3.6.2. Microwaves Methods
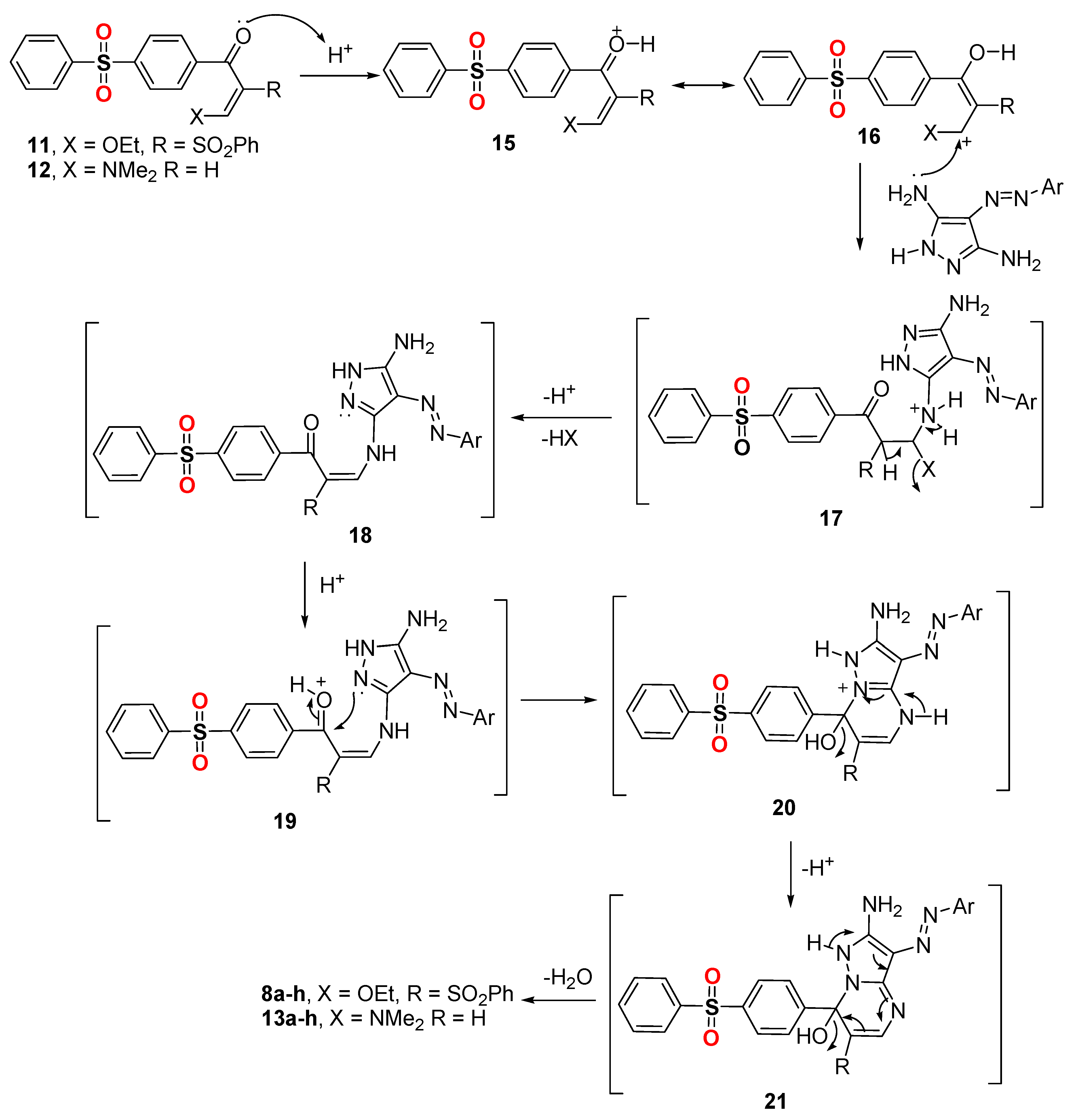
Method A: A mixture of Enaminone 6 or enaminone 12 (0.001 mol) and the appropriate arylazodiaminopyrazoles 7a–h (0.001 mol) in in 20 mL glacial acetic acid were mixed in a HP-500 process vial. The vail was capped properly and was irradiated by microwaves irradiation (800 W power) using pressurized conditions at 110 °C for 15 min. The reaction mixture was left to cool and the precipitated solid product was collected by filtration, washed with EtOH, dried, and finally recrystallized from DMF/EtOH to afford the corresponding pyrazolo[1,5-a]pyrimidines 8a–h or 13a–h. The physical and spectral data of the synthesized compounds 8a–h and 13a–h are listed below.
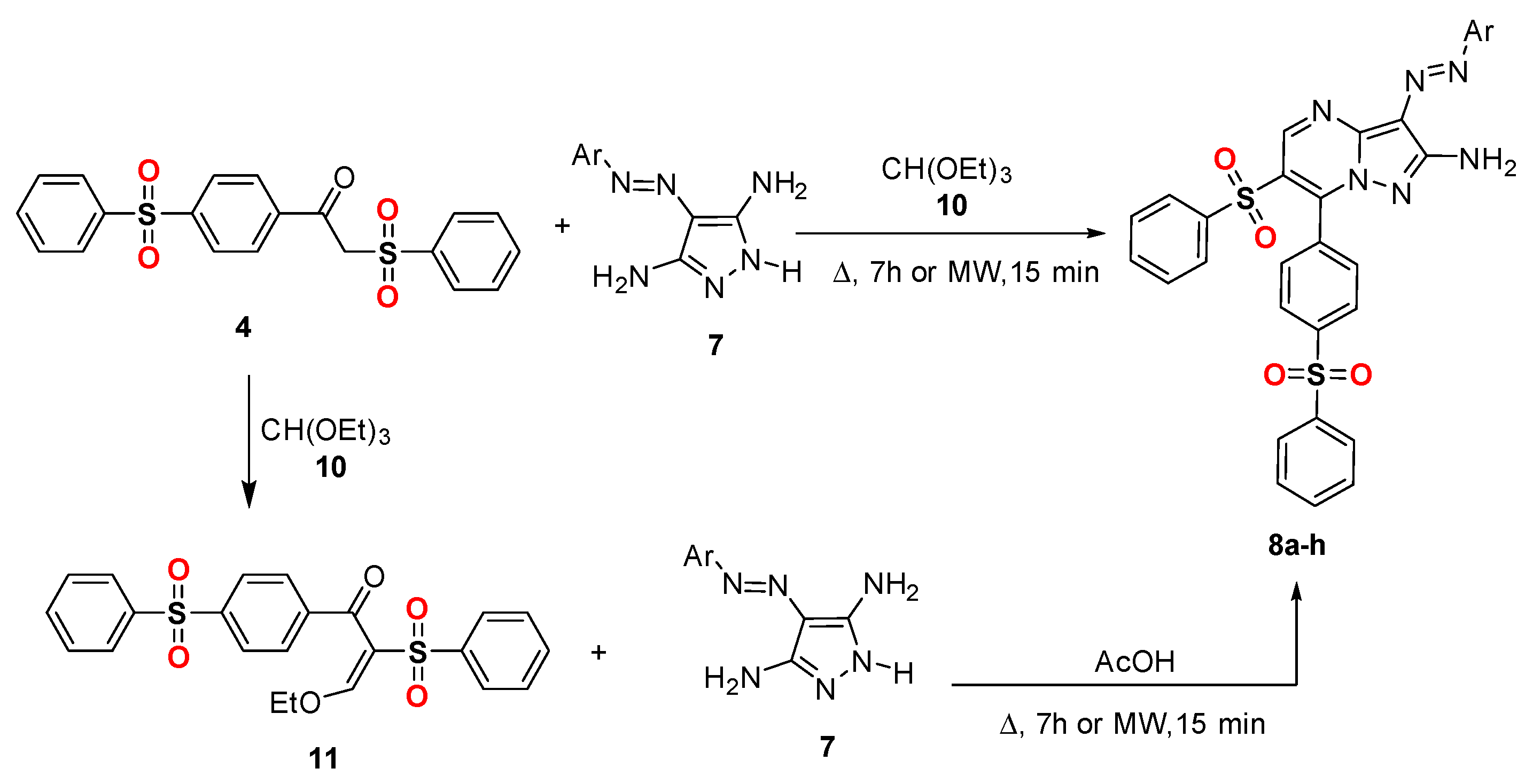
Method B (for compounds 8a–h only): A mixture of disulfone derivative 4 (0.4 g, 0.001 mol) and an equivalent molar ratio of the appropriate arylazodiaminopyrazoles 7a–h in triethylorthoformate (20 mL), was mixed in a HP-500 process vial. The vail was capped properly and was irradiated by microwaves irradiation (800 W power) using pressurized conditions at 110 °C for 15 min. The reaction mixture was left to cool and the precipitated solid product was collected by filtration, washed with EtOH, dried, and finally recrystallized from DMF/EtOH to afford the corresponding pyrazolo[1,5-a]pyrimidines 8a–h. The physical and spectral data of the synthesized compounds 8a–h and 13a–h are listed below.
Method C (for compounds 8a–h only): A mixture of compound 11 (0.001 mol) and the appropriate arylazodiaminopyrazoles 7a–h (0.001 mol) in in 20 mL glacial acetic acid were mixed in a HP-500 process vial. The vail was capped properly and was irradiated by microwaves irradiation (800 W power) using pressurized conditions at 110 °C for 15 min. The reaction mixture was left to cool and the precipitated solid product was collected by filtration, washed with EtOH, dried, and finally recrystallized from DMF/EtOH to afford the corresponding pyrazolo[1,5-a]pyrimidines 8a–h.
6-Benzenesulfonyl-7-(4-benzenesulfonyl-phenyl)-3-(4-chloro-phenylazo)-pyrazolo[1,5-a]pyrimidin-2-ylamine (8a)
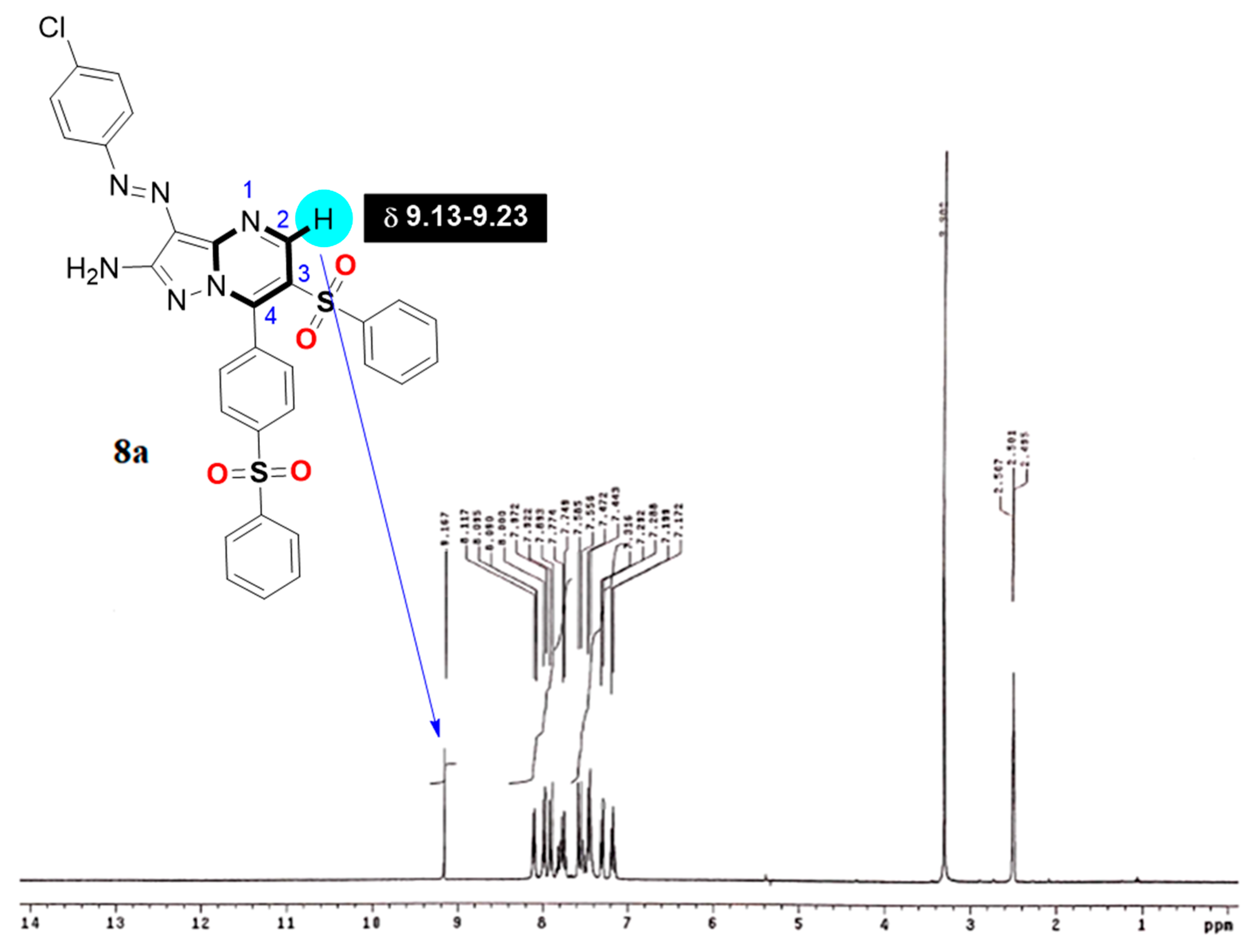
Yellow solid, mp.: 235–237 °C, IR ύ: 3464, 3362 (NH2), 3100 (sp2-CH), 2900 (sp3-CH), 1616 (C=N) cm−1; 1H NMR (DMSO-d6) δ: 7.17–7.89 (m, 12H, Ar-H and NH2), 7.56 (d, J = 8 Hz, 2H, Ar-H), 7.86 (d, J = 8 Hz, 2H, Ar-H), 7.92 (d, J = 8 Hz, 2H, Ar-H), 8.09 (d, J = 8 Hz, 2H, Ar-H), 9.17 (s, 1H, pyrimidine-H). 13C NMR (DMSO-d6) δ: 115.6, 120.6, 122.1, 123.0, 126.7, 127.0, 127.7, 128.9, 129.1, 129.9, 130.9, 132.3, 133.6, 134.1, 140.1, 140.5, 142.8, 144.9, 147.9, 148.7, 151.4, 153.9. Ms m/z (%) 628 (M+, 16), 557 (14), 521(28), 508 (93), 483 (15), 410 (38), 381 (98), 346 (16), 335 (100), 274 (17), 236 (27), 217 (13). Anal. Calcd. For: C30H21ClN6O4S2 (629.11) C, 57.27; H, 3.36; N, 13.36. Found: C, 57.09; H, 3.16; N, 13.27%.
6-Benzenesulfonyl-7-(4-benzenesulfonyl-phenyl)-3-(3-methyl-phenylazo)-pyrazolo[1,5-a]pyrimidin-2-ylamine (8b)
Pale brown solid, mp.: 233–235 °C, IR ύ: 3459, 3337 (NH2), 3096 (sp2-CH), 2980 (sp3-CH), 1609 (C=N) cm−1; 1H NMR (DMSO-d6) δ: 2.39 (s, 3H, CH3), 7.15–7.80 (m, 16H, Ar-H and NH2), 7.99 (d, J = 9 Hz, 2H, Ar-H), 8.09 (d, J = 9 Hz, 2H, Ar-H), and 9.16 (s, 1H, pyrimidine-H).13C NMR (DMSO-d6) δ: 20.9 (CH3), 115.4, 119.2, 121.5, 121.8, 126.8, 127.0, 127.8, 128.9, 129.1, 130.0, 130.2, 131.0, 132.4, 133.7, 134.2, 138.5, 140.2, 140.6, 142.7, 144.9, 147.8, 148.5, 152.7, and 154.0. Ms m/z (%) 610 (M+ + 2, 13), 608 (M+, 23), 593 (14), 541 (100), 532 (23), 517 (32), 489 (16), 391 (34), 326 (9), 235 (16), 217 (9), 140 (15), 129 (90), 95 (43), and 76 (10). Anal. Calcd. For: C31H24N6O4S2 (608.69) C, 61.17; H, 3.97; N, 13.81. Found: C, 61.03; H, 3.82; N, 13.69%.
6-Benzenesulfonyl-7-(4-benzenesulfonyl-phenyl)-3-(3-chloro-phenylazo)-pyrazolo[1,5-a]pyrimidin-2-ylamine (8c)
Yellow crystals, mp.: 260–262 °C, IR ύ: 3408, 3280 (NH2), 3068 (sp2-CH) 1616 (C=N); 1H NMR (DMSO-d6) δ: 3.57 (s, 2H, NH2), 7.16 (t, J = 7.65 Hz, 2H, Ar-H), 7.30 (d, J = 8.5 Hz, 2H, Ar-H), 7.46 (d, J = 7.65 Hz, 2H, Ar-H), 7.53–7.86 (m, 7H, Ar-H), 7.95 (s, 1H, Ar-H), 7.99 (d, J = 7.65 Hz, 2H, Ar-H), 8.10 (d, J = 7.65 Hz, 2H, Ar-H), and 9.19 (s, 1H, N=CH). 13C NMR (DMSO-d6) δ: 115.8, 120.4, 120.9, 122.4, 126.9, 127.1, 127.9, 128.8, 129.0, 130.0, 130.1, 131.0, 132.4, 133.8, 134.0, 134.3, 140.1, 140.6, 142.8, 145.1, 148.1, 148.8, 153.9, and 154.0. Ms m/z (%) 628 (M+−1, 18), 552 (27), 520 (11), 483 (17), 409 (26), 345 (15), 274 (10), 236 (15), 216 (17), 111 (8), and 72 (100). Anal. Calcd. For: C30H21ClN6O4S2 (629.11) C, 57.27; H, 3.36; N, 13.36. Found: C, 57.08; H, 3.21 N, 13.29%.
6-Benzenesulfonyl-7-(4-benzenesulfonyl-phenyl)-3-(2-chloro-phenylazo)-pyrazolo[1,5-a]pyrimidin-2-ylamine (8d)
Yellow solid, mp.: 250–252 °C, IR ύ:br. 3458 (NH2), 1612 (C=N); 1H NMR (DMSO-d6) 5.40 (s, 2H, NH2), 7.17–7.82 (m, 14H, Ar-H), 7.98 (d, J = 8 Hz, 2H, Ar-H), 8.09 (d, J = 8 Hz, 2H, Ar-H), and 9.22 (s, 1H, pyrimidine-H). 13C NMR (DMSO-d6) δ: 111.9, 116.4, 116.7, 122.7, 126.8, 127.8, 127.1, 127.6, 128.1, 128.9, 130.3, 130.7, 132.2, 132.3, 134.2, 138.0, 139.9, 140.5, 142.8, 145.2, 147.8, 149.0, 153.7, and 156.7. Ms m/z (%) 629 (M+, 32), 579 (100), 552 (49), 519 (17), 504 (35), 489 (15), 412 (7), 346 (34), 275 (8), 141 (6), and 111 (19). Anal. Calcd. For: C30H21ClN6O4S2 (629.11) C, 57.27; H, 3.36; N, 13.36. Found: C, 57.07; H, 3.30; N, 13.15%.
6-Benzenesulfonyl-7-(4-benzenesulfonyl-phenyl)-3-(3-methoxy-phenylazo)-pyrazolo[1,5-a]pyrimidin-2-ylamine (8e)
Dark yellow solid, mp.: 200–202 °C, IR ύ br. 3450 (NH2), 1618 (C=N); 1H NMR (DMSO-d6) δ: 3.57 (s, 3H, OCH3), 5.40 (s, 2H, NH2), 7.56–8.12 (m, 18H, Ar-H), and 9.17 (s, 1H, pyrimidine-H). Ms m/z (%) 624 (M+, 50), 453 (87), 439 (99), 318 (94), and 274 (100). Anal. Calcd.For: C31H24N6O5S2 (624.69) C, 59.60; H, 3.87; N, 13.45. Found: C, 59.46; H, 3.73; N, 13.21%.
6-Benzenesulfonyl-7-(4-benzenesulfonyl-phenyl)-3-phenylazo-pyrazolo[1,5-a]pyrimidin-2-ylamine (8f)
Dark yellow solid, mp.: 270–272 °C, IR ύ: br, 3456 (NH2), 1614 (C=N); 1H NMR (DMSO-d6) δ: 7.15–7.89 (m, 15H, Ar–H), 7.98 (d, J = 8 Hz, 2H, Ar-H), 8.09 (d, J = 8 Hz, 2H, Ar-H), and 9.18 (s, 1H, pyrimidine-H). 13C NMR (DMSO-d6) δ: 115.4, 121.6, 121.9, 126.9, 127.1, 127.9, 129.0, 129.2, 129.6, 130.1, 131.0, 132.5, 133.8, 134.3, 140.2, 140.6, 142.8, 145.0, 147.9, 148.6, 152.7, and 154.0. Ms m/z (%) 594 (M+, 20), 580 (18), 523 (22), 490 (32), 457 (49), 375 (100), 273 (28), 218 (28), 142 (24), and 77 (27). Anal. Calcd. For: C30H22N6O4S2 (594.66) C, 60.59; H, 3.73; N, 14.13. Found: C, 60.46; H, 3.61; N, 14.02%.
6-Benzenesulfonyl-7-(4-benzenesulfonyl-phenyl)-3-(2-nitro-phenylazo)-pyrazolo[1,5-a]pyrimidin-2-ylamine (8g)
Dark red solid, mp.: 285–287 °C, IR ύ: 3446, 3340 (NH2), 1620, 1585 (C=N).; 1H NMR (DMSO-d6) δ: 7.16–8.12 (m, 20H, Ar–H), 9.23 (s, 1H, pyrimidine-H). 13C NMR (DMSO-d6) δ:117.3, 117.4, 120.5, 123.3, 124.4, 126.8, 127.1, 127.8, 128.9, 129.5, 129.9, 130.9, 132.2, 133.6, 133.8, 134.2, 139.9, 140.6, 142.9, 144.6, 145.3, 146.0, 149.4, and 153.6. Ms m/z (%) 639 (M+, 23), 620 (19), 563 (15), 516 (13), 494 (14), 438 (99), 423 (32), 148 (21), 137 (17), 123 (12), 77 (8), and 60 (100). Anal. Calcd. For: C30H21N7O6S2 (639.66) C, 56.33; H, 3.31; N, 15.33. Found: C, 56.18; H, 3.29; N, 15.08%.
6-Benzenesulfonyl-7-(4-benzenesulfonyl-phenyl)-3-(4-methoxy-phenylazo)-pyrazolo[1,5-a]pyrimidin-2-ylamine (8h)
Pale brown solid, mp.: 220–222 °C (EtOH), IR ύ: br. 3450 (NH2), 1616 (C=N); 1H NMR (DMSO-d6) δ: 3.83 (s, 3H, OCH3), 7.05 (d, J = 8 Hz, 2H, Ar-H), 7.14–7.47 (m, 10H, Ar-H), 7.53 (s, 2H, NH2), 7.85 (d, J = 8 Hz, 2H, Ar-H), 7.97 (d, J = 8 Hz, 2H, Ar-H), 8.09 (d, J = 8 Hz, 2H, Ar-H), and 9.13 (s, 1H, pyrimidine-H). 13C NMR (DMSO-d6) δ: 55.5, 114.4, 115.0, 121.4, 123.1, 126.8, 127.0, 127.8, 128.9, 129.9, 130.9, 132.5, 133.6, 134.1, 140.3, 140.6, 142.8, 144.8, 146.8, 147.2, 148.2, 154.2, and 160.6. Ms m/z (%) 624 (M+, 24), 550 (16), 488 (18), 407 (47), 273 (38), 217 (44), 159 (100), 133 (6), and 80 (28). Anal. Calcd. For: C31H24N6O5S2 (624.69) C, 59.60; H, 3.87; N, 13.45. Found: C, 59.42; H, 3.70; N, 13.31%.
7-(4-Benzenesulfonyl-phenyl)-3-(4-chloro-phenylazo)-pyrazolo[1,5-a]pyrimidin-2-ylamine (13a)
Orange crystals, mp.: 280–282 °C (EtOH), IR ύ: 3421, 3298 (NH2), 3099 (sp2-CH), 2900 (sp3-CH), 1616 (C=N) cm−1; 1H NMR (DMSO-d6) δ: 7.30 (d, J = 3.9 Hz, 1H, Pyrimidine-H), 7.52 (d, J = 9 Hz, 2H, Ar-H), 7.68–7.70 (m, 7H, Ar-H and NH2), 7.83 (d, J = 9 Hz, 2H, Ar-H), 8.04–8.25 (m, 4H, Ar-H), 8.63 (d, J = 3.9 Hz, and 1H, Pyrimidine-H). 13C NMR (DMSO-d6) δ: 110.0, 114.9, 122.8, 127.4, 127.7, 129.18, 130.0, 131.0, 132.7, 134.2, 135.2, 140.6, 142.9, 143.5, 147.6, 150.9, 151.7, and 151.9. Ms m/z (%) 488 (M+, 14), 414 (14), 363 (24), 270 (17), 255 (29), 218 (13), 140 (9), 131 (100), 121 (74), 110 (24), and 102 (29). Anal. Calcd. For: C24H17ClN6O2S (488.95) C, 58.95; H, 3.50; N, 17.19. Found: C, 58.76; H, 3.41; N, 17.06%.
7-(4-Benzenesulfonyl-phenyl)-3-m-tolylazo-pyrazolo[1,5-a]pyrimidin-2-ylamine (13b)
Red solid, mp.: 235–237 °C (AcOH), IR ύ: 3421, 3274 (NH2), 3165 (sp2-CH), 2919 (sp3-CH), 1616 (C=N) cm−1; 1H NMR (DMSO-d6) δ: 2.39 (s, 3H, CH3), 7.18 (d, J = 7.65 Hz, 2H, Ar-H), 7.25 (s, 2H, NH2), 7.27 (d, J = 5.1 Hz, 1H, Pyrimidine-H), 7.37 (t, J = 7.65 Hz, 2H, Ar-H), 7.62 (d, J = 7.65 Hz, 1H, Ar-H), 7.64 (s, 1H, Ar-H), 7.69 (t, J = 7.65 Hz, 1H, Ar-H), 7.75 (t, J = 7.65 Hz, 1H, Ar-H), 8.06 (d, J = 7.65 Hz, 1H, Ar-H), 8.18 (d, J = 7.65 Hz, 2H, Ar-H), 8.23 (d, J = 7.65 Hz, 2H, Ar-H), 8.63 (d, J = 5.1 Hz, 1H, Pyrimidine-H). 13C NMR (DMSO-d6) δ: 20.9 (CH3), 109.5, 114.6, 118.9, 120.5, 121.2, 127.3, 127.6, 128.8, 129.3, 129.9, 134.0, 135.2, 138.4, 140.6, 142.9, 143.3, 147.5, 150.6, 151.8, 152.9. Ms m/z (%) 468 (M+, 8), 454 (14), 395 (11), 377 (15), 311 (12), 250 (7), 215 (7), 171 (74), 142 (2), 91 (14), 81 (100). Anal. Calcd. For: C25H20N6O2S (468.53) C, 64.09; H, 4.30; N, 17.94. Found: C, 63.89; H, 4.21; N, 17.85%.
7-(4-Benzenesulfonyl-phenyl)-3-(3-chloro-phenylazo)-pyrazolo[1,5-a]pyrimidin-2-ylamine (13c)
Red crystals, mp.: 245–247 °C (AcOH), IR ύ: 3421, 3274 (NH2), 3067 (sp2-CH), 1616 (C=N) cm−1; 1H NMR (DMSO-d6) δ: 7.26–8.23 (m, 16H, Ar-H and NH2), 8.61 (d, J = 4.5 Hz, 1H, pyrimidine-H).13C NMR (DMSO-d6) δ: 110.0, 115.1, 120.2, 120.3, 127.3, 127.6, 127.8, 129.9, 130.5, 130.9, 133.8, 134.0, 135.1, 140.6, 142.9, 143.4, 147.7, 150.9, 151.9, and 154.2. Ms m/z (%) 488 (M+, 8), 458 (19), 412 (19), 377 (11), 273 (44), 249 (100), 218(7), 161 (14), 143 (37), 111 (59), and 108 (74). Anal. Calcd. For: C24H17ClN6O2S (488.95) C, 58.95; H, 3.50; N, 17.19. Found: C, 58.88; H, 3.41; N, 17.01%.
7-(4-Benzenesulfonyl-phenyl)-3-(2-chloro-phenylazo)-pyrazolo[1,5-a]pyrimidin-2-ylamine (13d)
Orange crystals, mp.: 280–282 °C (AcOH), IR ύ: 3398, 3298 (NH2), 3164 (sp2-CH), 2997 (sp3-CH), 1615 (C=N) cm−1; 1H NMR (DMSO-d6) δ: 7.32 (d, J = 4 Hz, 1H, pyrimidine-H), 7.46 (s, 2H, NH2), 7.67–8.24 (m, 13H, Ar-H), and 8.66 (d, J = 4 Hz, 1H, pyrimidine-H). 13C NMR (DMSO-d6) δ: 110.6, 116.4, 116.43, 127.4, 127.7, 128.0, 129.8, 130.0, 130.3, 131.1, 131.5, 134.2, 135.1, 140.6, 143.0, 143.8, 147.8, 148.2, 151.3, and 151.9. Ms m/z (%) 488 (M+, 8), 452 (13), 411 (100), 378 (28), 364 (20), 272 (30), 254 (17), 213 (25), 111 (26), and 90 (27). Anal. Calcd. For: C24H17ClN6O2S (488.95) C, 58.95; H, 3.50; N, 17.19. Found: C, 58.82; H, 3.34; N, 17.06%.
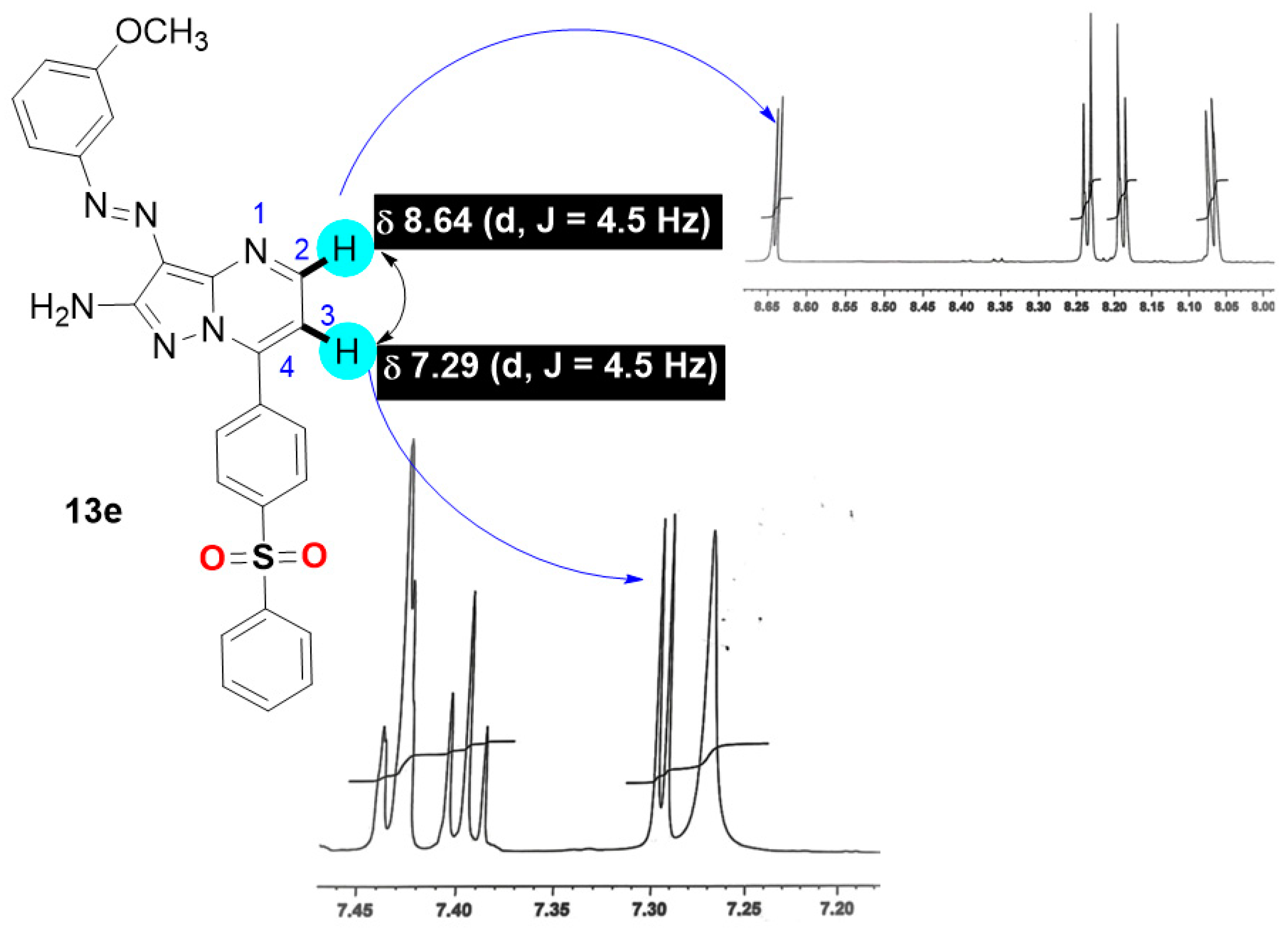
7-(4-Benzenesulfonyl-phenyl)-3-(3-methoxy-phenylazo)-pyrazolo[1,5-a]pyrimidin-2-ylamine (13e)
Red solid, mp.: 258–260 °C (AcOH), IR ύ: 3437, 3298 (NH2), 3155 (sp2-CH), 2909 (sp3-CH), 1612 (C=N) cm−1; 1H NMR (DMSO-d6) δ: 3.83 (s, 3H, OCH3), 6.94–6.95 (m, 1H, Ar-H), 7.27 (s, 2H, NH2), 7.29 (d, J = 4.5 Hz, 1H, pyrimidine-H), 7.39–7.44 (m, 3H, Ar-H), 7.68 (t, J = 7.65 Hz, 2H, Ar-H), 7.74 (t, J = 6.8 Hz, 1H, Ar-H), 8.06 (d, J = 7.65 Hz, 2H, Ar-H), 8.18 (d, J = 8.5 Hz, 2H, Ar-H), 8.23 (d, J = 8.5 Hz, 2H, Ar-H), and 8.64 (d, J = 4.5 Hz, 1H, pyrimidine-H). 13C NMR (DMSO-d6) δ: 55.2, 105.5, 109.6, 114.1, 114.6, 114.9, 127.3, 127.6, 129.7, 129.9, 130.9, 134.0, 135.2, 140.6, 142.9, 143.3, 147.5, 150.7, 151.9, 154.2, and 160.0. Ms m/z (%) 484 (M+, 15), 454 (24), 407 (11), 379 (17), 360 (100), 349 (12), 327 (10), 267 (7), 218 (15), 160 (7), 136 (41), and 78(17). Anal. Calcd. For: C25H20N6O3S (484.53) C, 61.97; H, 4.16; N, 17.34. Found: C, 61.76; H, 4.00; N, 17.15%.
7-(4-Benzenesulfonyl-phenyl)-3-phenylazo-pyrazolo[1,5-a]pyrimidin-2-ylamine (13f)
Red solid, mp.: 215–217 °C (AcOH), IR ύ: 3420, 3270 (NH2), 3162 (sp2-CH), 2998 (sp3-CH), 1616 (C=N) cm−1; 1H NMR (DMSO-d6) δ: 7.22–8.24 (m, 17H, Ar-H and NH2), 8.60 (d, J = 4.5 Hz, 1H, pyrimidine-H). 13C NMR (DMSO-d6) δ: 114.7, 121.1, 127.3, 127. 6, 129.0, 129.2, 129.9, 130.9, 131.1, 134.0, 135.1, 140.6, 142.9, 143.3, 147.5, 150.6, 151.8, and 152.9. Ms m/z (%) 454 (M+, 19), 438 (27), 364 (7), 361 (27), 349 (16), 313 (14), 237 (10), 217 (14), 208 (27), and 106 (100). Anal. Calcd. For: C24H18N6O2S (454.50) C, 63.42; H, 3.99; N, 18.49. Found: C, 63.32; H, 3.88; N, 18.32%.
7-(4-Benzenesulfonyl-phenyl)-3-(2-nitro-phenylazo)-pyrazolo[1,5-a]pyrimidin-2-ylamineylamine (13g)
Red solid, mp.: 265–267 °C (AcOH), IR ύ: 3410, 3294 (NH2), 3165 (sp2-CH), 2900 (sp3-CH), 1617 (C=N) cm−1; 1H NMR (DMSO-d6) δ: 7.46 (d, J = 4.5 Hz, 1H, pyrimidine-H), 7.60 (t, J = 7 Hz, 1H, Ar-H), 7.73–7.83 (m, 6H, Ar-H and NH2), 7.95 (dd, J = 8, 1.7Hz, 1H, Ar-H), 8.05 (dd, J = 8, 1.7Hz, 1H, Ar-H), 8.11 (dd, J = 8, 1.7Hz, 2H, Ar-H), 8.24 (d, J = 8.5 Hz, 2H, Ar-H), 8.27 (d, J = 8.5 Hz, 2H, Ar-H), and 8.76 (d, J = 4.5 Hz, 1H, pyrimidine-H). Ms m/z (%) 499 (M+, 58), 453 (11), 422 (28), 378 (27), 362 (5), 218 (6), 208 (100), 151(11), 142 (5), 122 (10), and 77 (30). Anal. Calcd. For: C24H17N7O4S (499.50) C, 57.71; H, 3.43; N, 19.63. Found: C, 57.56; H, 3.40; N, 19.52%.
7-(4-Benzenesulfonyl-phenyl)-3-(4-methoxy-phenylazo)-pyrazolo[1,5-a]pyrimidin-2-ylamine (13h)
Red, yield (94%), mp.: 255–257 °C (AcOH), IR ύ: 3447, 3337 (NH2), 3066 (sp2-CH), 2900 (sp3-CH), 1611 (C=N) cm−1; 1H NMR (DMSO-d6) δ: 3.83 (s, 3H, OCH3), 7.06 (d, J = 8.5 Hz, 2H, Ar-H), 7.17 (s, 2H, NH2), 7.24 (d, J = 5.1 Hz, 1H, pyrimidine-H), 7.68 (t, J = 7.65 Hz, 2H, Ar-H), 7.74 (t, J = 7.65 Hz, 1H, Ar-H), 7.81 (d, J = 8.5 Hz, 2H, Ar-H), 8.06 (d, J = 8.5 Hz, 2H, Ar-H), 8.18 (d, J = 8.5 Hz, 2H, Ar-H), 8.23 (d, J = 8.5 Hz, 2H, Ar-H), and 8.61(d, J = 4.5 Hz, 1H, pyrimidine-H). 13C NMR (DMSO-d6) δ: 55.4, 109.1, 114.1, 114.3, 122.6, 127.3, 127.6, 129.9, 130.8, 134.0, 135.3, 140.6, 142.9, 143.2, 147.1, 147.2, 150.4, 151.9, 159.9. Ms m/z (%) 484 (M+, 22), 454 (16), 406 (23), 377 (24), 351 (90), 327 (22), 251 (50), 134(5), 124 (43), 105 (19), and 58 (100). Anal. Calcd. For: C25H20N6O3S (484.53) C, 61.97; H, 4.16; N, 17.34. Found: C, 61.78; H, 4.03; N, 17.25%.