Andean Prumnopitys Andina (Podocarpacae) Fruit Extracts: Characterization of Secondary Metabolites and Potential Cytoprotective Effect
Abstract
1. Introduction
2. Results and Discussion
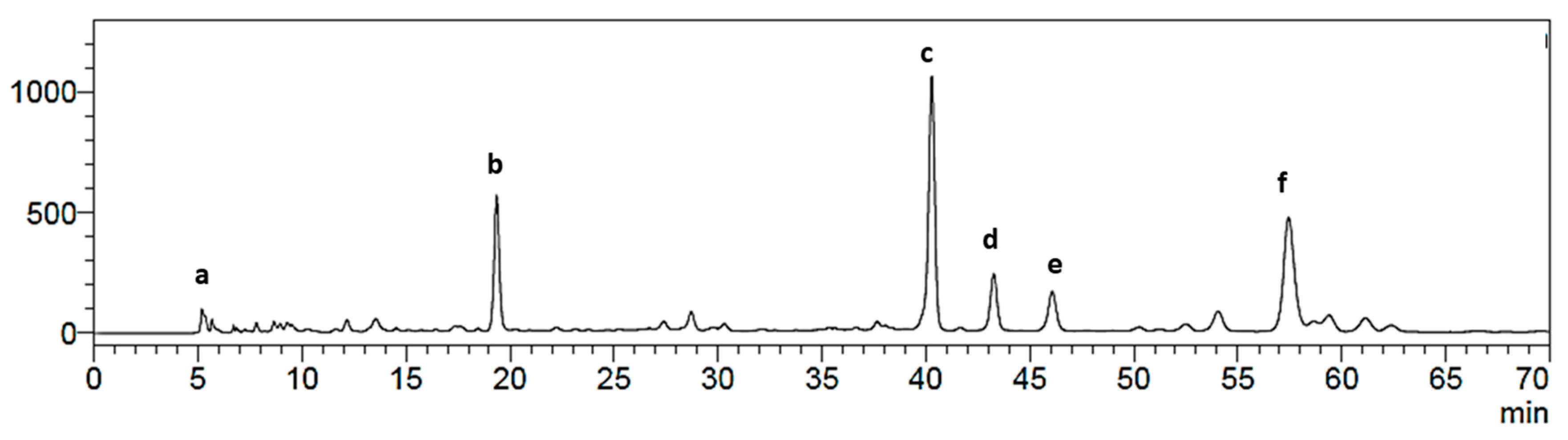
2.1. Isolation of Main Constituents from the Fruit Extract
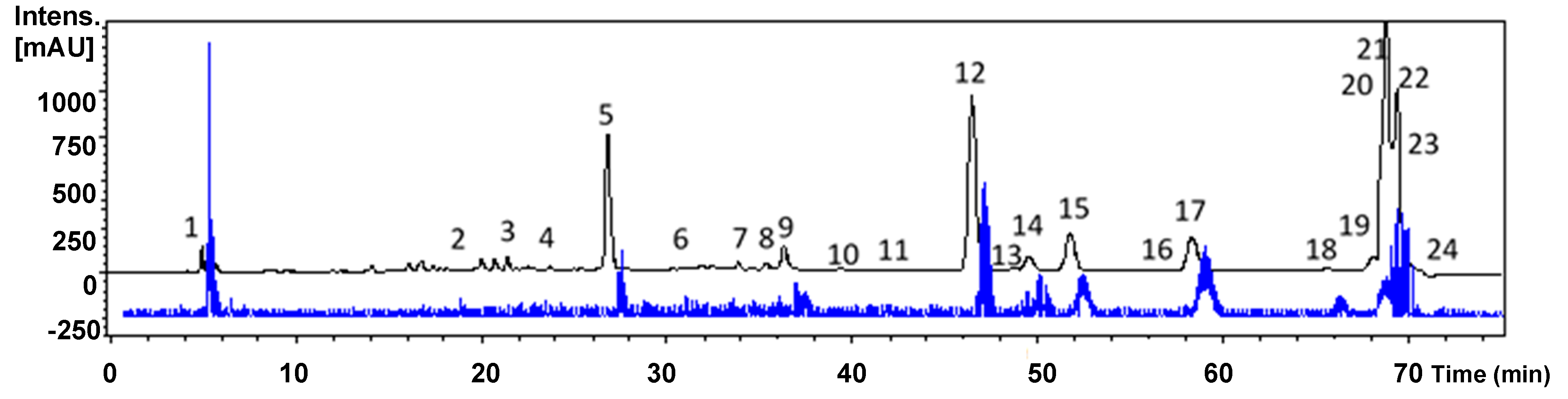
2.2. High-Performance Liquid Chromatography Diode-Array Detector Mass Spectrometry (HPLC-DAD-MS) Profile
2.2.1. Simple Phenolics
2.2.2. Phenylpropanoids
2.2.3. Flavonoids
2.2.4. Other Compounds
2.3. Total Phenolics (TP) and Total Flavonoid (TF) Content
2.4. Antioxidant Activity
2.5. Inhibition of Carbohydrate- and Lipid-Metabolizing Enzymes
2.6. Determination of Thiobarbituric Acid Reactive Substances (TBARS) after Simulated Gastric Digestion
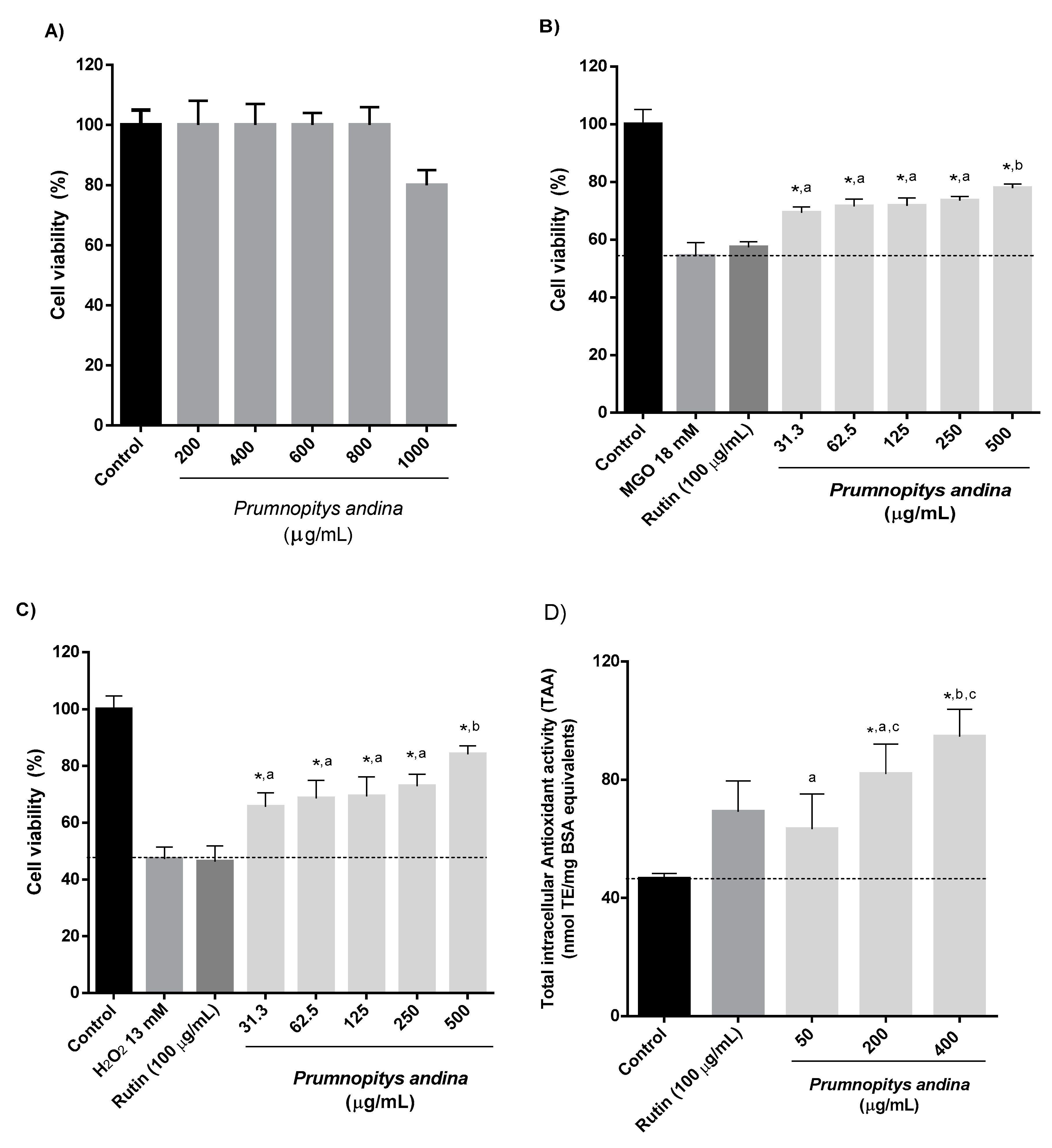
2.7. Cell-Based Experiments
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Plant Material
3.3. Extract Fingerprinting by HPLC-DAD
3.4. Isolation and Characterization of Main Compounds from P. andina Fruit Extract
3.4.1. Sephadex LH-20 Permeation/Column Chromatography
3.4.2. Counter-Current Chromatography (CCC) Separation
3.5. Instrumental Analysis
3.6. HPLC-DAD-MS/MS Analysis
3.7. Total Phenolic and Total Flavonoid Content
3.8. Antioxidant Activity
3.9. Inhibition of Carbohydrate- and Lipid-Metabolizing Enzymes
3.9.1. α-Glucosidase
3.9.2. α-Amylase
3.9.3. Lipase
3.10. Determination of Thiobarbituric Acid Reactive Substances (TBARS) in a Simulated Gastric Digestion Model
3.11. Cell-Based Experiments
3.11.1. Cell Culture
3.11.2. Determination of the Cytotoxicity of P. andina XAD7 Fruit Extracts in Human Gastric Epithelial Cells (AGS)
3.11.3. Cytoprotection Against H2O2 or Methylglyoxal (MGO)-Induced Stress
3.11.4. Intracellular Total Antioxidant Activity (TAA)
3.12. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 20-HE | 20 hydroxyecdysone |
| AAPH | 2,2′-azobis(2-amidinopropane) dihydrochloride |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) |
| ACN | acetonitrile |
| AGS | adenocarcinoma gastric epithelial cells |
| amu | atomic mass units |
| AUC | area under the curve |
| BuOH | butanol |
| CCC | counter-current chromatography |
| CE | catechin equivalents |
| COX | cyclooxygenase |
| DAD | diode-array detector |
| DEPT | Distortionless Enhancement by Polarization Transfer |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| FBS | fetal bovine serum |
| FRAP | ferric reducing antioxidant power |
| GAE | gallic acid equivalents |
| HPLC | high-performance liquid chromatography |
| HMBC | Heteronuclear Multiple Bond Correlation |
| HMQC | Heteronuclear Multiple-Quantum Correlation |
| iNOS | inducible nitric oxide synthase |
| LP | lower phase |
| LPS | lipopolysaccharide |
| MDA | malondialdehyde |
| MGO | methylglyoxal |
| MS | mass spectrometry |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NMR | nuclear magnetic resonance |
| PBS | phosphate buffer saline |
| QTOF | quadrupole time of flight |
| ORAC | oxygen radical absorbance capacity |
| SGF | simulated gastric fluid |
| TAA | total intracellular antioxidant activity |
| TBARS | thiobarbituric acid reactive species |
| TE | trolox equivalents |
| TEAC | trolox equivalent antioxidant capacity |
| TF | total flavonoid content |
| TLC | thin layer chromatography |
| TP | total phenolic content |
| UP | upper phase |
References
- The Plant List. Available online: http://www.theplantlist.org/1.1/browse/G/Podocarpaceae/ (accessed on 6 March 2019).
- Abdillahi, H.S.; Stafford, G.I.; Finnie, J.F.; Van Staden, J. Ethnobotany, phytochemistry and pharmacology of Podocarpus sensu latissimo (s.l.). South African, J. Bot. 2010, 76, 1–24. [Google Scholar] [CrossRef]
- Gosling, P.G.; Ives, L.M.; Cunningham, V.J.; Hechenleitner, P.; Brownless, P.; Thomas, P.; Martinez, C. Preliminary advice on fruit handling, seed treatment and “germination” of embryos of Prumnopitys andina. Sibbaldia J. Bot. Garden Hortic. 2005, 3, 41–50. [Google Scholar]
- Marticorena, C.; Rodríguez, R. Flora de Chile. I. Pteridophyta-Gymnospermae; Universidad de Concepción: Concepción, Chile, 1995; pp. 1–351. [Google Scholar]
- Rodríguez, J.A.; Theoduloz, C.; Yáñez, T.; Becerra, J.; Schmeda-Hirschmann, G. Gastroprotective and ulcer-healing effect of ferruginol in mice and rats: Assessment of its mechanisms of action using in vitro models. Life Sci. 2006, 78, 2503–2509. [Google Scholar] [CrossRef]
- Smith, E.C.J.; Wareham, N.; Sloh, M.; Gibbons, S. 2β-Acetoxyferruginol - A new antibacterial abietane diterpene from the bark of Prumnopitys andina. Phytochem. Lett. 2008, 1, 49–53. [Google Scholar] [CrossRef]
- Netzel, M.; Netzel, G.; Tian, Q.; Schwartz, S.; Konczak, I. Sources of antioxidant activity in Australian native fruits. Identification and quantification of anthocyanins. J. Agric. Food Chem. 2006, 54, 9820–9826. [Google Scholar] [CrossRef]
- Netzel, M.; Netzel, G.; Tian, Q.; Schwartz, S.; Konczak, I. Native Australian fruits-A novel source of antioxidants for food. Innov. Food Sci. Emerg. Technol. 2007, 8, 339–346. [Google Scholar] [CrossRef]
- Tan, A.C.; Konczak, I.; Ramzan, I.; Sze, D.M.Y. Antioxidant and cytoprotective activities of native Australian fruit polyphenols. Food Res. Int. 2011, 44, 2034–2040. [Google Scholar] [CrossRef]
- Indrianingsih, A.W.; Tachibana, S.; Itoh, K. In vitro evaluation for antioxidant and α-glucosidase inhibitory assay of several tropical and subtropical plants. Procedia Environ. Sci. 2015, 28, 639–648. [Google Scholar] [CrossRef]
- Winterhalter, P. Application of countercurrent chromatography (CCC) to the analysis of natural pigments. Trends Food Sci. Technol. 2007, 18, 507–513. [Google Scholar] [CrossRef]
- Ito, Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A 2005, 1065, 145–168. [Google Scholar] [CrossRef]
- Girault, J.P.; Lafont, R. The complete 1H-NMR assignment of ecdysone and 20-hydroxyecdysone. J. Insect Physiol. 1988, 34, 701–706. [Google Scholar] [CrossRef]
- Suksamrarn, A.; Pattanaprateep, P. Selective acetylation of 20-hydroxyecdysone partial synthesis of some minor ecdysteroids and analogues. Tetrahedron 1995, 51, 10633–10650. [Google Scholar] [CrossRef]
- Galbraith, M.N.; Horn, D.H.S. Insect moulting hormones: Crustecdysone (20-hydroxyecdysone) from Podocarpus elatus. Aust. J. Chem. 1969, 22, 1045–1057. [Google Scholar] [CrossRef]
- Destrez, B.; Pinel, G.; Monteau, F.; Lafont, R.; Le Bizec, B. Detection and identification of 20-hydroxyecdysone metabolites in calf urine by liquid chromatography-high resolution or tandem mass spectrometry measurements and establishment of their kinetics of elimination after 20-hydroxyecdysone administration. Anal. Chim. Acta 2009, 637, 178–184. [Google Scholar] [CrossRef]
- Courtheyn, D.; Le Bizec, B.; Brambilla, G.; De Brabander, H.F.; Cobbaert, E.; Van de Wiele, M.; Vercammen, J.; De Wasch, K. Recent developments in the use and abuse of growth promoters. Anal. Chim. Acta 2002, 473, 71–82. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Da Silva, N.A.; Rodrigues, E.; Mercadante, A.Z.; de Rosso, V.V. Phenolic compounds and carotenoids from four fruits native from the Brazilian Atlantic Forest. J. Agric. Food Chem. 2014, 62, 5072–5084. [Google Scholar] [CrossRef]
- Krasteva, I.; Nikolov, S. Flavonoids in Astragalus corniculatus. Quim. Nova 2008, 31, 59–60. [Google Scholar] [CrossRef]
- Geng, P.; Sun, J.; Zhang, M.; Li, X.; Harnly, J.M.; Chen, P. Comprehensive characterization of C-glycosyl flavones in wheat (Triticum aestivum L.) germ using UPLC-PDA-ESI/HRMS and mass defect filtering. J. Mass Spectrom. 2016, 51, 914–930. [Google Scholar] [CrossRef]
- Abdillahi, H.S.; Finnie, J.F.; Van Staden, J. Anti-inflammatory, antioxidant, anti-tyrosinase and phenolic contents of four Podocarpus species used in traditional medicine in South Africa. J. Ethnopharmacol. 2011, 136, 496–503. [Google Scholar] [CrossRef]
- López-Alarcón, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef]
- Jiménez-Aspee, F.; Quispe, C.; Soriano, M.P.D.C.; Fuentes-González, J.; Hünecke, E.; Theoduloz, C.; Schmeda-Hirschmann, G. Antioxidant activity and characterization of constituents in copao fruit (Eulychnia acida) Phil., Cactaceae) by HPLC-DAD-MS/MS. Food Res. Int. 2014, 62, 286–298. [Google Scholar] [CrossRef]
- Jiménez-Aspee, F.; Theoduloz, C.; Vieira, M.N.; Rodríguez-Werner, M.; Schmalfuss, E.; Winterhalter, P.; Schmeda-Hirschmann, G. Phenolics from the Patagonian currants Ribes spp.: Isolation, characterization and cytoprotective effect in human AGS cells. J. Funct. Foods 2016, 26, 11–26. [Google Scholar] [CrossRef]
- Rasheed, D.M.; Porzel, A.; Frolov, A.; El Seedi, H.R.; Wessjohann, L.A.; Farag, M.A. Comparative analysis of Hibiscus sabdariffa (roselle) hot and cold extracts in respect to their potential for α-glucosidase inhibition. Food Chem. 2019, 250, 236–244. [Google Scholar] [CrossRef]
- McDougall, G.J.; Shapiro, F.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Different polyphenolic components of soft fruits inhibit alpha-amylase and alpha-glucosidase. J. Agric. Food Chem. 2005, 53, 2760–2766. [Google Scholar] [CrossRef]
- McDougall, G.J.; Kulkarni, N.N.; Stewart, D. Berry polyphenols inhibit pancreatic lipase activity in vitro. Food Chem. 2009, 115, 193–199. [Google Scholar] [CrossRef]
- Kanner, J. Dietary advanced lipid oxidation endproducts are risk factors to human health. Mol. Nutr. Food Res. 2007, 51, 1094–1101. [Google Scholar] [CrossRef]
- Kanner, J.; Selhub, J.; Shpaizer, A.; Rabkin, B.; Shacham, I.; Tirosh, O. Redox homeostasis in stomach medium by foods: The Postprandial Oxidative Stress Index (POSI) for balancing nutrition and human health. Redox Biol. 2017, 12, 929–936. [Google Scholar] [CrossRef]
- Kanner, J.; Gorelik, S.; Roman, S.; Kohen, R. Protection by polphenols of post-prandial human plasma and low-density lipoprotein modification: The stomach as a bioreactor. J. Agric. Food Chem. 2012, 60, 8790–9796. [Google Scholar] [CrossRef]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Rad. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 14, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation production, metabolism and signalling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Cheli, F.; Baldi, A. Nutrition-based health: Cell-based bioassays for food antioxidant activity evaluation. J. Food Sci. 2011, 76, R197–R205. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Rafter, J.; Jenner, A. Health promotion by flavonoids, tocopherols, tocotrienols and other phenols: Direct or indirect effects? Antioxidant or not? Am. J. Clin. Nutr. 2005, S1, 268S–276S. [Google Scholar] [CrossRef]
- Speciale, A.; Anwar, S.; Canali, R.; Chirafisi, J.; Saija, A.; Virgili, F.; Cimino, F. Cyanidin-3-O-glucoside counters the response to TNF-alpha of endothelial cells by activating Nrf2 pathway. Mol. Nutr. Food Res. 2013, 57, 1979–1987. [Google Scholar] [CrossRef]
- Kahle, K.; Kraus, M.; Scheppach, M.; Ackermann, M.; Ridder, F.; Richling, E. Studies on apple and blueberry fruit constituents: Do the polyphenols reach the colon after ingestion? Mol. Nutr. Food Res. 2006, 50, 418–423. [Google Scholar] [CrossRef]
- He, J.; Wallace, T.C.; Keatley, K.E.; Failla, M.L.; Giusti, M.M. Stability of black raspberry anthocyanins in the digestive tract lumen and transport efficiency into gastric and small intestinal tissues in the rat. J. Agric. Food Chem. 2009, 57, 3141–3148. [Google Scholar] [CrossRef]
- Schantz, M.; Mohn, C.; Baum, M.; Richling, E. Antioxidative efficiency of an anthocyanin rich bilberry extract in the human colon tumor cell lines Caco-2 and HT-29. J. Berry Res. 2010, 1, 25–33. [Google Scholar]
- Wang, L.; Ding, L.; Xue, C.; Ma, S.; Du, Z.; Zhang, T.; Liu, J. Corn gluten hydrolysate regulates the expressions of antioxidant defense and ROS metabolism relevant genes in H2O2-induced HepG2 cells. J. Funct. Foods 2018, 42, 362–370. [Google Scholar] [CrossRef]
- Chow, J.M.; Shen, S.C.; Huan, S.K.; Lin, H.Y.; Chen, Y.C. Quercetin, but not rutin and quercitrin, prevention of H2O2-induced apoptosis via anti-oxidant activity and heme oxygenase 1 gene expression in macrophages. Biochem. Pharmacol. 2005, 69, 1839–1851. [Google Scholar] [CrossRef]
- Zhang, J.; Stanley, R.A.; Adaim, A.; Melton, LD.; Skinner, MA. Free radical scavenging and cytoprotective activities of phenolic antioxidants. Mol. Nutr. Food Res. 2006, 50, 996–1005. [Google Scholar] [CrossRef]
- Tan, A.C.; Hou, D.X.; Konczak, I.; Tanigawa, S.; Ramzan, I.; Sze, D.M.Y. Native Australian fruit polyphenols inhibit COX-2 and iNOS expression in LPS-activated murine macrophages. Food Res. Int. 2011, 44, 2362–2367. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymol. 1999, 299, 152–178. [Google Scholar]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Theoduloz, C.; Burgos-Edwards, A.; Schmeda-Hirschmann, G.; Jiménez-Aspee, F. Effect of polyphenols from wild Chilean currants (Ribes spp.) on the activity of intracellular antioxidant enzymes in human gastric AGS cells. Food Biosci. 2018, 24, 80–88. [Google Scholar] [CrossRef]
- Oki, T.; Matsui, T.; Osajima, Y. Inhibitory effect of α-glucosidase inhibitors varies according to its origin. J. Agric. Food Chem. 1999, 47, 550–553. [Google Scholar] [CrossRef]
- Bernfeld, P. Amylase, α and β. In Methods in Enzymology; Colowick, S.P., Kaplan, N.O., Eds.; Academic Press: New York, NY, USA, 1955; pp. 149–158. [Google Scholar]
- Picard, S.; Parthasarathy, S.; Fruebis, J.; Witztum, J.L. Aminoguanidine inhibits oxidative modification of low density lipoprotein protein and the subsequent increase in uptake by macrophage scavenger receptors. PNAS 1992, 89, 6876–6880. [Google Scholar] [CrossRef]
- Ferrari, D.; Speciale, A.; Cristani, M.; Fratantonio, D.; Molonia, M.S.; Ranaldi, G.; Saija, A.; Cimino, F. Cyanidin-3-O-glucoside inhibits NF-kB signalling in intestinal epithelial cells exposed to TNF-α and exerts protective effects via Nrf2 pathway activation. Toxicol. Lett. 2016, 264, 51–58. [Google Scholar] [CrossRef]
Sample Availability: Samples of the main compounds are available from the authors. |

 20-HE | 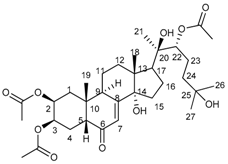 20-HEAc | ||||
|---|---|---|---|---|---|
| H | 20-HE | 20-HEAc | C | 20-HE | 20-HEAc |
| 1 | 1.33 m, 1.68 m | 1.50 m, 1.85 m | 1 | 35.97 t | 34.02 t |
| 2 | 3.87 dt (12.0, 3.2) | 5.03 dt (12.4, 3.2) | 2 | 67.31 d | 68.61 d |
| 3 | 3.97 br s | 5.33 br s | 3 | 67.12 d | 67.00 d |
| 4 | 1.62–1.64 m | 1.55 m, 2.03 m | 4 | 31.44 t | 31.73 t |
| 5 | 2.40 dd (12.0, 4.4) | 2.36 dd | 5 | 50.38 d | 50.95 d |
| 6 | 6 | 205.06 s | 201.99 s | ||
| 7 | 5.83 br s | 5.84 sbr | 7 | 120.74 d | 121.63 d |
| 8 | 8 | 166.59 s | 164.39 s | ||
| 9 | 3.17 dd br (8.4, 8.4) | 3.08 t (7.6) | 9 | 33.70 d | 33.56 d |
| 10 | 10 | 37.87 s | 38.39 s | ||
| 11 | 1.68 m, 1.86 m | 1.63 m, 1.77 m | 11 | 20.10 t | 20.53 t |
| 12 | 1.76 m, 2.14 ddd (12.8, 8.0, 4.4) | 1.83 m, 2.08 m | 12 | 31.11 t | 31.04 t |
| 13 | 13 | 48.03 s | 47.49 s | ||
| 14 | 14 | 83.84 s | 84.57 s | ||
| 15 | 1.49 m, 1.87 m | 1.68 m, 1.78 m | 15 | 30.39 t | 29.24 t |
| 16 | 1.17 m, 1.55 m | 1.45 m, 1.71 m | 16 | 25.94 t | 24.79 t |
| 17 | 2.39 m | 2.35 m | 17 | 49.13 d | 49.50 d |
| 18 | 0.91 s | 0.82 s | 18 | 16.66 q | 17.46 q |
| 19 | 0.99 s | 1.00 s | 19 | 23.02 q | 23.81 q |
| 20 | 20 | 76.53 s | 77.34 s | ||
| 21 | 1.22 s | 1.19 | 21 | 19.67 q | |
| 22 | 3.35 d (13.6) | 4.82 d (9.2) | 22 | 77.02 d | 79.63 d |
| 23 | 1.6–1.7 m | 1.6–1.7 m | 23 | 20.10 t | 20.36 t |
| 24 | 1.34 m, 1.70 m | 1.38–1.48 m | 24 | 40.98 t | 40.27 t |
| 25 | 25 | 69.92 s | 70.60 s | ||
| 26 | 1.22 s | 1.21 s | 26 | 28.31 q | 30.06 q |
| 27 | 1.21 s | 1.20 s | 27 | 27.59 q | 28.85 q |
| Acetate | Acetate | ||||
| C=O | 172.49 s, 170.48 s, 170.20 s | ||||
| CH3 | 2.08 s, 2.08 s, 1.98 s | CH3 | 21.14 q (2C), 21.10 q | ||
| Peak | Rt [min] | λmax [nm] | [M−H]− | MS2 | Tentative I Dentification |
|---|---|---|---|---|---|
| 1 | 4.6 | 326, 300sh, 285 | 341.01 | 178.82 (91.4), 161.0 (38.9), 143.0 (13.4) | Caffeic acid β-glucoside 1 *,# |
| 2 | 19.2 | 280 | 299.13 | 136.80 (100) | Hydroxybenzoic acid hexoside |
| 3 | 21.3 | - | 315.20 | 152.84 (100), 108.8 (4) | Dihydroxybenzoic acid hexoside |
| 4 | 23.8 | 326. 292 sh | 514.99 | 352.66 (100). 190.70 (66) | 3,5-dicaffeoylquinic acid |
| 5 | 27.1 | 321, 285 | 515.21 | 352.80 (32). 341.90 (24) 323.00 (100). 190.99 (32). 178.90 (8) | Dicaffeoylquinic acid |
| 6 | 30.8 | 330, 277 | 497.39 | 334.93 (100), 178.90 (10) | Caffeoylshikimic acid hexoside |
| 7 | 34.1 | 321, 288 | 341.28 | 178.89 (100), 135.00 (7) | Caffeic acid hexoside 2 |
| 8 | 35.6 | 325, 300sh | 352.99 | 190.91 (100), 179.3 (0.4) | 3-caffeoylquinic acid ** |
| 9 | 36.2 | 320sh, 285 | 449.30 | 286.94 (100). 269.0 (39). 259.0 (45) | Dihydrokaempferol hexoside |
| 10 | 39.3 | 312, 292sh | 337.08 | 190.89 (100), 163.5 (2) | 5-p-coumaroylquinic acid |
| 11 | 42.1 | 332sh, 294 | 465.03 | 338.95 (24), 284.94 (100). 150.91 (36) | Taxifolin hexoside |
| 12 | 46.1 | 249 | 479.37 | 319.90, 159.30 | 20-Hydroxyecdysone * |
| 13 | 46.3 | 352, 248 | 447.28 | 356.90 (44), 326.97 (100). 287.00 (29). 259.10 (18) | Orientin ** |
| 14 | 49.7 | 354, 248 | 609.14 | 446.97 (100), 285.0 (10) | Kaempferol dihexoside |
| 15 | 52.0 | 320, 248 | 355.38 | 192.90 (100) | Hydroxymethoxycinnamic acid hexoside |
| 16 | 57.4 | 344, 248 | 431.08 | 310.99 (100) | Apigenin-C-hexoside |
| 17 | 58.9 | 353, 280 | 609.30 | 342.87 (6). 300.85 (100) | Quercetin rutinoside *,**,# |
| 18 | 65.8 | - | 447.28 | 284.90 (100) | Kaempferol hexoside |
| 19 | 68.2 | 341, 325, 280 | 463.23 | 343.00 (1.3). 300.88 (100) | Quercetin hexoside |
| 20 | 68.6 | 350, 300sh | 433.18 | 300.88 (100) | Quercetin pentoside |
| 21 | 69.1 | 350, 297 sh | 593.34 | 284.95 (100) | Kaempferol rutinoside |
| 22 | 70.0 | 354, 280sh | 623.29 | 315.02 (100) | Isorhamnetin rutinoside |
| 23 | 70.2 | - | 505.54 | 462.98 (32). 300.91 (100) | Quercetin acetyl glucoside ** |
| 24 | 72.1 | - | 519.36 | 314.92 (100) | Isorhamnetin acetylhexoside |
| Samples | Yield of Extraction (%) | TP (g GAE/100g XAD7 Extract) | TF (g CE/100g XAD7 Extract) | DPPH (SC50, µg XAD7 Extract/mL) | FRAP (µmol TE/g XAD7 Extract) | TEAC (µM TE/g XAD7 Extract) | ORAC (µmol TE/g XAD7 Extract) |
|---|---|---|---|---|---|---|---|
| 2016 | nd | 4.1 ± 0.1 a | 2.4 ± 0.1 a | 93.6 ± 2.9 a | 183.0 ± 5.0 a | 206.8 ± 11.2 a | 859.7 ± 28.9 a |
| 2017a | 1.1 | 2.0 ± 0.0 b | 1.3 ± 0.0 b | >100 | inactive | inactive | 371.2 ± 17.3 b |
| 2017b | 0.4 | 7.1 ± 0.1 c | 5.3 ± 0.1 c | 33.1 ± 0.4 b | 359.8 ± 10.3 b | inactive | 867.4 ± 48.3 a |
| 2018 | 0.3 | 7.3 ± 0.1 c | 5.0 ± 0.1 d | 67.6 ± 1.9 c | 473.3 ± 8.8 c | 514.7 ± 10.6 b | 1921.2 ± 149.8 c |
| Quercetin * | - | - | - | 7.8 ± 0.3 | 1077.2 ± 16.4 | 8157.9 ± 22.1 | 22561.6 ± 808.8 |
| Samples | α-Glucosidase (IC50, µg XAD7 Extract/mL) | α-Amylase (IC50, µg XAD7 Extract/mL) | Pancreatic Lipase (IC50, µg XAD7 Extract/mL) |
|---|---|---|---|
| 2016 | 8.7 ± 0.3 a | inactive | inactive |
| 2017a | 4.8 ± 0.4 b | inactive | inactive |
| 2017b | 4.6 ± 0.1 b | inactive | inactive |
| 2018 | 5.7 ± 0.2 c | inactive | inactive |
| Acarbose * | 137.7 ± 1.3 µg/mL | 28.5 ± 0.3 µg/mL | ND |
| Orlistat | ND | ND | 0.04 ± 0.00 µg/mL |
| Fraction | α-Glucosidase Inhibition | α-Amylase Inhibiton | Pancreatic Lipase Inhibition |
|---|---|---|---|
| 1–6 | 39.6 ± 2.5% | inactive | inactive |
| 7–8 (20-OH-ecdysone) | 40.1 ± 1.3% | inactive | inactive |
| 9–12 | 28.8 ± 2.1 µg/mL | inactive | inactive |
| 13–14 | 3.4 ± 0.1 µg/mL | inactive | inactive |
| 15–18 | 4.7 ± 0.2 µg/mL | inactive | inactive |
| 19–20 | 2.9 ± 0.1 µg/mL | inactive | inactive |
| 21–22 (Caffeic acid) | 11.4 ± 0.7 µg/mL | inactive | inactive |
| 23–24 | 9.9 ± 0.3 µg/mL | inactive | inactive |
| 25–26 | 7.8 ± 0.2 µg/mL | inactive | inactive |
| 27–28 | 8.4 ± 0.5 µg/mL | inactive | inactive |
| 29–30 | 37.3 ± 1.1 µg/mL | inactive | inactive |
| 31–32 (Rutin) | 44.8 ± 1.6% | inactive | inactive |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Aspee, F.; Theoduloz, C.; Pormetter, L.; Mettke, J.; Ávila, F.; Schmeda-Hirschmann, G. Andean Prumnopitys Andina (Podocarpacae) Fruit Extracts: Characterization of Secondary Metabolites and Potential Cytoprotective Effect. Molecules 2019, 24, 4028. https://doi.org/10.3390/molecules24224028
Jiménez-Aspee F, Theoduloz C, Pormetter L, Mettke J, Ávila F, Schmeda-Hirschmann G. Andean Prumnopitys Andina (Podocarpacae) Fruit Extracts: Characterization of Secondary Metabolites and Potential Cytoprotective Effect. Molecules. 2019; 24(22):4028. https://doi.org/10.3390/molecules24224028
Chicago/Turabian StyleJiménez-Aspee, Felipe, Cristina Theoduloz, Lisa Pormetter, Judith Mettke, Felipe Ávila, and Guillermo Schmeda-Hirschmann. 2019. "Andean Prumnopitys Andina (Podocarpacae) Fruit Extracts: Characterization of Secondary Metabolites and Potential Cytoprotective Effect" Molecules 24, no. 22: 4028. https://doi.org/10.3390/molecules24224028
APA StyleJiménez-Aspee, F., Theoduloz, C., Pormetter, L., Mettke, J., Ávila, F., & Schmeda-Hirschmann, G. (2019). Andean Prumnopitys Andina (Podocarpacae) Fruit Extracts: Characterization of Secondary Metabolites and Potential Cytoprotective Effect. Molecules, 24(22), 4028. https://doi.org/10.3390/molecules24224028






