Abstract
A chiral primary amine-salicylamide is used as an organocatalyst for the enantioselective conjugate addition of α,α-disubstituted aldehydes to maleimides and nitroalkenes. The reactions are performed in deep eutectic solvents as reaction media at room temperature, leading to the corresponding adducts with enantioselectivities up to 88% (for maleimides) and 80% (for nitroalkenes). Catalyst and solvent can be recovered and reused.
1. Introduction
Currently, traditional volatile organic compounds (VOCs) are the common solvents for performing organic reactions although, from an environmental point of view, they show many intrinsic drawbacks, such as accumulation in the atmosphere due to their low boiling points, flammability, toxicity and non-biodegradability. For all these reasons several greener and friendlier synthetic methodologies based on alternative reaction media have been developed, all of them having certain number of advantages, as well as disadvantages [1,2].
Among these alternative reaction media, deep eutectic solvents (DESs) are attracting a growing interest [3]. A DES is a combination of two or three compounds, Lewis or Brønsted acids and bases containing a variety of anionic and/or cationic species, which interacts through hydrogen bonds forming a eutectic mixture with a melting point lower than the individual components [3]. DESs are non-volatile, show a low ecological footprint, are economical, essentially nontoxic and easily recyclable, therefore research concerning their use as environmentally friendly neoteric solvents in organic synthesis is growing rapidly [4,5,6,7,8,9,10,11].
In addition, probably the most attractive methodology for the enantioselective preparation of functionalized molecules in organic synthesis is the use of asymmetric organocatalysis, since metal-free small organic compounds are used as catalysts under usually very mild and simple reaction conditions [12,13]. This methodology maintains sustainability in organic synthesis and provides many advantages, such as accessibility, inexpensive catalysts and reduced toxicity. However, most of these asymmetric processes are carried out using environmentally unfriendly VOCs as reaction media, although efforts have been devoted to achieving more sustainable synthetic procedures [14].
Therefore, the combination of asymmetric organocatalysis and the use of DESs as solvents would show quite powerful and promising in order to achieve the enantioselective preparation of compounds of interest in an environmentally friendly manner. However, the application of DESs in asymmetric organocatalysis is still in its infancy, very few examples being reported (Figure 1). Thus, enantioselective aldol reactions have been performed in DESs using the amino acids L-proline (1) [15,16] and L-isoleucine (2) [17] as organocatalysts, as well as a combination of diaryl prolinols 3 [18] or 4 [19] and an enzyme. In addition, some enantioselective conjugate additions have been performed in DESs, such as the reaction of isobutyraldehyde and β-nitrostyrene and other conjugate addition reactions using 9-amino-9-deoxy-epi-quinine (5) as catalyst [20], the reaction of 1,3-dicarbonyl compounds with β-nitrostyrenes organocatalyzed by 2-amino benzimidazole 6 [21], and the reaction of aldehydes and maleimides organocatalyzed by primary-amine monocarbamate 7 [22]. Moreover, benzimidazole ent-6 has also been used as catalyst for the α-amination of 1,3-dicarbonyl compounds in DESs [23].
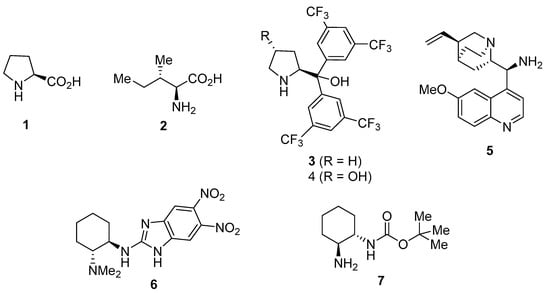
Figure 1.
Chiral organocatalysts employed in enantioselective reactions performed in deep eutectic solvents (DESs).
Recently, our group has employed a simple chiral primary amine-monosalicylamide from trans-cyclohexane-1,2-diamine 8 (Figure 2) and ent-8 as organocatalysts in the enantioselective Michael addition of the “difficult” α,α-disubstituted aldehydes to maleimides [24] and β-nitroalkenes [25], obtaining the corresponding succinimides and γ-nitroaldehydes, respectively, in excellent chemical yields and enantioselections, but working in conventional VOCs as reaction media. The enantioselective preparation of succinimides and γ-nitroaldehydes shows interest, as the succinimide moiety is present in natural products and drug candidates [26,27,28], and also can be transformed into interesting compounds such as γ-lactams [29], which are important in the treatment of HIV [30] and neurological disorders [31,32]. In addition, γ-nitroaldehydes are precursors of γ-aminobutyric acid analogues (GABAs), which exhibit many pharmacological activities including antidepressant, anticonvulsant, anxiolytic and others [33,34], as well as can be potent drugs in the treatment of neurodegenerative disorders [35].
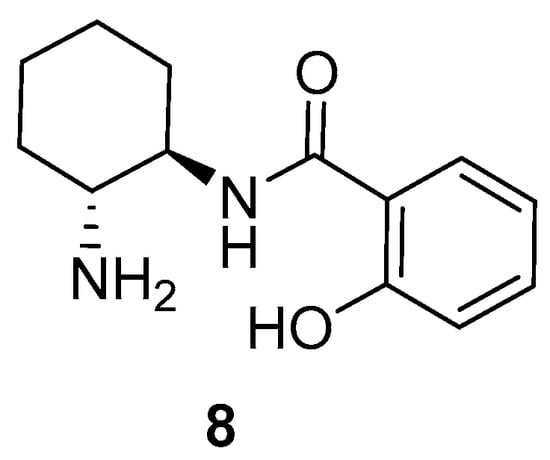
Figure 2.
Organocatalyst employed in this study.
Therefore, the asymmetric preparation of these compounds using 8 as catalyst would gain considerably from an environmentally point if the mentioned preparations could be performed in DESs. Thus, we present now the enantioselective addition of aldehydes to maleimides and nitroalkenes organocatalyzed by 8 using DESs as reaction media.
2. Results and Discussion
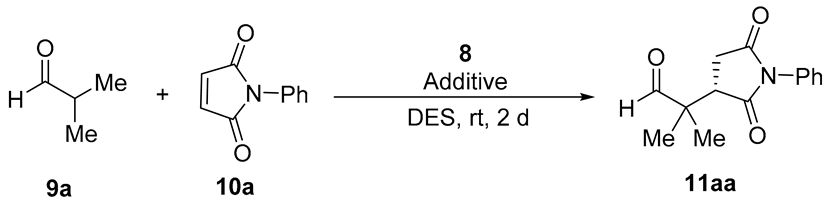
The primary amine-salicylamide 8 was prepared as described by monoamidation of (1R,2R)-cyclohexane-1,2-diamine with phenyl salicylate in refluxing propan-2-ol [25]. Initially, we explored the reaction of aldehydes with maleimides in DESs, using the model conjugate addition of isobutyraldehyde (9a) to N-phenylmaleimide (10a) (Table 1).

Table 1.
Screening and optimization of the reaction conditions for the model enantioselective conjugate addition of isobutyraldehyde to N-phenylmaleimide in DESs organocatalyzed by 8.
Thus, the reaction organocatalyzed by 8 (10 mol%) carried out in several choline chloride (ChCl)-containing DESs (1:2 molar ratio) at room temperature afforded the corresponding substituted succinimide (S)-11aa after 2 d reaction time (Table 1, entries 1–4). The (S) absolute configuration of the final adduct was determined by comparison of the elution order of the corresponding enantiomers in chiral HPLC with those in the literature [36]. The DES resulting in a higher enantioselection was the formed by the mixture 1ChCl/2EG (EG = ethylene glycol), which gave 11aa in 80% ee (Table 1, entry 3). The use of a mixture of 1Ph3MePBr/2Gly as DES gave a similar enantioselectivity, but slightly lower conversion (Table 1, entry 5). In addition, we also assayed the influence of the addition of some acid or basic additives. Thus, acid additives improved the enantioselectivity of the reaction (Table 1, entries 6–9), the best ee for 11aa (88%) being obtained when 4-nitrobenzoic acid was used (Table 1, entry 8). The addition of a basic additive such as imidazole gave very similar results, but the addition of 4-(dimethylamino)pyridine (DMAP) gave rise to decomposition products. We also increased and lowered the loading of organocatalyst and the best additive but without achieving better results (Table 1, entries 12–14).
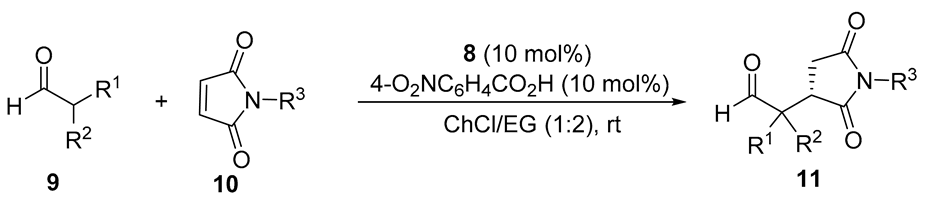
Next, we extended this enantioselective reaction to other maleimides 10 under the best conditions [8 (10 mol%), 4-O2NC6H4CO2H (10 mol%), 1ChCl/2EG, rt], the results being summarized in Table 2. The absolute configuration of the known succinimides 11 was assigned in accordance with the elution order of the enantiomers in chiral HPLC (see the Experimental Section). Thus, when 9a reacted with N-aryl maleimides 10b and 10c bearing electron-donating groups on the phenyl ring, such as 4-methyl and 4-methoxy, adducts 11ab and 11ac were obtained in 66 and 86% ee, respectively (Table 2, entries 2 and 3). When N-aryl maleimides 10d and 10e, bearing chloro and bromo groups at the para-position, were used, succinimides 11ad and 11ae were isolated in similar 81 and 78% ee, respectively (Table 2, entries 4 and 5). Interestingly, the reaction of isobutyraldehyde with maleimides non-N-arylated such as N-methylmaleimide (10f) or even the simple maleimide (10g), gave rise to the corresponding succinimides 11af and 11ag in almost quantitative yield and with enantioselectivities of 78 and 73%, respectively (Table 2, entries 6 and 7). We also employed cyclohexanecarbaldehyde (9b) as reacting aldehyde with N-phenylmaleimide (10a), although isolated yield and enantioselectivity for the corresponding adduct 11ba diminished (Table 2, entry 8). Moreover, we also explored the performance of organocatalyst 7 (Figure 1), instead of 8, in the case of entry 1 (Table 2), but we obtained adduct 11aa in only 73% ee.

Table 2.
Enantioselective conjugate addition of aldehydes to maleimides organocatalyzed by 8 in a DES.
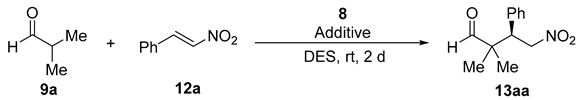
Next, we explored the suitability of DESs as reaction media in the reaction of isobutyraldehyde (9a) with β-nitrostyrene (12a) organocatalyzed by 8 (10 mol%) to give γ-nitroaldehyde 13aa (Table 3). Thus, when ChCl-including mixtures were employed as DESs, adduct 13aa was obtained (Table 3, entries 1–4), the (R) absolute configuration of the final adduct being determined by comparison of the elution order of the corresponding enantiomers in chiral HPLC with those in the literature [37]. However, the results were rather disappointing, the conversions being low-to-very low. The conversion rose up dramatically when employing as DES the mixture 1Ph3MePBr/2Gly, but the enantioselection was very poor (Table 3, entry 5). The addition of different acid or basic additives when the DESs 1ChCl/2Urea, 1ChCl/2Gly or 1ChCl/2EG were used did not give higher conversions, whereas the addition of additives when the DES 1Ph3MePBr/2Gly was employed did not afford a higher enantioselectivity. However, in the case of using 1ChCl/2H2O, we observed that the presence of basic additives certainly had an influence on the conversion and the enantioselectivity to 13aa (Table 3, entries 6–8). Thus, the use of 1,4-diazabicyclo[2.2.2]octane (DABCO) (10 mol%) as basic additive gave rise to quantitative conversion to 13aa with 55% ee (Table 3, entry 7), whereas the use of DMAP (10 mol%) afforded also quantitative conversion and 75% ee (Table 3, entry 8). On the contrary, the addition of acid additives such as benzoic acid or hexanedioic acid (HDA) lowered down the conversion dramatically (Table 3, entries 9 and 10). Finally, lowering or increasing the amount of organocatalyst 8 and DMAP gave a lower conversion or enantioselection (Table 3, entries 11 and 12).

Table 3.
Screening and optimization of the reaction conditions for the model enantioselective conjugate addition of isobutyraldehyde to trans-β-nitrostyrene in DES organocatalyzed by 8.
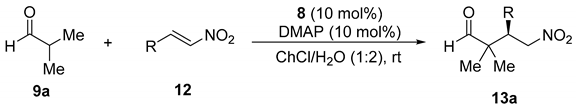
Using the above obtained more efficient reaction conditions [8 (10 mol%), DMAP (10 mol%), 1ChCl/2H2O, rt], we explored the use of other β-nitroolefins in the enantioselective conjugate addition reaction with isobutyraldehyde (Table 4). The absolute configuration of the known γ-nitroaldehydes 13a was assigned in accordance with the elution order of the enantiomers in chiral HPLC when compared to the literature (see the Experimental Section).

Table 4.
Enantioselective conjugate addition of isobutyraldehyde to nitroalkenes organocatalyzed by 8 in a DES.
Thus, the reaction of 9a with nitroalkenes 12b and 12c, bearing electron-releasing groups such as 4-methyl or 4-methoxy in the aromatic ring, were used, the corresponding adducts 13ab and 13ac were isolated with similar enantioselectivities (Table 4, entries 2 and 3). The presence of a dioxolane system on the aromatic ring in the nitroolefin 12d raised the enantioselection up to 75% (Table 4, entry 4), whereas this was lowered down to 68% when 12e containing three methoxy groups was used as electrophile (Table 2, entry 5). When a 4-fluoro group was present (12f) the corresponding 13af was isolated in 72% ee, whereas a chloro group at 2-position (12g) afforded adduct 13ag in 80% ee (Table 4, entries 6 and 7). The presence of a 4-chloro (12h) gave 13ah in only a 53% ee (Table 4, entry 8), whereas the presence of bromo groups in 2- and 4- position gave the corresponding products with enantioselectivities of 80 and 70%, respectively (Table 4, entries 9 and 10). In addition, an electron-withdrawing group such as the 4-trifluoromethyl (12k) gave a 70% enantioselectivity for 13ak (Table 4, entry 11). Moreover, when the nitroalkene 12l, bearing a 2-naphthyl group, was employed as Michael acceptor, the corresponding adduct 13al was obtained in 75% ee (Table 4, entry 12), whereas the use of a 2-furanyl-containing nitroalkene 12m gave rise to adduct 13am in 76% ee (Table 4, entry 13). Furthermore, we also explored the behavior of organocatalyst 7 (Figure 1) when used instead of 8 in the reaction described in entry 1 (Table 4) but adduct 13aa was obtained in a lower 69% ee.
The possibility of reusing the DES is very important in a synthetic methodology performed using these neoteric solvents. Therefore, we explored the reusability of the DES, and the catalytic system, by carrying out different reaction cycles of the model conjugate addition reactions performed under the best reaction conditions depicted in entry 1 of Table 2 and Table 4.
Thus, we explored the reusability of the system in the model reaction of isobutyraldehyde (9a) and N-phenylmaleimide (10a) (Table 2, entry 1). Once the reaction was finished, 2-methyltetrahydrofuran (2-MeTHF) was added and the resulting mixture was stirred vigorously. After the two layers settled down, the upper layer, containing the final adduct 11aa, was separated. 1H NMR analysis of this crude revealed that the 4-nitrobenzoic acid used as additive, as well as a small amount of ethylene glycol, were also extracted from the DES, although no traces of the organocatalyst were observed. Refreshing the catalytic system by the addition of new additive (but no new chiral organocatalyst 8) to the recovered DES allowed to obtain 11aa in a second reaction cycle with similar conversion and identical enantioselectivity than when used for the first time. Following this recovery procedure, the DES containing the organocatalyst 8 was used in the third cycle, but the conversion diminished, although the enantioinduction remained similar (Table 5).

Table 5.
Recycle experiments in the reaction of 9a and 10a. Conversions and ee’s of 11aa after consecutive reaction cycles.
In addition, we also explored the reusability of the system DES/organocatalyst in the model reaction of isobutyraldehyde (9a) and β-nitrostyrene (12a) in 1ChCl/2H2O (Table 4, entry 1). Thus, performing a similar extraction than in the previous case using 2-MeTHF, we could also observe (1H NMR) the necessity of adding new additive (DMAP) after the first reaction cycle, but no leaching of organocatalyst 8 was detected. Again, refreshing the catalytic system by the addition of new DMAP as additive (but no new 8) to the recovered DES, allowed to obtain 13aa in a second reaction cycle with identical enantioselectivity than in the first one (Table 6). The DES containing the organocatalyst 8 was reused in an additional cycle with a decrease in the conversion but essentially without diminishing the achieved enantioinduction (Table 6). The reason for the observed decrease in this case (and in the former) is not totally clear. Probably, the structure of the DES results somehow degraded in the extraction/recovery process.

Table 6.
Recycle experiments in the reaction of 9a and 12a. Conversions and ee’s of 13aa after consecutive reaction cycles.
3. Experimental Section
3.1. General Information
All the reagents and solvents employed were of the best grade available and were used without further purification. Isobutyraldehyde was distilled. Organocatalyst 8 was obtained as reported [25]. Nitroolefins 12 were purchased or prepared according to a described procedure [38]. The 1H and 13C spectra were recorded at room temperature on a Bruker Oxford (Bruker, Billerica, MA, USA) AV300 at 300 MHz and on a Bruker Oxford AV400 at 101 MHz, respectively, using TMS as internal standard. Absolute configurations for adducts 11 and 13a were determined according to the order of elution of their enantiomers in chiral HPLC. Reference racemic samples of adducts 11 and 13a were obtained by performing the conjugate addition reactions using 4-methoxybenzylamine (20 mol%) as organocatalyst in toluene as solvent at room temperature.
3.2. General Procedure for the Preparation of DESs
A mixture of the two components, with the specified molar ratio, was added to a round bottom flask and the mixture was stirred for 60 min in a temperature range between 65 and 80 °C, obtaining the corresponding DES [39].
3.3. General Procedure for the Enantioselective Conjugate Addition of Aldehydes to Maleimides
To a mixture of catalyst 8 (4.7 mg, 0.02 mmol), 4-nitrobenzoic acid (3.3 mg, 0.02 mmol) and maleimide 10 (0.2 mmol) in ChCl/EG (1/2 molar ratio, 0.5 mL) was added the aldehyde 9 (0.4 mmol), and the reaction was vigorously stirred at rt until completion (TLC) (Table 2). After this period, HCl 2N (10 mL) was added and the reaction product was extracted with AcOEt (3 × 10 mL). The combined organic phases were washed with saturated NaHCO3 (10 mL) and brine (10 mL), dried over MgSO4 and, after filtration, the solvent was evaporated under reduced pressure (15 torr) to get the crude product, which was purified by flash column chromatography on silica gel (n-hexane/AcOEt gradients). The adducts 11 were identified by comparison of their NMR data with those of the literature (Supplementary Materials, NMR spectra). Their enantiomeric excesses were determined by chiral HPLC on the reaction crude, using the conditions described in each case (Supplementary Materials, HPLC chromatograms).
2-(2,5-Dioxo-1-phenylpyrrolidin-3-yl)-2-methylpropanal (11aa) [36]. White solid (48 mg, 98%); 1H NMR (CDCl3): δH = 9.52 (s, 1H), 7.51−7.43 (m, 2H), 7.42–7.36 (m, 1H), 7.31−7.26 (m, 2H), 3.15 (dd, J = 9.5, 5.5 Hz, 1H), 2.98 (dd, J = 18.3, 9.5 Hz, 1H), 2.62 (dd, J = 18.3, 5.5 Hz, 1H), 1.33 (s, 3H), 1.29 (s, 3H) ppm; 13C NMR (CDCl3): δC = 202.9, 177.0, 174.9, 131.9, 129.3, 128.9, 126.7, 48.7, 45.1, 32.0, 20.5, 19.8 ppm; HPLC: Chiralcel OD-H, λ = 240 nm, n-hexane/2-propanol, 80:20, 1.0 mL/min, tr (major) = 25.0 min, tr (minor) = 30.6 min.
2-(2,5-Dioxo-1-(p-tolyl)pyrrolidin-3-yl)-2-methylpropanal (11ab) [36]. White solid (46 mg, 88%); 1H NMR (CDCl3): δH = 9.49 (s, 1H), 7.31−7.22 (m, 2H), 7.17−7.08 (m, 2H), 3.12 (dd, J = 9.5, 5.5 Hz, 1H), 2.93 (dd, J = 18.3, 9.5 Hz, 1H), 2.57 (dd, J = 18.3, 5.5 Hz, 1H), 2.37 (s, 3H), 1.28 (s, 3H), 1.24 (s, 3H) ppm; 13C NMR (CDCl3): δC = 202.9, 177.1, 175.0, 138.8, 129.9, 129.2, 126.4, 48.5, 45.0, 31.8, 21.2, 20.3, 19.4 ppm; HPLC: Chiralcel OD-H, λ = 230 nm, n-hexane/2-propanol, 80:20, 1.0 mL/min, tr (major) = 19.4 min, tr (minor) = 22.6 min.
2-(1-(4-Methoxyphenyl)-2,5-dioxopyrrolidin-3-yl)-2-methylpropanal (11ac) [40]. White solid (54 mg, 98%); 1H NMR (CDCl3): δH = 9.52 (s, 1H), 7.23−7.14 (m, 2H), 7.02−6.93 (m, 2H), 3.82 (s, 3H), 3.14 (dd, J = 9.5, 5.4 Hz, 1H), 2.97 (dd, J = 18.2, 9.5 Hz, 1H), 2.60 (dd, J = 18.2, 5.4 Hz, 1H), 1.32 (s, 3H), 1.28 (s, 3H) ppm; 13C NMR (CDCl3): δC = 202.9, 177.3, 175.2, 159.7, 127.9, 124.5, 114.6, 55.6, 48.6, 45.1, 31.9, 20.4, 19.7 ppm; HPLC: Chiralpak AS-H, λ = 240 nm, n-hexane/2-propanol, 80:20, 1.0 mL/min, tr (major) = 31.0 min, tr (minor) = 34.8 min.
2-(1-(4-Chlorophenyl)-2,5-dioxopyrrolidin-3-yl)-2-methylpropanal (11ad) [36]. White solid (33 mg, 59%); 1H NMR (CDCl3): δH = 9.49 (s, 1H), 7.51−7.38 (m, 2H), 7.31−7.20 (m, 2H), 3.11 (dd, J = 9.5, 5.4 Hz, 1H), 2.97 (dd, J = 18.1, 9.5 Hz, 1H), 2.61 (dd, J = 18.1, 5.4 Hz, 1H), 1.36 (s, 3H), 1.29 (s, 3H) ppm; 13C NMR (CDCl3): δC = 202.8, 176.8, 174.6, 134.6, 130.4, 129.5, 127.9, 48.8, 45.1, 32.1, 20.6, 20.0 ppm; HPLC: Chiralcel OD-H, λ = 230 nm, n-hexane/2-propanol, 80:20, 1.0 mL/min, tr (major) = 21.2 min, tr (minor) = 35.6 min.
2-(1-(4-Bromophenyl)-2,5-dioxopyrrolidin-3-yl)-2-methylpropanal (11ae) [36]. White solid (55 mg, 85%); 1H NMR (CDCl3): δH = 9.48 (s, 1H), 7.64−7.55 (m, 2H), 7.23−7.14 (m, 2H), 3.11 (dd, J = 9.5, 5.5 Hz, 1H), 2.97 (dd, J = 18.2, 9.5 Hz, 1H), 2.61 (dd, J = 18.2, 5.5 Hz, 1H), 1.35 (s, 3H), 1.28 (s, 3H) ppm; 13C NMR (CDCl3): δC = 202.8, 176.7, 174.5, 132.5, 130.9, 128.2, 122.7, 48.8, 45.1, 32.1, 20.6, 20.0 ppm; HPLC: Chiralcel OD-H, λ = 240 nm, n-hexane/2-propanol, 80:20, 1.0 mL/min, tr (major) = 22.2 min, tr (minor) = 34.9 min.
2-Methyl-2-(1-methyl-2,5-dioxopyrrolidin-3-yl)propanal (11af) [22]. White solid (36 mg, 98%); 1H NMR (CDCl3): δH = 9.51 (s, 1H), 3.05 (dd, J = 9.2, 5.2 Hz, 1H), 2.99 (s, 3H), 2.83 (dd, J = 18.2, 9.2 Hz, 1H), 2.45 (dd, J = 18.2, 5.2 Hz, 1H), 1.22 (s, 3H), 1.21 (s, 3H) ppm; 13C NMR (CDCl3): δC = 202.9, 177.9, 175.9, 48.0, 45.1, 31.5, 24.9, 20.1, 19.2 ppm; HPLC: Chiralpak AS-H, λ = 210 nm, n-hexane/2-propanol, 80:20, 1.0 mL/min, tr (minor) = 11.5 min, tr (major) = 12.5 min.
2-(2,5-Dioxopyrrolidin-3-yl)-2-methylpropanal (11ag) [22]. White solid (33 mg, 98%); 1H NMR (CDCl3): δH = 9.49 (s, 1H), 9.03 (br. s, 1H), 3.10 (dd, J = 7.7, 5.5 Hz, 1H), 2.85 (dd, J = 18.3, 7.7 Hz, 1H), 2.50 (dd, J = 18.3, 5.5 Hz, 1H), 1.24 (s, 3H), 1.23 (s, 3H) ppm; 13C NMR (CDCl3): δC = 203.0, 178.5, 176.4, 48.1, 46.4, 32.9, 20.2, 19.4 ppm; HPLC: Chiralpak AD-H, λ = 210 nm, n-hexane/2-propanol, 80:20, 1.0 mL/min, tr (minor) = 16.8 min, tr (major) = 21.2 min.
1-(2,5-Dioxo-1-phenylpyrrolidin-3-yl)cyclohexane-1-carbaldehyde (11ba) [36]. White solid (36 mg, 63%); 1H NMR (CDCl3): δH = 9.54 (s, 1H), 7.56−7.33 (m, 3H), 7.33−7.26 (m, 2H), 3.22 (dd, J = 9.4, 6.0 Hz, 1H), 2.88 (dd, J = 18.1, 9.4 Hz, 1H), 2.68 (dd, J = 18.1, 6.0 Hz, 1H), 1.96 (m, 2H), 1.60 (m, 8H) ppm; 13C NMR (CDCl3): δC = 204.7, 177.2, 174.9, 132.1, 129.3, 128.8, 126.8, 52.3, 42.9, 31.7, 28.8, 28.3, 25.3, 21.6, 21.4 ppm; HPLC: Chiralcel OD-H, λ = 240 nm, n-hexane/2-propanol, 80:20, 1.0 mL/min, tr (major) = 23.9 min, tr (minor) = 30.9 min.
3.4. General Procedure for the Enantioselective Conjugate Addition of Isobutyraldehyde to Nitroalkenes
To a mixture of catalyst 8 (4.7 mg, 0.02 mmol), DMAP (2.4 mg, 0.02 mmol) and nitroalkene 12 (0.2 mmol) in ChCl/H2O (1/2 molar ratio, 0.5 mL) was added isobutyraldehyde 9a (37 µL, 28.8 mg, 0.4 mmol), and the reaction was vigorously stirred at rt until completion (TLC) (Table 4). After this period, HCl 2N (10 mL) was added and the reaction product was extracted with AcOEt (3 × 10 mL). The combined organic phases were washed with saturated NaHCO3 (10 mL) and brine (10 mL), dried over MgSO4 and, after filtration, the solvent was evaporated under reduced pressure (15 torr) to get the crude product, which was purified by flash column chromatography on silica gel (n-hexane/AcOEt gradients). The adducts 13a were identified by comparison of their NMR data with those of the literature (Supplementary Materials, NMR spectra). Their enantiomeric excesses were determined by chiral HPLC on the reaction crude, using the conditions described in each case (Supplementary Materials, HPLC chromatograms).
2,2-Dimethyl-4-nitro-3-phenylbutanal (13aa) [37]. Yellow oil (41 mg, 92%); 1H NMR (CDCl3): δH = 9.53 (s, 1H), 7.39−7.28 (m, 3H), 7.24−7.15 (m, 2H), 4.86 (dd, J = 13.0, 11.2 Hz, 1H), 4.69 (dd, J = 13.0, 4.3 Hz, 1H), 3.78 (dd, J = 11.2, 4.3 Hz, 1H), 1.14 (s, 3H), 1.01 (s, 3H) ppm; 13C NMR (CDCl3): δC = 204.4, 135.5, 129.2, 128.9, 128.3, 76.5, 48.7, 48.4, 21.8, 19.1 ppm; HPLC: Chiralcel OD-H, λ = 210 nm, n-hexane/2-propanol, 80:20, 1.0 mL/min, tr (major) = 12.2 min, tr (minor) = 17.4 min.
2,2-Dimethyl-4-nitro-3-(p-tolyl)butanal (13ab) [41]. Yellow oil (28 mg, 60%); 1H NMR (CDCl3): δH = 9.53 (s, 1H), 7.18–7.03 (m, 4H), 4.83 (dd, J = 12.9, 11.3 Hz, 1H), 4.67 (dd, J = 12.9, 4.3 Hz, 1H), 3.74 (dd, J = 11.3, 4.3 Hz, 1H), 2.32 (s, 3H), 1.13 (s, 3H), 1.00 (s, 3H) ppm; 13C NMR (CDCl3): δC = 204.5, 138.1, 132.4, 129.6, 129.1, 76.6, 48.5, 48.4, 21.8, 21.2, 19.1 ppm; HPLC: Chiralcel OD-H, λ = 240 nm, n-hexane/2-propanol, 80:20, 1.0 mL/min, tr (major) = 9.2 min, tr (minor) = 12.4 min.
3-(4-Methoxyphenyl)-2,2-dimethyl-4-nitrobutanal (13ac) [37]. Yellow oil (37 mg, 74%); 1H NMR (CDCl3): δH = 9.52 (s, 1H), 7.17−7.05 (m, 2H), 6.91−6.79 (m, 2H), 4.81 (dd, J = 12.8, 11.3 Hz, 1H), 4.66 (dd, J = 12.8, 4.3 Hz, 1H), 3.79 (s, 3H), 3.73 (dd, J = 11.3, 4.3 Hz, 1H), 1.12 (s, 3H), 1.00 (s, 3H) ppm; 13C NMR (CDCl3): δC = 204.5, 159.5, 130.3, 127.3, 114.3, 76.7, 55.4, 48.5, 48.1, 21.7, 19.1 ppm; HPLC: Chiralcel OD-H, λ = 240 nm, n-hexane/2-propanol, 80:20, 1.0 mL/min, tr (major) = 12.1 min, tr (minor) = 16.4 min.
3-(Benzo[d] [1,3] dioxol-5-yl)-2,2-dimethyl-4-nitrobutanal (13ad) [25]. Yellow oil (36 mg, 67%); 1H NMR (CDCl3): δH = 9.51 (s, 1H), 6.75 (d, J = 7.9 Hz, 1H), 6.72−6.61 (m, 2H), 5.96 (s, 2H), 4.78 (dd, J = 12.9, 11.3 Hz, 1H), 4.65 (dd, J = 12.9, 4.3 Hz, 1H), 3.69 (dd, J = 11.3, 4.3 Hz, 1H), 1.13 (s, 3H), 1.02 (s, 3H) ppm; 13C NMR (CDCl3): δC = 204.4, 148.1, 147.6, 129.1, 122.8, 109.3, 108.5, 101.4, 76.7, 48.5 (2xC), 21.8, 19.2 ppm; HPLC: Chiralcel OD-H, λ = 230 nm, n-hexane/2-propanol, 80:20, 1.0 mL/min, tr (major) = 15.8 min, tr (minor) = 20.5 min.
2,2-Dimethyl-4-nitro-3-(3,4,5-trimethoxyphenyl)butanal (13ae) [25]. Yellow oil (45 mg, 73%); 1H NMR (CDCl3): δH = 9.52 (s, 1H), 6.38 (s, 2H), 4.85 (dd, J = 13.0, 11.2 Hz, 1H), 4.69 (dd, J = 13.0, 4.3 Hz, 1H), 3.84 (s, 6H), 3.83 (s, 3H), 3.69 (dd, J = 11.2, 4.3 Hz, 1H), 1.16 (s, 3H), 1.06 (s, 3H) ppm; 13C NMR (CDCl3): δC = 204.5, 153.6, 138.1, 131.2, 106.6, 76.6, 61.0, 56.4, 49.2, 48.4, 22.0, 19.6 ppm; HPLC: Chiralcel OD-H, λ = 210 nm, n-hexane/2-propanol, 80:20, 1.0 mL/min, tr (major) = 15.5 min, tr (minor) = 17.8 min.
3-(4-Fluorophenyl)-2,2-dimethyl-4-nitrobutanal (13af) [37]. Yellow oil (76 mg, 78%); 1H NMR (CDCl3): δH = 9.51 (s, 1H), 7.24−7.13 (m, 2H), 7.09−6.98 (m, 2H), 4.82 (dd, J = 13.0, 11.3 Hz, 1H), 4.69 (dd, J = 13.0, 4.3 Hz, 1H), 3.78 (dd, J = 11.3, 4.3 Hz, 1H), 1.13 (s, 3H), 1.01 (s, 3H) ppm; 13C NMR (CDCl3): δC = 204.1, 163.6, 161.6, 130.9, 130.8, 116.0, 115.8, 76.5, 48.4, 48.1, 21.8, 19.1 ppm; HPLC: Chiralcel OD-H, λ = 210 nm, n-hexane/2-propanol, 80:20, 1.0 mL/min, tr (major) = 10.0 min, tr (minor) = 15.6 min.
3-(2-Chlorophenyl)-2,2-dimethyl-4-nitrobutanal (13ag) [42]. Yellow oil (25 mg, 49%); 1H NMR (CDCl3): δH = 9.55 (s, 1H), 7.45–7.39 (m, 1H), 7.31–7.26 (m, 2H), 7.23 (m, 1H), 4.97–4.48 (m, 3H), 1.17 (s, 3H), 1.07 (s, 3H) ppm; 13C-NMR (CDCl3): δC = 203.9, 135.9, 133.9, 130.6, 129.3, 128.4, 127.3, 76.3, 49.2, 42.6, 21.0, 18.8 ppm; HPLC: Chiralcel OD-H, λ = 210 nm, n-hexane/2-propanol, 80:20, 1.0 mL/min, tr (major) = 9.7 min, tr (minor) = 23.2 min.
3-(4-Chlorophenyl)-2,2-dimethyl-4-nitrobutanal (13ah) [37]. Yellow oil (45 mg, 89%); 1H NMR (CDCl3): δH = 9.50 (s, 1H), 7.35−7.29 (m, 2H), 7.18−7.11 (m, 2H), 4.83 (dd, J = 13.1, 11.3 Hz, 1H), 4.69 (dd, J = 13.1, 4.2 Hz, 1H), 3.77 (dd, J = 11.3, 4.2 Hz, 1H), 1.13 (s, 3H), 1.01 (s, 3H) ppm; 13C-NMR (CDCl3): δC = 204.0, 134.3, 134.1, 130.5, 129.1, 76.3, 48.3, 48.1, 21.9, 19.1 ppm; HPLC: Chiralcel OD-H, λ = 210 nm, n-hexane/2-propanol, 80:20, 1.0 mL/min, tr (major) = 11.2 min, tr (minor) = 16.4 min.
3-(2-Bromophenyl)-2,2-dimethyl-4-nitrobutanal (13ai) [41]. Yellow oil (42 mg, 70%); 1H NMR (CDCl3): δH = 9.55 (s, 1H), 7.61 (dd, J = 8.0, 1.2 Hz, 1H), 7.36–7.25 (m, 2H), 7.15 (ddd, J = 8.0, 6.8, 2.2 Hz, 1H), 4.84 (dd, J = 13.2, 11.3 Hz, 1H), 4.72 (dd, J = 13.2, 4.1 Hz, 1H), 4.62 (dd, J = 11.3, 4.1 Hz, 1H), 1.17 (s, 3H), 1.09 (s, 3H) ppm; 13C-NMR (CDCl3): δC = 203.9, 135.7, 134.0, 129.6, 128.5, 128.0, 127.2, 76.6, 49.2, 45.4, 21.1, 18.9 ppm; HPLC: Chiralcel OD-H, λ = 230 nm, n-hexane/2-propanol, 80:20, 1.0 mL/min, tr (major) = 10.5 min, tr (minor) = 25.6 min.
3-(4-Bromophenyl)-2,2-dimethyl-4-nitrobutanal (13aj) [37]. Yellow oil (50 mg, 83%); 1H NMR (CDCl3): δH = 9.50 (s, 1H), 7.51−7.42 (m, 2H), 7.15−7.04 (m, 2H), 4.82 (dd, J = 13.1, 11.3 Hz, 1H), 4.69 (dd, J = 13.1, 4.2 Hz, 1H), 3.76 (dd, J = 11.3, 4.2 Hz, 1H), 1.12 (s, 3H), 1.01 (s, 3H) ppm; 13C NMR (CDCl3): δC = 203.9, 134.7, 132.0, 130.9, 122.4, 76.2, 48.2, 48.1, 21.9, 19.1 ppm; HPLC: Chiralcel OD-H, λ = 230 nm, n-hexane/2-propanol, 80:20, 1.0 mL/min, tr (major) = 12.4 min, tr (minor) = 17.4 min.
2,2-Dimethyl-4-nitro-3-(4-(trifluoromethyl)phenyl)butanal (13ak) [43]. Yellow oil (49 mg, 85%); 1H NMR (CDCl3): δH = 9.50 (s, 1H), 7.61 (d, J = 8.1 Hz, 2H), 7.36 (d, J = 8.1 Hz, 2H), 4.89 (dd, J = 13.3, 11.4 Hz, 1H), 4.74 (dd, J = 13.3, 4.1 Hz, 1H), 3.87 (dd, J = 11.4, 4.1 Hz, 1H), 1.15 (s, 3H), 1.03 (s, 3H) ppm; 13C NMR (CDCl3): δC = 203.7, 139.9, 129.7, 125.9 (2xC), 125.8 (2xC), 76.1, 48.4, 48.3, 22.0, 19.1 ppm; HPLC: Chiralcel OD-H, λ = 210 nm, n-hexane/2-propanol, 80:20, 1.0 mL/min, tr (major) = 10.4 min, tr (minor) = 16.2 min.
2,2-Dimethyl-3-(naphthalen-2-yl)-4-nitrobutanal (13al) [37]. Yellow oil (36 mg, 67%); 1H NMR (CDCl3): δH = 9.55 (s, 1H), 7.84−7.77 (m, 3H), 7.66 (d, J = 1.4 Hz, 1H), 7.52−7.45 (m, 2H), 7.31 (dd, J = 8.6, 1.9 Hz, 1H), 4.98 (dd, J = 13.1, 11.3 Hz, 1H), 4.76 (dd, J = 13.1, 4.2 Hz, 1H), 3.95 (dd, J = 11.3, 4.2 Hz, 1H), 1.17 (s, 3H), 1.04 (s, 3H) ppm; 13C NMR (CDCl3): δC = 204.4, 133.2, 133.1, 133.0, 128.6, 128.5, 128.0, 127.8, 126.7, 126.6, 126.5, 76.5, 48.8, 48.6, 22.0, 19.2 ppm; HPLC: Chiralcel OD-H, λ = 280 nm, n-hexane/2-propanol, 75:25, 1.0 mL/min, tr (major) = 15.6 min, tr (minor) = 18.1 min.
3-(Furan-2-yl)-2,2-dimethyl-4-nitrobutanal (13am) [37]. Yellow oil (32 mg, 75%); 1H NMR (CDCl3): δH = 9.52 (s, 1H), 7.37 (d, J = 1.9 Hz, 1H), 6.32 (dd, J = 3.2, 1.9 Hz, 1H), 6.22 (dd, J = 7.1, 3.2 Hz, 1H), 4.76 (dd, J = 12.9, 11.0 Hz, 1H), 4.59 (dd, J = 12.9, 3.9 Hz, 1H), 3.92 (dd, J = 11.0, 3.9 Hz, 1H), 1.18 (s, 3H), 1.05 (s, 3H) ppm; 13C NMR (CDCl3): δC = 203.6, 149.9, 142.9, 110.6, 109.8, 75.0, 48.3, 42.4, 21.3, 19.3 ppm; HPLC: Chiralcel OD-H, λ = 230 nm, n-hexane/2-propanol, 80:20, 1.0 mL/min, tr (major) = 8.1 min, tr (minor) = 11.8 min.
3.5. General Procedure for Recycling Experiments
To a mixture of catalyst 8 (4.7 mg, 0.02 mmol), additive (4-nitrobenzoic acid or DMAP, 0.02 mmol) and N-phenylmaleimide 10a (34.6 mg, 0.2 mmol) or β-nitrostyrene 12a (29.8 mg, 0.2 mmol) in the corresponding DES (0.5 mL) was added isobutyraldehyde 9a (37 µL, 28.8 mg, 0.4 mmol), and the reaction was vigorously stirred for 2 days at rt. After this period, 2-MeTHF (3 mL) was added and the mixture was stirred for 10 min at rt. The stirring was stopped to allow phase separation and the upper organic layer was removed with a pipette. This extractive procedure was repeated two more times combining the organic extracts, which were washed with water (3 × 5 mL), dried over MgSO4, filtered and evaporated under reduced pressure (15 torr) to afford the reaction product. The residual volatile organic solvent present in the DES phase was removed under vacuum evaporation (15 torr) and the catalytic system was regenerated by addition of new additive (0.02 mmol) (in the case of the reaction of isobutyraldehyde and N-phenylmaleimide, 140 μL of additional ethylene glycol were added). The next reaction cycle was performed with the DES mixture adding isobutyraldehyde and N-phenylmaleimide or β-nitrostyrene. Once the reaction was finished, the reaction mixture was subjected again to the above-described procedure and further reaction cycles were repeated using the recycled DES phase containing 8.
4. Conclusions
We concluded that a primary amine-salicylamide, prepared by a simple monoamidation of an enantiomerically pure trans-cyclohexane-1,2-diamine, could act as chiral organocatalyst suitable for the enantioselective conjugate addition of aldehydes to maleimides or nitroolefins carried out in deep eutectic mixtures as “green” solvents. Good yields and enantioselectivities could be achieved working in choline chloride/ethylene glycol (for maleimides) and choline chloride/water (for nitroolefins), the presence of acid and basic additives, respectively, being necessary. The eutectic solvent containing the organocatalyst could be recycled and reused affording similar enantioselectivities.
Supplementary Materials
The following are available online, NMR spectra and HPLC chromatograms.
Author Contributions
Conceptualization, R.C.; Investigation, A.T.-C., A.S.-L. and E.S.; Methodology, R.C. and A.T.-C.; Project administration, R.C.; Supervision, R.C.; Writing – original draft, R.C.
Funding
We thank the financial support from the Spanish Ministerio de Economía, Industria y Competitividad (CTQ201788171-P and PGC2018-096616-B-I00) and the University of Alicante (VIGROB-173, UADIF18-20 and UAUSTI18-05).
Acknowledgments
E.S. thanks the Manchester Metropolitan University (U.K.) for an ERASMUS+ fellowship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Clark, J.H.; Hunt, A.; Topi, C.; Paggiola, G.; Sherwood, J. Sustainable Solvents: Perspectives from Research, Business and International Policy; Royal Society of Chemistry: London, UK, 2017. [Google Scholar]
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Brohl, A.; Hallett, J.P. Green and sustainable solvents in chemical processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef] [PubMed]
- Marcus, Y. Deep Eutectic Solvents; Springer: Cham, Switzerland, 2019. [Google Scholar]
- García-Alvarez, J. Deep eutectic mixtures: Promising sustainable solvents for metal-catalyzed and metal-mediated organic reactions. Eur. J. Inorg. Chem. 2015, 5147–5157. [Google Scholar] [CrossRef]
- Liu, P.; Hao, J.-W.; Mo, L.-P.; Zhang, Z.-H. Recent advances in the application of deep eutectic solvents as sustainable media as well as catalysts in organic reactions. RSC Adv. 2015, 5, 48675–48704. [Google Scholar] [CrossRef]
- Alonso, D.A.; Baeza, A.; Chinchilla, R.; Guillena, G.; Pastor, I.M.; Ramón, D.J. Deep eutectic solvents: The organic reaction medium of the century. Eur. J. Org. Chem. 2016, 612–632. [Google Scholar] [CrossRef]
- Guajardo, N.; Müller, C.R.; Schrebler, R.; Carlesi, C.; Domínguez de María, P. Deep eutectic solvents for organocatalysis, biotransformations, and multistep organocatalyst/enzyme combinations. ChemCatChem 2016, 8, 1020–1027. [Google Scholar] [CrossRef]
- Khandelwal, S.; Tailor, Y.K.; Kumar, M. Deep eutectic solvents (DESs) as eco-friendly and sustainable solvent/catalyst systems in organic transformations. J. Mol. Liq. 2016, 215, 345–386. [Google Scholar] [CrossRef]
- Longo, L.S.; Craveiro, M.V. Deep eutectic solvents as unconventional media for multicomponent reactions. J. Braz. Chem. Soc. 2018, 29, 1999–2025. [Google Scholar] [CrossRef]
- Florindo, C.; Branco, L.C.; Marrucho, I.M. Quest for green-solvent design: From hydrophilic to hydrophobic (deep) eutectic solvents. ChemSusChem 2019, 12, 1549–1559. [Google Scholar] [CrossRef]
- Peng, L.; Hu, Z.; Lu, Q.; Tang, Z.; Jiao, Y.; Xu, X. DESs: Green solvents for transition metal catalyzed organic reactions. Chin. Chem. Lett. 2019. [Google Scholar] [CrossRef]
- Dalko, P.I. Comprehensive Enantioselective Organocatalysis: Catalysts, Reactions, and Applications; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Karame, I.; Srour, H. Recent Advances in Organocatalysis; IntechOpen: London, UK, 2016. [Google Scholar]
- Hernández, J.G.; Juaristi, E. Recent efforts directed to the development of more sustainable asymmetric organocatalysis. Chem. Commun. 2012, 48, 5396–5409. [Google Scholar] [CrossRef]
- Martínez, R.; Berbegal, L.; Guillena, G.; Ramón, D.J. Bio-renewable enantioselective aldol reaction in natural deep eutectic solvents. Green Chem. 2016, 18, 1724–1730. [Google Scholar] [CrossRef]
- Brenna, D.; Massolo, E.; Puglisi, A.; Rossi, S.; Celentano, G.; Benaglia, M.; Capriati, V. Towards the development of continuous, organocatalytic, and stereoselective reactions in deep eutectic solvents. Beilstein J. Org. Chem. 2016, 12, 2620–2626. [Google Scholar] [CrossRef]
- Fanjul-Mosteirin, N.; Concellón, C.; del Amo, V. L-Isoleucine in a choline chloride/ethylene glycol deep eutectic solvent: A reusable reaction kit for the asymmetric cross-aldol carboligation. Org. Lett. 2016, 18, 4266–4269. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.R.; Meiners, I.; Domínguez de María, P. Highly enantioselective tandem enzyme-organocatalyst crossed aldol reactions with acetaldehyde in deep-eutectic-solvents. RSC Adv. 2014, 4, 46097–46101. [Google Scholar] [CrossRef]
- Müller, C.R.; Rosen, A.; Domínguez de María, P. Multi-step enzyme-organocatalyst C-C bond forming reactions in deep-eutectic-solvents: Towards improved performances by organocatalyst design. Sustain. Chem. Process. 2015, 3, 1–8. [Google Scholar] [CrossRef]
- Massolo, E.; Palmieri, S.; Benaglia, M.; Capriati, V.; Perna, F.M. Stereoselective organocatalysed reactions in deep eutectic solvents: Highly tunable and biorenewable reaction media for sustainable organic synthesis. Green Chem. 2016, 792–797. [Google Scholar] [CrossRef]
- Ñiguez, D.R.; Guillena, G.; Alonso, D.A. Chiral 2-Aminobenzimidazoles in Deep Eutectic Mixtures: Recyclable Organocatalysts for the Enantioselective Michael Addition of 1,3-Dicarbonyl Compounds to β-Nitroalkenes. ACS Sustain. Chem. Eng. 2017, 5, 10649–10656. [Google Scholar] [CrossRef]
- Flores-Ferrándiz, J.; Chinchilla, R. Organocatalytic enantioselective conjugate addition of aldehydes to maleimides in deep eutectic solvents. Tetrahedron Asymmetry 2017, 28, 302–306. [Google Scholar] [CrossRef]
- Ñiguez, D.R.; Khazaeli, P.; Alonso, D.A.; Guillena, G. Deep eutectic mixtures as reaction media for the enantioselective organocatalyzed α-amination of 1,3-dicarbonyl compounds. Catalysts 2018, 8, 217. [Google Scholar] [CrossRef]
- Torregrosa-Chinillach, A.; Moragues, A.; Pérez-Furundarena, H.; Chinchilla, R.; Gómez-Bengoa, E.; Guillena, G. Enantioselective Michael addition of aldehydes to maleimides organocatalyzed by a chiral primary amine-salicylamide. Molecules 2018, 23, 3299. [Google Scholar] [CrossRef]
- Martínez-Guillén, J.R.; Flores-Ferrándiz, J.; Gómez, C.; Gómez-Bengoa, E.; Chinchilla, R. Asymmetric conjugate addition of α,α-disubstituted aldehydes to nitroalkenes organocatalyzed by chiral monosalicylamides from trans-cyclohexane-1,2-diamines. Molecules 2018, 23, 141. [Google Scholar] [CrossRef] [PubMed]
- Freiberg, C.; Brunner, N.A.; Schiffer, G.; Lampe, T.; Pohlmann, J.; Brands, M.; Raabe, M.; Haebich, D.; Ziegelbauer, K. Identification and characterization of the first class of potent bacterial Acetyl-CoA carboxylase inhibitors with antibacterial activity. J. Biol. Chem. 2004, 279, 26066–26073. [Google Scholar] [CrossRef] [PubMed]
- Isaka, M.; Rugseree, N.; Maithip, P.; Kongsaeree, P.; Prabpai, S.; Thebtaranonth, Y. Hirsutellones A-E, antimycobacterial alkaloids from the insect pathogenic fungus Hirsutella nivea body centered cubic 2594. Tetrahedron 2005, 61, 5577–5583. [Google Scholar] [CrossRef]
- Uddin, J.; Ueda, K.; Siwu, E.R.O.; Kita, M.; Uemura, D. Cytotoxic labdane alkaloids from an ascidian Lissoclinum sp.: Isolation, structure elucidation, and structure-activity relationship. Bioorg. Med. Chem. 2006, 14, 6954–6961. [Google Scholar] [CrossRef] [PubMed]
- Nöth, J.; Frankowski, K.J.; Neuenswander, B.; Aubé, J.; Reiser, O. Efficient synthesis of γ-lactams by a tandem reductive amination/lactamization sequence. J. Comb. Chem. 2008, 10, 456–459. [Google Scholar] [CrossRef]
- Kazmierski, W.M.; Andrews, W.; Furfine, E.; Spaltenstein, A.; Wright, L. Discovery of potent pyrrolidone-based HIV-1 protease inhibitors with enhanced drug-like properties. Bioorg. Med. Chem. Lett. 2004, 14, 5689–5692. [Google Scholar] [CrossRef]
- Das Sarma, K.; Zhang, J.; Huang, Y.; Davidson, J.G. Amino acid esters and amides for reductive amination of mucochloric acid: Synthesis of novel γ-lactams, short peptides and antiseizure agent Levetiracetam (Keppra). Eur. J. Org. Chem. 2006, 3730–3737. [Google Scholar] [CrossRef]
- Tang, K.; Zhang, J.-T. The effects of (-)-clausenamide on functional recovery in transient focal cerebral ischemia. Neurol. Res. 2002, 24, 473–478. [Google Scholar] [CrossRef]
- Aboul-Enein, M.N.; El-Azzouny, A.A.; Saleh, O.A.; Maklad, Y.A. On chemical structures with potent antiepileptic/anticonvulsant profile. Mini Rev. Med. Chem. 2012, 12, 671–700. [Google Scholar] [CrossRef]
- Andresen, H.; Aydin, B.E.; Mueller, A.; Iwersen-Bergmann, S. An overview of gamma-hydroxybutyric acid: Pharmacodynamics, pharmacokinetics, toxic effects, addiction, analytical methods, and interpretation of results. Drug Test Anal. 2011, 3, 560–568. [Google Scholar] [CrossRef]
- Gajcy, K.; Lochynski, S.; Librowski, T. A role of GABA analogues in the treatment of neurological diseases. Curr. Med. Chem. 2010, 17, 2338–2347. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.-W.; Liu, X.-F.; Liu, J.-T.; Liu, Z.-J.; Tao, J.-C. Highly enantioselective Michael addition of α,α-disubstituted aldehydes to maleimides catalyzed by new primary amine-squaramide bifunctional organocatalysts. Tetrahedron Lett. 2017, 58, 4487–4490. [Google Scholar] [CrossRef]
- Ma, Z.-W.; Liu, X.-F.; Sun, B.; Huang, X.-H.; Tao, J.-C. Chiral primary amine-squaramide catalyzed highly enantioselective Michael addition of isobutyraldehyde to nitroolefins. Synthesis 2017, 49, 1307–1314. [Google Scholar] [CrossRef]
- Rodríguez, J.M.; Pujol, M.D. Straightforward synthesis of nitroolefins by microwave- or ultrasound-assisted Henry reaction. Tetrahedron Lett. 2011, 52, 2629–2632. [Google Scholar] [CrossRef]
- García, G.; Aparicio, S.; Ullah, R.; Atilhan, M. Deep eutectic solvents: Physicochemical properties and gas separation applications. Energy Fuels 2015, 29, 2616–2644. [Google Scholar] [CrossRef]
- Miura, T.; Masuda, A.; Ina, M.; Nakashima, K.; Nishida, S.; Tada, N.; Itoh, A. Asymmetric Michael reactions of α,α-disubstituted aldehydes with maleimides using a primary amine thiourea organocatalyst. Tetrahedron Asymmetry 2011, 22, 1605–1609. [Google Scholar] [CrossRef]
- Avila-Ortiz, C.G.; Díaz-Corona, L.; Jiménez-González, E.; Juaristi, E. Asymmetric Michael addition organocatalyzed by α,β-dipeptides under solvent-free reaction conditions. Molecules 2017, 22, 1328. [Google Scholar] [CrossRef]
- Reyes-Rangel, G.; Vargas-Caporali, J.; Juaristi, E. In search of diamine analogs of the α,α-diphenyl prolinol privileged chiral organocatalyst. Synthesis of diamine derivatives of α,α-diphenyl-(S)-prolinol and their application as organocatalysts in the asymmetric Michael and Mannich reactions. Tetrahedron 2016, 72, 379–391. [Google Scholar] [CrossRef]
- Porta, R.; Coccia, F.; Annunziata, R.; Puglisi, A. Comparison of different polymer- and silica-supported 9-amino-9-deoxy-epi-quinines as recyclable organocatalysts. ChemCatChem 2015, 7, 1490–1499. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 7 and 8 are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).