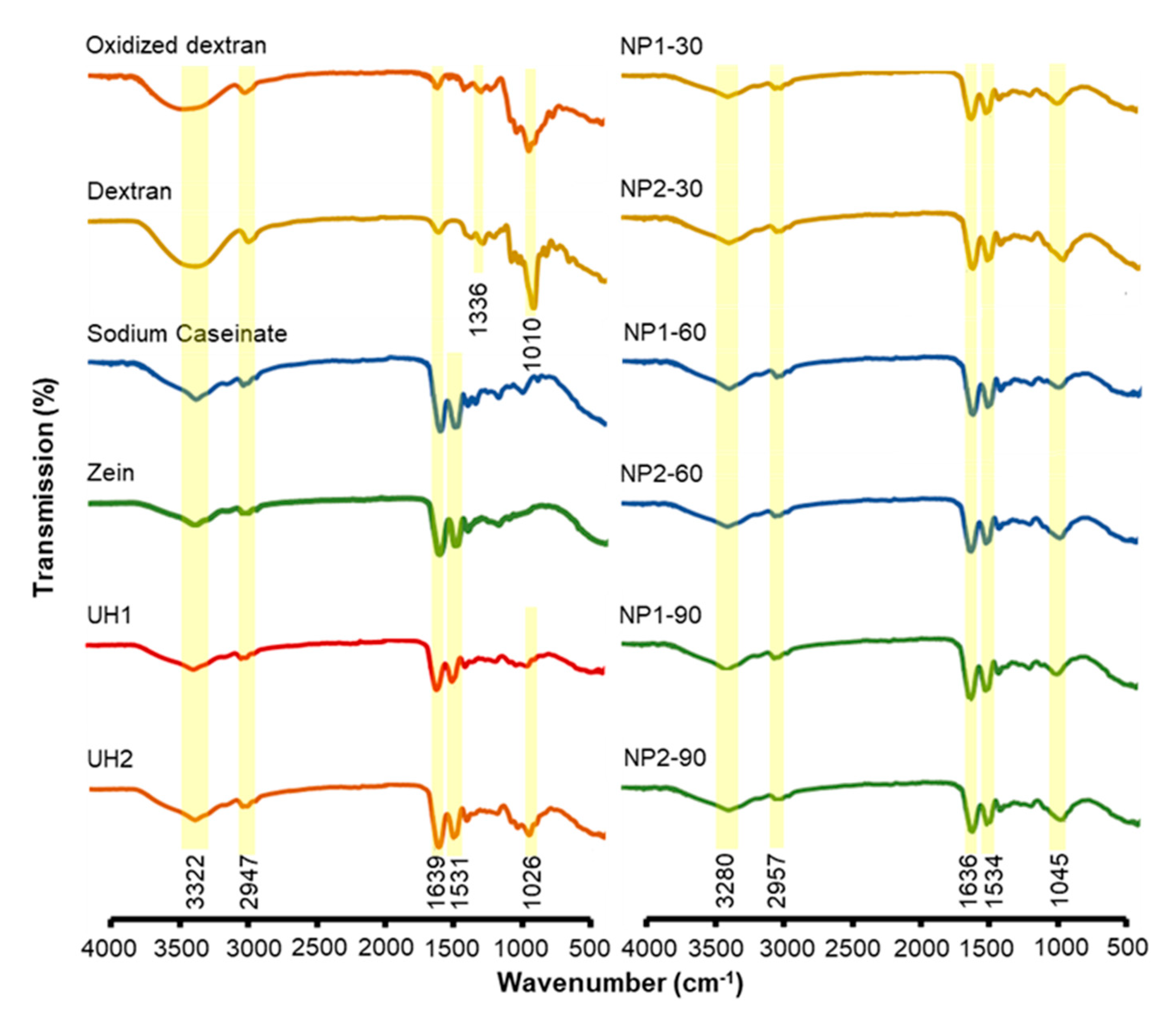
2.2. FTIR Study
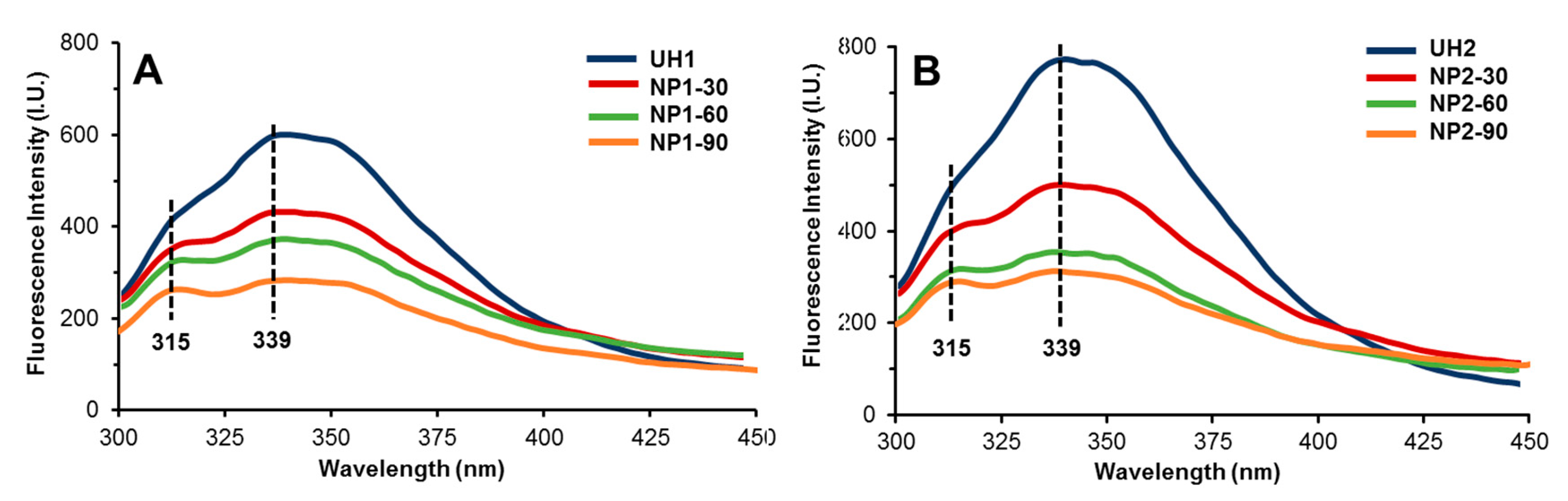
2.3. Fluorescence Study
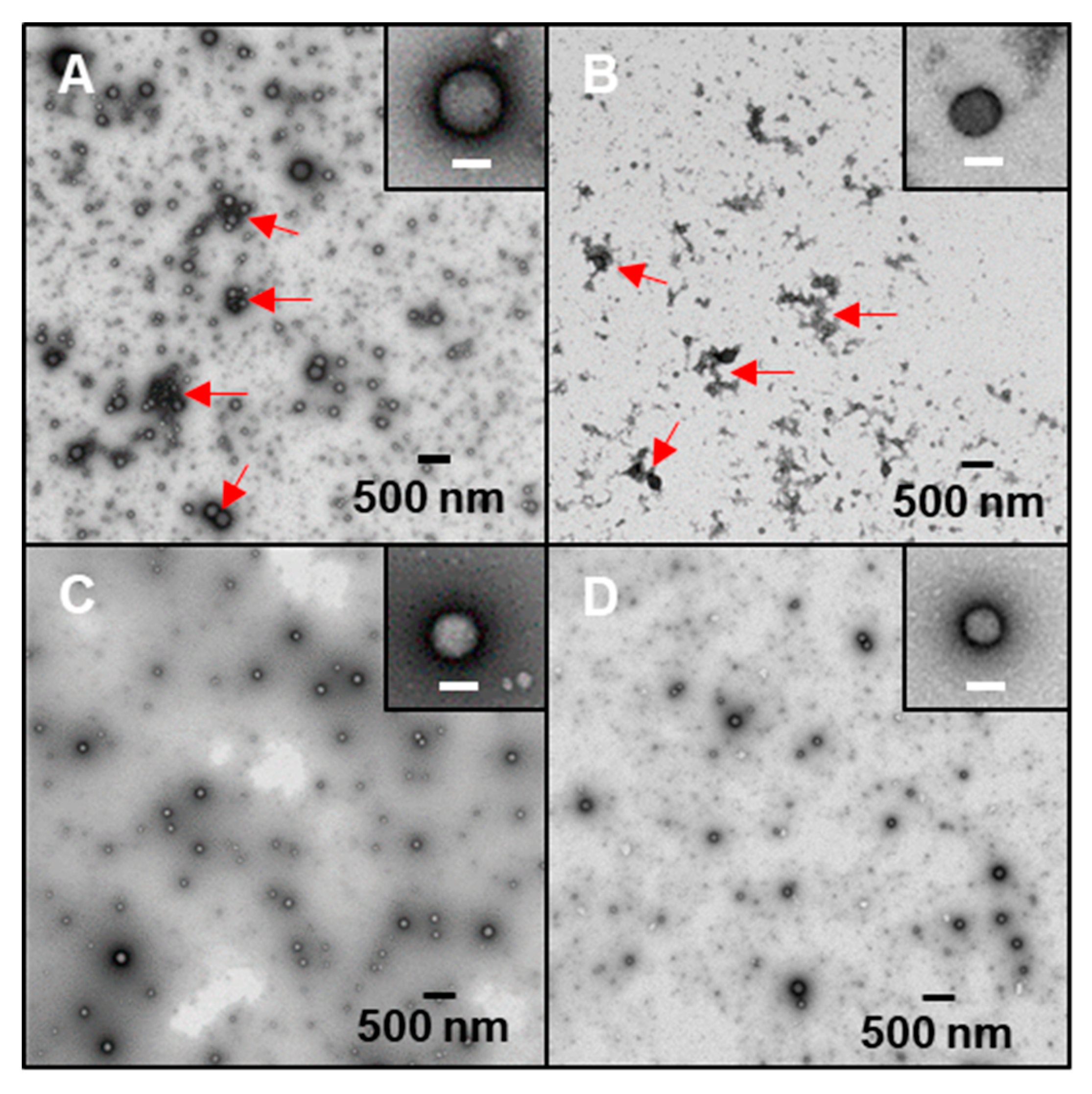
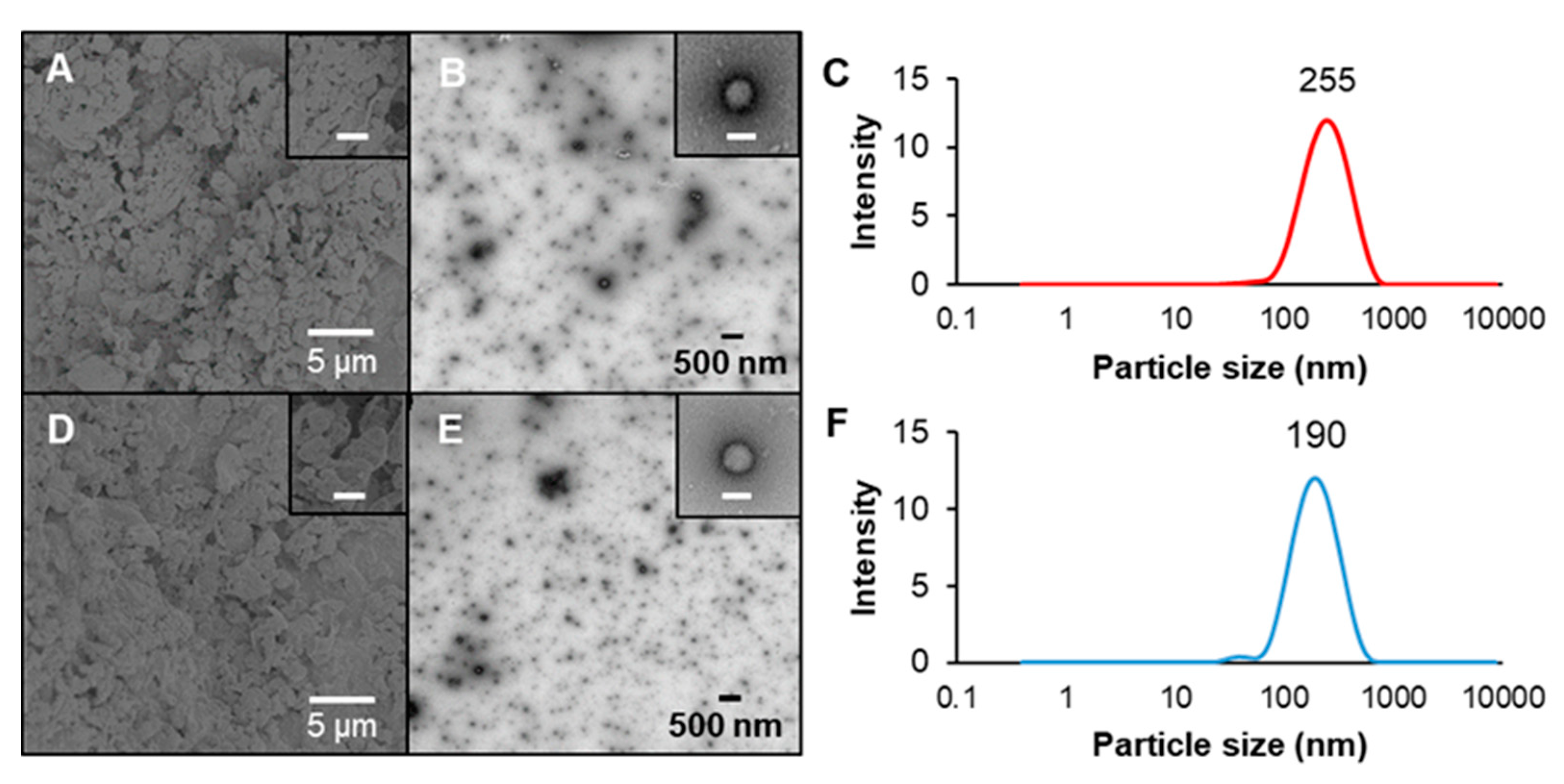
2.4. Morphology of Freshly Prepared Nanoparticles
2.5. Spray-Drying and Redispersion of Nanoparticles
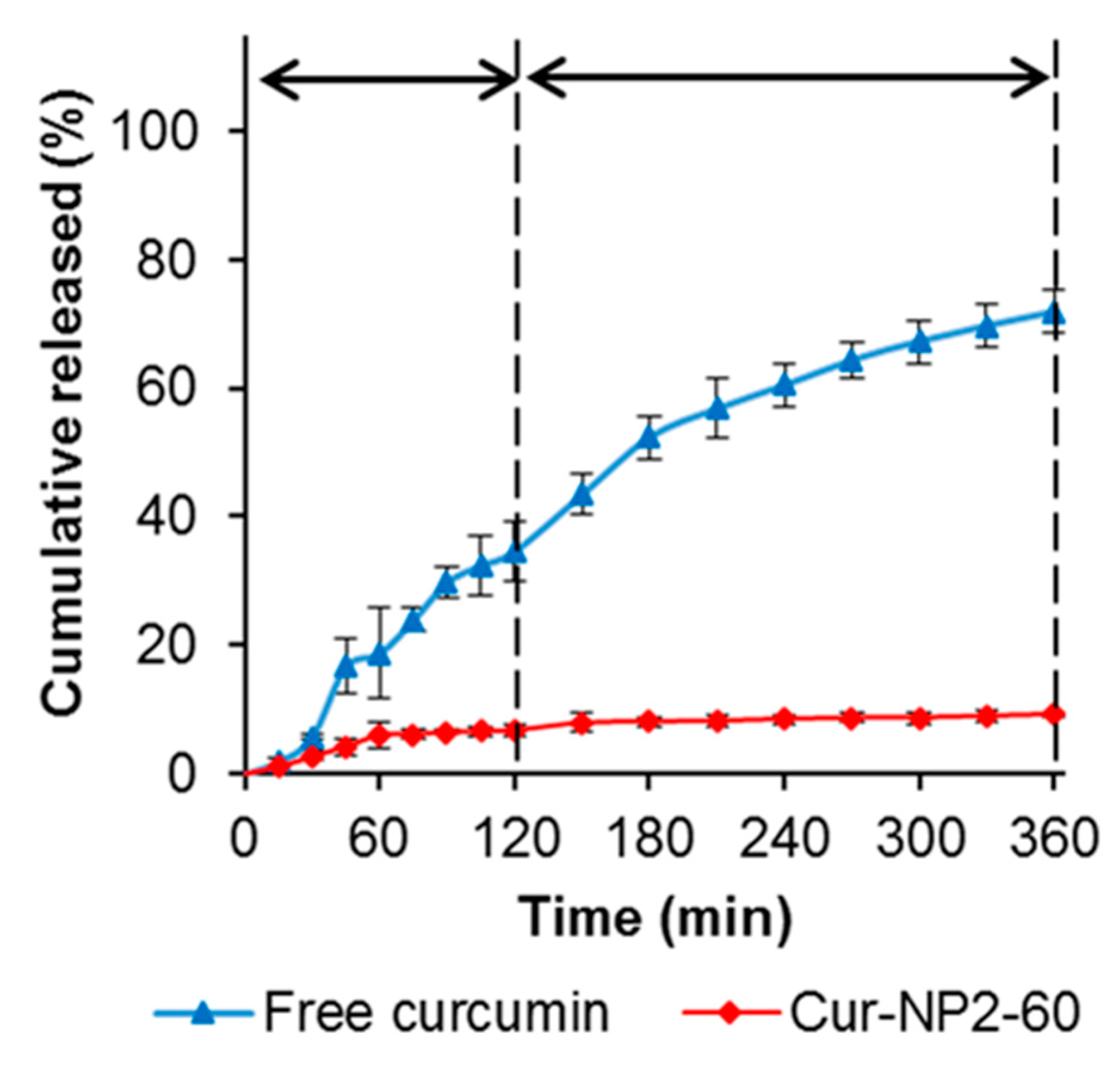
2.6. Encapsulation and Kinetic Release Profile of Curcumin
3. Materials and Methods
3.1. Materials
3.2. Preparation of Oxidized Dextran, Zein, and Sodium Caseinate
3.3. Preparation of Complex Nanoparticles
3.4. Particulate Properties
3.5. Intrinsic Fluorescence Property
3.6. Fourier Transform Infrared Spectroscopy
3.7. Stability in Simulated Gastric and Intestinal Fluid
3.8. Morphological Observation
3.9. Spray-Drying and Redispersion
3.10. Encapsulation and Release Studies
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Patel, A.R.; Velikov, K.P. Colloidal delivery systems in foods: A general comparison with oral drug delivery. LWT Food Sci. Technol. 2011, 44, 1958–1964. [Google Scholar] [CrossRef]
- Wang, T.; Luo, Y. Biological fate of ingested lipid-based nanoparticles: Current understanding and future directions. Nanoscale 2019, 11, 11048–11063. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Bae, M.; Lee, J.Y.; Luo, Y. Solid lipid-polymer hybrid nanoparticles prepared with natural biomaterials: A new platform for oral delivery of lipophilic bioactives. Food Hydrocoll. 2018, 84, 581–592. [Google Scholar] [CrossRef]
- Jones, O.G.; McClements, D.J. Recent progress in biopolymer nanoparticle and microparticle formation by heat-treating electrostatic protein–polysaccharide complexes. Adv. Colloid Interf. Sci. 2011, 167, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.H.; Emam-Djomeh, Z.; Sabatino, P.; Meeren, P.V. Nanocomplexes arising from protein-polysaccharide electrostatic interaction as a promising carrier for nutraceutical compounds. Food Hydrocoll. 2015, 50, 16–26. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Zein-based micro- and nano-particles for drug and nutrient delivery: A review. J. Appl. Polym. Sci. 2014, 131, 40696. [Google Scholar] [CrossRef]
- Bisharat, L.; Berardi, A.; Perinelli, D.R.; Bonacucina, G.; Casettari, L.; Cespi, M.; AlKhatib, H.S.; Palmieri, G.F. Aggregation of zein in aqueous ethanol dispersions: Effect on cast film properties. Int. J. Biol. Macromol. 2018, 106, 360–368. [Google Scholar] [CrossRef]
- Kasaai, M.R. Zein and zein -based nano-materials for food and nutrition applications: A review. Trends Food Sci. Technol. 2018, 79, 184–197. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, H.; Liu, C.; Zhu, J.; Xu, Y.; Zhang, S.; Fan, M.; Zhang, D.; Zhang, Y.; Zhang, Z.; et al. Fabrication of stable zein nanoparticles by chondroitin sulfate deposition based on antisolvent precipitation method. Int. J. Biol. Macromol. 2019, 139, 30–39. [Google Scholar] [CrossRef]
- Veneranda, M.; Hu, Q.; Wang, T.; Luo, Y.; Castro, K.; Madariaga, J.M. Formation and characterization of zein-caseinate-pectin complex nanoparticles for encapsulation of eugenol. LWT Food Sci. Technol. 2018, 89, 596–603. [Google Scholar] [CrossRef]
- Luo, Y.; Teng, Z.; Wang, T.T.; Wang, Q. Cellular uptake and transport of zein nanoparticles: Effects of sodium caseinate. J. Agric. Food Chem. 2013, 61, 7621–7629. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zhang, Y.; Huang, G.; Liu, J.; Slavin, M.; Yu, L.L. Zein-caseinate composite nanoparticles for bioactive delivery using curcumin as a probe compound. Food Hydrocoll. 2018, 83, 25–35. [Google Scholar] [CrossRef]
- Li, H.; Xu, Y.; Sun, X.; Wang, S.; Wang, J.; Zhu, J.; Wang, D.; Zhao, L. Stability, bioactivity, and bioaccessibility of fucoxanthin in zein-caseinate composite nanoparticles fabricated at neutral pH by antisolvent precipitation. Food Hydrocoll. 2018, 84, 379–388. [Google Scholar] [CrossRef]
- Chang, C.; Wang, T.; Hu, Q.; Luo, Y. Zein/caseinate/pectin complex nanoparticles: Formation and characterization. Int. J. Biol. Macromol. 2017, 104, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Wang, T.; Hu, Q.; Zhou, M.; Xue, J.; Luo, Y. Pectin coating improves physicochemical properties of caseinate/zein nanoparticles as oral delivery vehicles for curcumin. Food Hydrocoll. 2017, 70, 143–151. [Google Scholar] [CrossRef]
- Chang, C.; Wang, T.; Hu, Q.; Luo, Y. Caseinate-zein-polysaccharide complex nanoparticles as potential oral delivery vehicles for curcumin: Effect of polysaccharide type and chemical cross-linking. Food Hydrocoll. 2017, 72, 254–262. [Google Scholar] [CrossRef]
- Hu, Q.; Bae, M.; Fleming, E.; Lee, J.Y.; Luo, Y. Biocompatible polymeric nanoparticles with exceptional gastrointestinal stability as oral delivery vehicles for lipophilic bioactives. Food Hydrocoll. 2019, 89, 386–395. [Google Scholar] [CrossRef]
- Wang, X.; Xiong, Y.L. Oxidative polyaldehyde production: A novel approach to the conjugation of dextran with soy peptides for improved emulsifying properties. J. Food Sci. Technol. 2016, 53, 3215–3224. [Google Scholar] [CrossRef]
- Oliver, S.; Yee, E.; Kavallaris, M.; Vittorio, O.; Boyer, C. Water soluble antioxidant dextran–quercetin conjugate with potential anticancer properties. Macromol. Biosci. 2018, 18, 1700239. [Google Scholar] [CrossRef]
- Xin, Y.; Yuan, J. Schiff’s base as a stimuli-responsive linker in polymer chemistry. Polym. Chem. 2012, 3, 3045–3055. [Google Scholar] [CrossRef]
- Shang, J.; Gao, X. Nanoparticle counting: Towards accurate determination of the molar concentration. Chem. Soc. Rev. 2014, 43, 7267–7278. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Luo, Y.; Wang, Q. Carboxymethyl chitosan–soy protein complex nanoparticles for the encapsulation and controlled release of vitamin D3. Food Chem. 2013, 141, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Maia, J.; Ferreira, L.; Carvalho, R.; Ramos, M.A.; Gil, M.H. Synthesis and characterization of new injectable and degradable dextran-based hydrogels. Polymer 2005, 46, 9604–9614. [Google Scholar] [CrossRef]
- Jamwal, S.; Dautoo, U.K.; Ranote, S.; Dharela, R.; Chauhan, G.S. Enhanced catalytic activity of new acryloyl crosslinked cellulose dialdehyde-nitrilase Schiff base and its reduced form for nitrile hydrolysis. Int. J. Biol. Macromol. 2019, 131, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Acharya, D.P.; Sanguansri, L.; Augustin, M.A. Binding of resveratrol with sodium caseinate in aqueous solutions. Food Chem. 2013, 141, 1050–1054. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wan, Z.L.; Yang, X.Q.; Wang, J.M.; Guo, J.; Lin, Y. Colloidal complexation of zein hydrolysate with tannic acid: Constructing peptides-based nanoemulsions for alga oil delivery. Food Hydrocoll. 2016, 54, 40–48. [Google Scholar] [CrossRef]
- Rodea-González, D.A.; Cruz-Olivares, J.; Román-Guerrero, A.; Rodríguez-Huezo, M.E.; Vernon-Carter, E.J.; Pérez-Alonso, C. Spray-dried encapsulation of chia essential oil (Salvia hispanica L.) in whey protein concentrate-polysaccharide matrices. J. Food Eng. 2012, 111, 102–109. [Google Scholar] [CrossRef]
- Wang, T.; Hu, Q.; Zhou, M.; Xia, Y.; Nieh, M.P.; Luo, Y. Development of “all natural” layer-by-layer redispersible solid lipid nanoparticles by nano spray drying technology. Eur. J. Pharm. Biopharm. 2016, 107, 273–285. [Google Scholar] [CrossRef]
- Vega, C.; Roos, Y.H. Invited review: Spray-dried dairy and dairy-like emulsions—compositional considerations. J. Dairy Sci. 2006, 89, 383–401. [Google Scholar] [CrossRef]
- Patel, A.R.; Bouwens, E.C.M.; Velikov, K.P. Sodium caseinate stabilized zein colloidal particles. J. Agri. Food Chem. 2010, 58, 12497–12503. [Google Scholar] [CrossRef]
- Chen, H.; Zhong, Q. Processes improving the dispersibility of spray-dried zein nanoparticles using sodium caseinate. Food Hydrocoll. 2014, 35, 358–366. [Google Scholar] [CrossRef]
- Xue, J.; Wang, T.; Hu, Q.; Zhou, M.; Luo, Y. Insight into natural biopolymer-emulsified solid lipid nanoparticles for encapsulation of curcumin: Effect of loading methods. Food Hydrocoll. 2018, 79, 110–116. [Google Scholar] [CrossRef]
- Wang, T.; Ma, X.; Lei, Y.; Luo, Y. Solid lipid nanoparticles coated with cross-linked polymeric double layer for oral delivery of curcumin. Colloid Surf. B Biointerfaces 2016, 148, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xue, J.; Wusigale; Wang, T.; Hu, Q.; Luo, Y. Carboxymethylation of phytoglycogen and its interactions with caseinate for the preparation of nanocomplex. Food Hydrocoll. 2020, 100, 105390. [Google Scholar] [CrossRef]
- Amani, S.; Mohamadnia, Z.; Mahdavi, A. pH-responsive hybrid magnetic polyelectrolyte complex based on alginate/BSA as efficient nanocarrier for curcumin encapsulation and delivery. Int. J. Biol. Macromol. 2019, 141, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Huang, L.; Jin, W.; Ge, P.; Shah, B.R.; Zhu, D.; Jing, J. Encapsulation and release behavior of curcumin based on nanoemulsions-filled alginate hydrogel beads. Int. J. Biol. Macromol. 2019, 134, 210–215. [Google Scholar] [CrossRef]
- Zhou, M.; Hu, Q.; Wang, T.; Xue, J.; Luo, Y. Alginate hydrogel beads as a carrier of low density lipoprotein/pectin nanogels for potential oral delivery applications. Int. J. Biol. Macromol. 2018, 120, 859–864. [Google Scholar] [CrossRef]
- Muangsiri, W.; Kirsch, L.E. The protein-binding and drug release properties of macromolecular conjugates containing daptomycin and dextran. Int. J. Pharm. 2006, 315, 30–43. [Google Scholar] [CrossRef]
- Pan, K.; Luo, Y.; Gan, Y.; Baek, S.J.; Zhong, Q. pH-driven encapsulation of curcumin in self-assembled casein nanoparticles for enhanced dispersibility and bioactivity. Soft Matter 2014, 10, 6820–6830. [Google Scholar] [CrossRef]
- Wang, T.; Xue, J.; Hu, Q.; Zhou, M.; Luo, Y. Preparation of lipid nanoparticles with high loading capacity and exceptional gastrointestinal stability for potential oral delivery applications. J. Colloid Interface Sci. 2017, 507, 119–130. [Google Scholar] [CrossRef]
- Kakkar, V.; Singh, S.; Singla, D.; Kaur, I.P. Exploring solid lipid nanoparticles to enhance the oral bioavailability of curcumin. Mol. Nutr. Food Res. 2011, 55, 495–503. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the complex nanoparticles are available from the authors. |
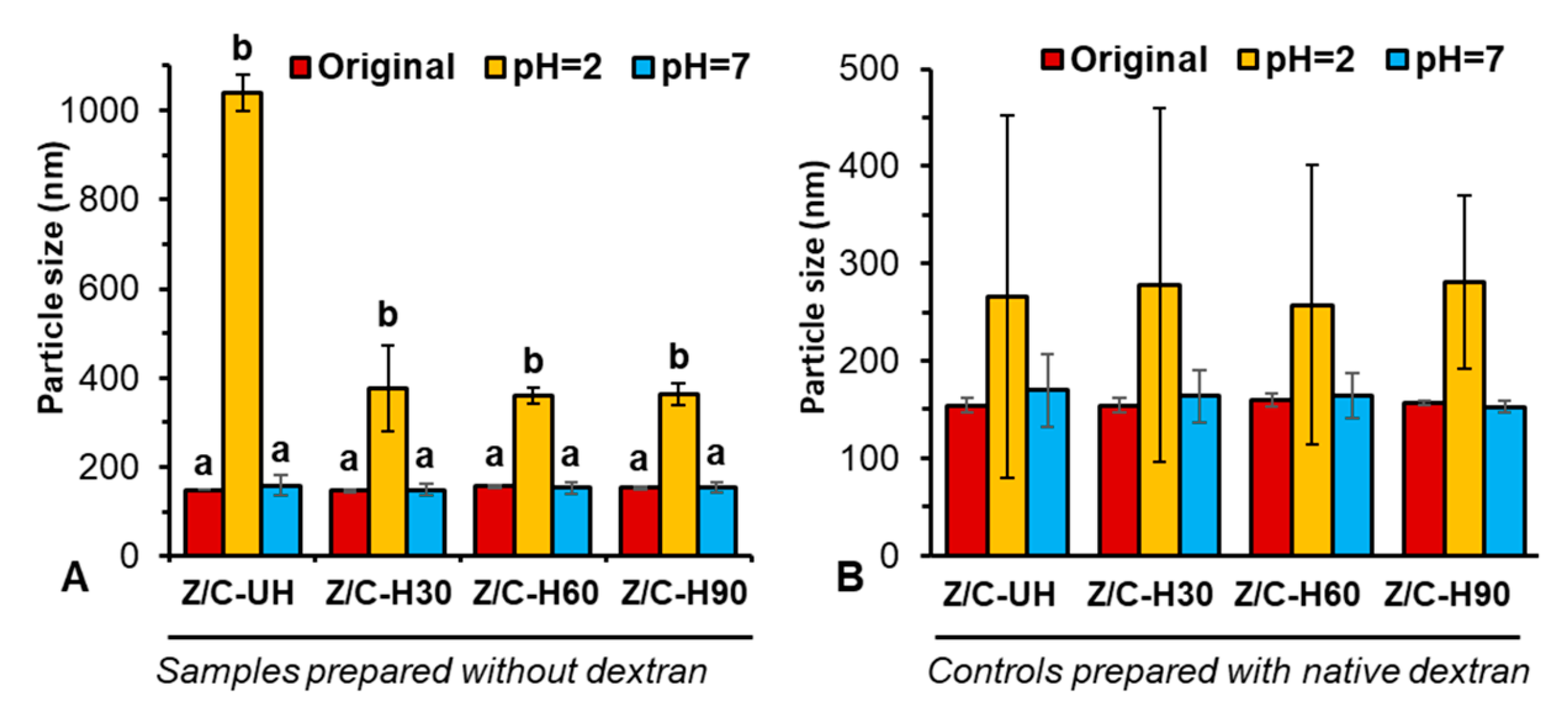
| Sample | Original | pH 2 | pH 7 | |||
|---|---|---|---|---|---|---|
| Size (nm) | PDI | Size (nm) | PDI | Size (nm) | PDI | |
| UH1 | 147 ± 8 | 0.09 ± 0.01 | 571 ± 172 | 0.48 ± 0.10 | 95 ± 2 | 0.32 ± 0.00 |
| UH2 | 152 ± 8 | 0.09 ± 0.01 | 578 ± 17 | 0.37 ± 0.02 | 94 ± 4 | 0.34 ± 0.05 |
| NP1-30 | 150 ± 3 | 0.10 ± 0.02 | 172 ± 6 | 0.34 ± 0.04 | 100 ± 4 | 0.29 ± 0.01 |
| NP2-30 | 148 ± 5 | 0.08 ± 0.02 | 163 ± 6 | 0.27 ± 0.02 | 104 ± 4 | 0.28 ± 0.01 |
| NP1-60 | 143 ± 2 | 0.08 ± 0.01 | 164 ± 14 | 0.32 ± 0.01 | 103 ± 9 | 0.29 ± 0.01 |
| NP2-60 | 150 ± 8 | 0.09 ± 0.02 | 151 ± 3 | 0.26 ± 0.03 | 105 ± 2 | 0.29 ± 0.01 |
| NP1-90 | 149 ± 4 | 0.09 ± 0.02 | 159 ± 3 | 0.29 ± 0.003 | 108 ± 7 | 0.28 ± 0.01 |
| NP2-90 | 146 ± 4 | 0.11 ± 0.01 | 150 ± 2 | 0.25 ± 0.01 | 110 ± 6 | 0.29 ± 0.00 |
| Loading Method | Sample | Size (nm) | PDI | Zeta Potential (mV) | Encapsulation Efficiency (%) |
|---|---|---|---|---|---|
| Method 1 | Cur NP1-60 | 179 ± 9 | 0.13 ± 0.03 | −46 ± 1 | 90 ± 0.3 |
| Cur-NP2-60 | 172 ± 6 | 0.13 ± 0.02 | −44 ± 5 | 95 ± 0.8 | |
| Method 2 | Cur-NP1-60 | 144 ± 3 | 0.10 ± 0.02 | −43 ± 1 | 92 ± 0.2 |
| Cur-NP2-60 | 143 ± 1 | 0.11 ± 0.02 | −50 ± 1 | 95 ± 0.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez, N.J.; Hu, Q.; Luo, Y. Oxidized Dextran as a Macromolecular Crosslinker Stabilizes the Zein/Caseinate Nanocomplex for the Potential Oral Delivery of Curcumin. Molecules 2019, 24, 4061. https://doi.org/10.3390/molecules24224061
Rodriguez NJ, Hu Q, Luo Y. Oxidized Dextran as a Macromolecular Crosslinker Stabilizes the Zein/Caseinate Nanocomplex for the Potential Oral Delivery of Curcumin. Molecules. 2019; 24(22):4061. https://doi.org/10.3390/molecules24224061
Chicago/Turabian StyleRodriguez, Nikolas J., Qiaobin Hu, and Yangchao Luo. 2019. "Oxidized Dextran as a Macromolecular Crosslinker Stabilizes the Zein/Caseinate Nanocomplex for the Potential Oral Delivery of Curcumin" Molecules 24, no. 22: 4061. https://doi.org/10.3390/molecules24224061
APA StyleRodriguez, N. J., Hu, Q., & Luo, Y. (2019). Oxidized Dextran as a Macromolecular Crosslinker Stabilizes the Zein/Caseinate Nanocomplex for the Potential Oral Delivery of Curcumin. Molecules, 24(22), 4061. https://doi.org/10.3390/molecules24224061







