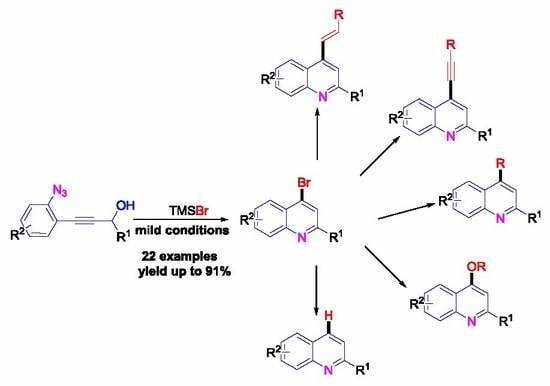
TMSBr-Promoted Cascade Cyclization of ortho-Propynol Phenyl Azides for the Synthesis of 4-Bromo Quinolines and Its Applications
Abstract
1. Introduction
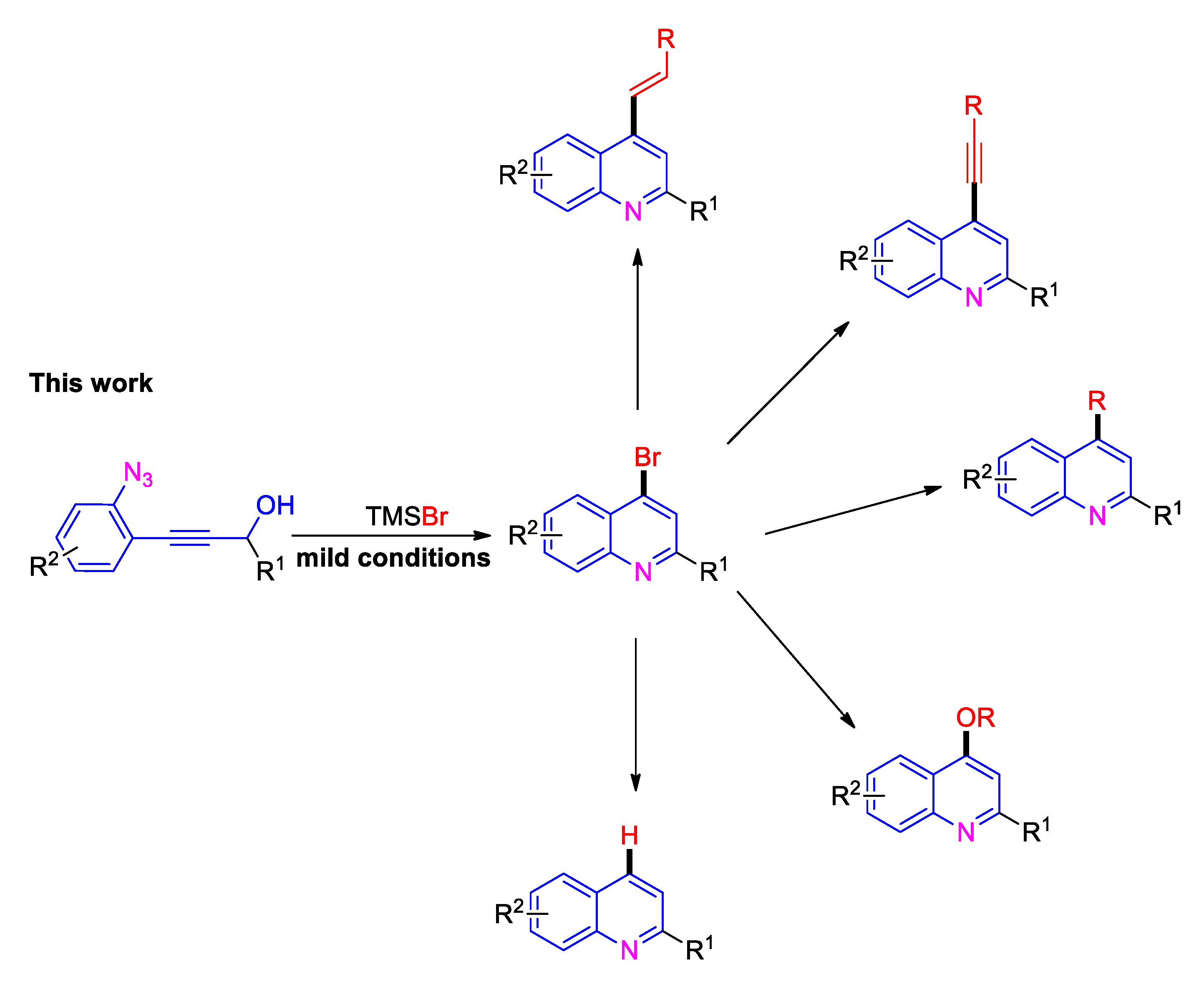
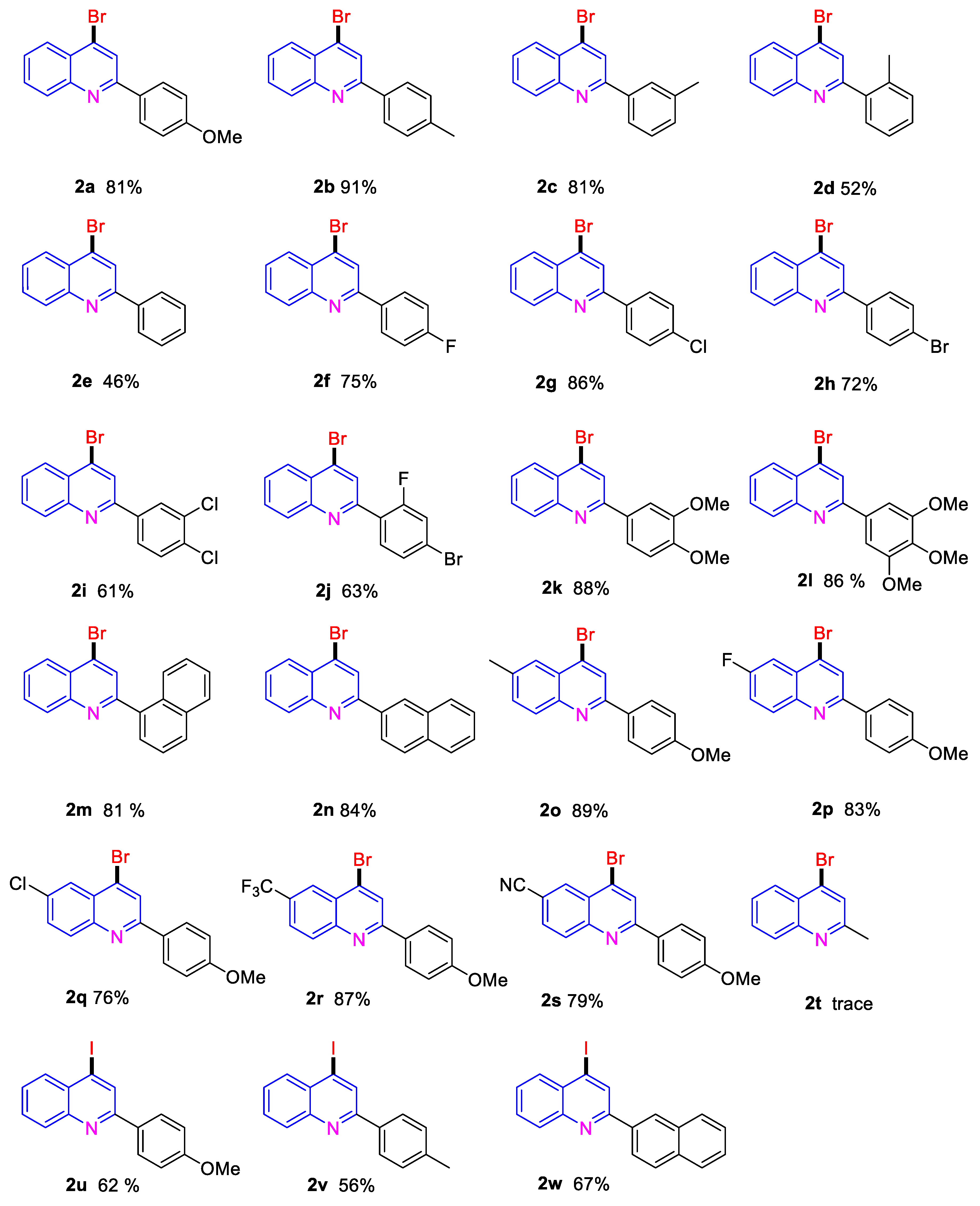
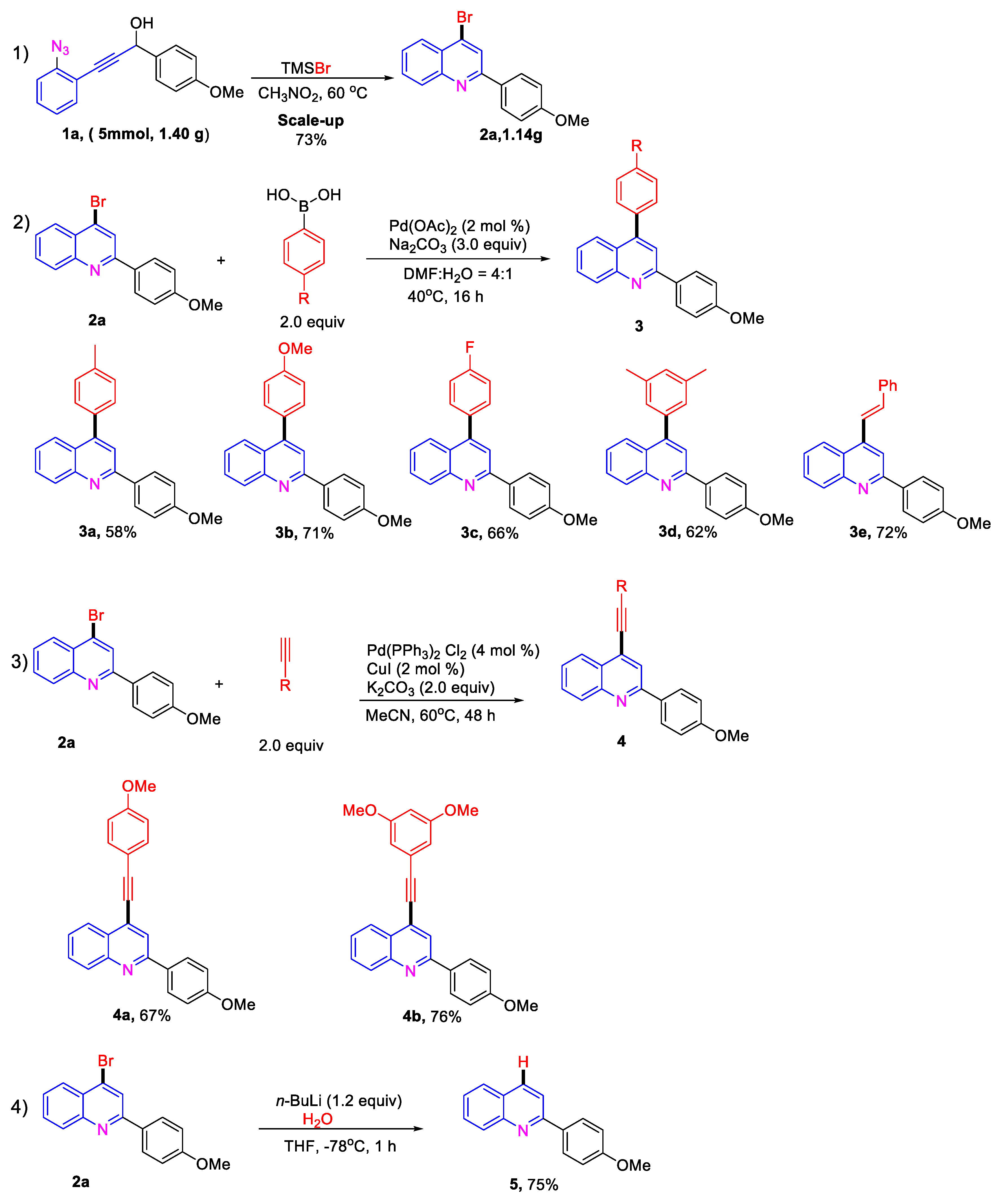
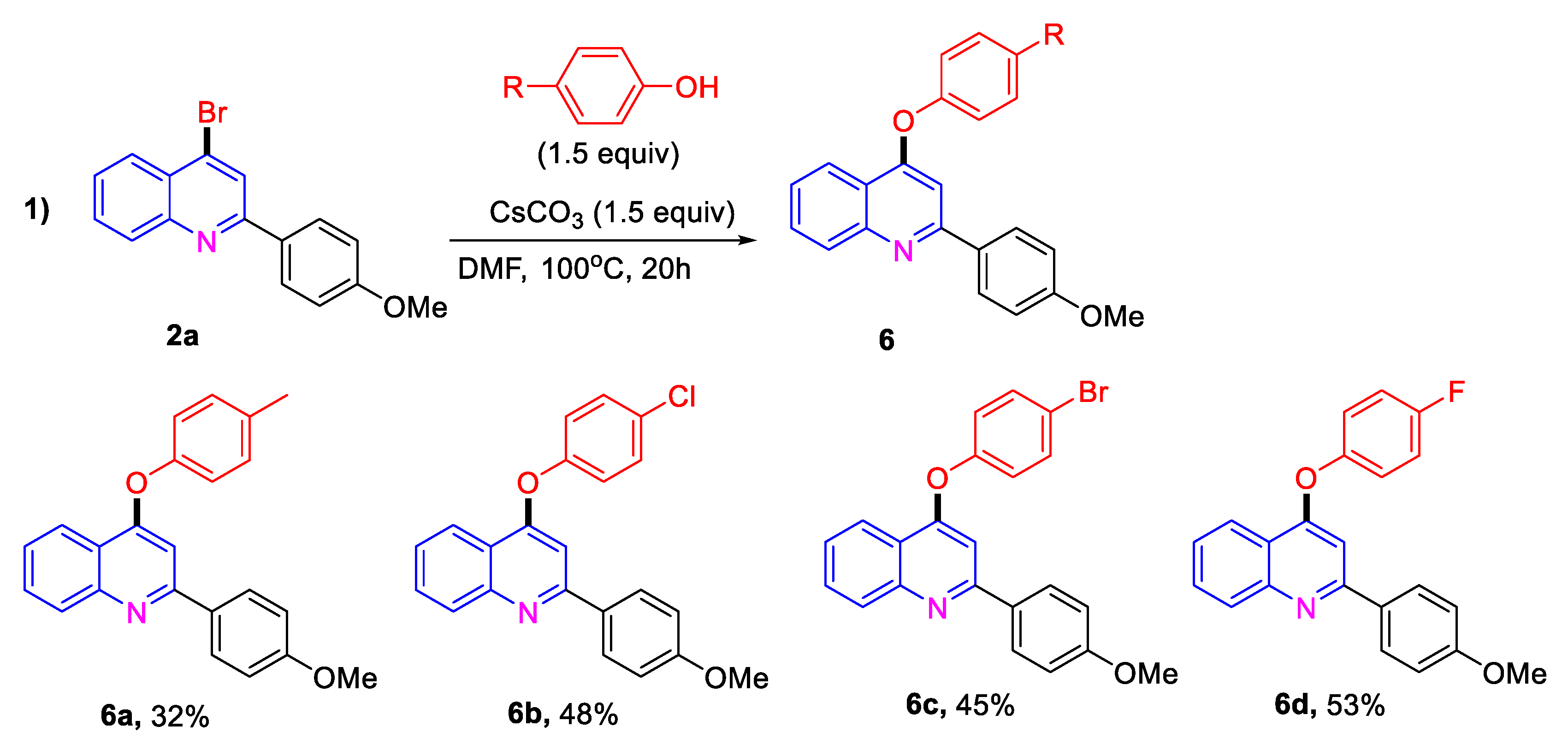
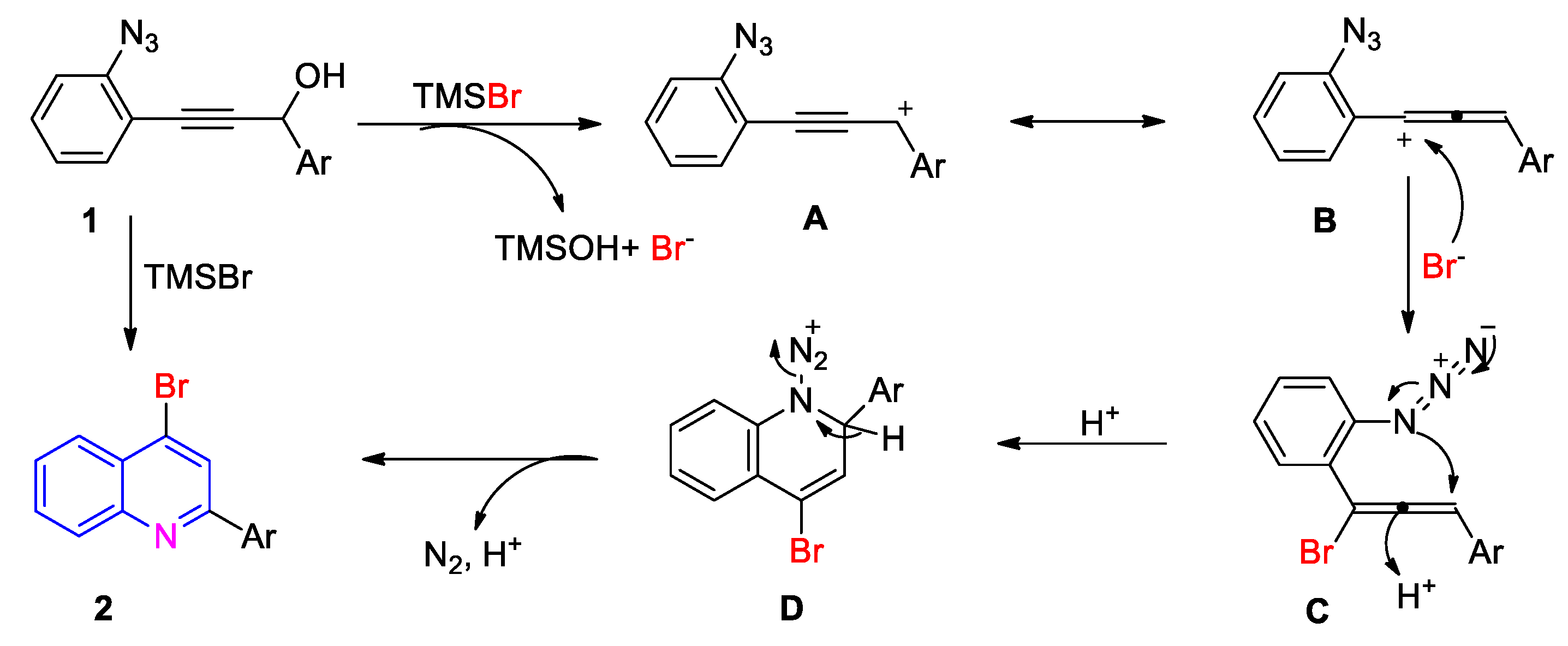
2. Results and Discussion
3. Materials and Methods
3.1. General Remarks
3.2. General Procedure for the Construction of 4-Bromo Quinolines 2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Joule, J.A.; Mills, K. Heterocylic Chemistry, 4th ed.; Blackwell Science, Ltd.: Oxford, UK, 2000; p. 121. [Google Scholar]
- Balasubraimanan, M.; Keay, J.G. Comprehensive Heterocyclic Chemistry II; Katritzky, A.R., Rees, C.W., Scriven, E.F.V., Eds.; Pergamon Press: Oxford, UK, 1996; Volume 5, p. 245. [Google Scholar]
- Michael, J.P. Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 2008, 25, 166–187. [Google Scholar] [CrossRef]
- Boa, A.N.; Canavan, S.P.; Hirst, P.R.; Ramsey, C.; Stead, A.M.W.; McConkey, G.A. Synthesis of brequinar analogue inhibitors of malaria parasite dihydroorotate dehydrogenase. Bioorg. Med. Chem. 2005, 13, 1945–1967. [Google Scholar] [CrossRef]
- Diao, Y.; Lu, W.; Jin, H.; Zhu, J.; Han, L.; Xu, M.; Gao, R.; Shen, X.; Zhao, Z.; Liu, X.; et al. Discovery of Diverse Human Dihydroorotate Dehydrogenase Inhibitors as Immunosuppressive Agents by Structure-Based Virtual Screening. J. Med. Chem. 2012, 55, 8341–8349. [Google Scholar] [CrossRef]
- Munier-Lehmann, H.; Lucas-Hourani, M.; Guillou, S.; Helynck, O.; Zanghi, G.; Noel, A.; Tangy, F.; Vidalain, P.; Janin, Y.L. Original 2-(3-Alkoxy-1H-pyrazol-1-yl)pyrimidine Derivatives as Inhibitors of Human Dihydroorotate Dehydrogenase (DHODH). J. Med. Chem. 2015, 58, 860–877. [Google Scholar] [CrossRef]
- Das, P.; Deng, X.; Zhang, L.; Roth, M.G.; Fontoura, B.M.A.; Phillips, M.A.; De Brabander, J.K. SAR-Based Optimization of a 4-Quinoline Carboxylic Acid Analogue with Potent Antiviral Activity. ACS Med. Chem. Lett. 2013, 4, 517–521. [Google Scholar] [CrossRef]
- Strekowski, L.; Say, M.; Zegrocka, O.; Tanious, F.A.; Wilson, W.D.; Manzel, L.; Macfarlane, D.E. Bis-4-aminoquinolines: Novel triple-helix DNA intercalators and antagonists of immunostimulatory CpG-oligodeoxynucleotides. Bioorg. Med. Chem. 2003, 11, 1079–1085. [Google Scholar] [CrossRef]
- Gogoi, S.; Zhao, C.-G. Organocatalyzed enantioselective synthesis of 6-amino-5-cyanodihydropyrano[2,3-c]pyrazoles. Tetrahedron Lett. 2009, 50, 2252–2255. [Google Scholar] [CrossRef]
- Tan, B.; Shi, Z.; Chua, J.P.; Zhong, G. Control of Four Stereocenters in an Organocatalytic Domino Double Michael Reaction: Efficient Synthesis of Multisubstituted Cyclopentanes. Org. Lett. 2008, 10, 3425–3428. [Google Scholar] [CrossRef]
- Wang, B.; Wu, F.; Wang, Y.; Liu, X.; Deng, L. Control of Diastereoselectivity in Tandem Asymmetric Reactions Generating Nonadjacent Stereocenters with Bifunctional Catalysis by Cinchona Alkaloids. J. Am. Chem. Soc. 2007, 129, 768–769. [Google Scholar] [CrossRef]
- Biddle, M.M.; Lin, M.; Scheidt, K.A. Catalytic Enantioselective Synthesis of Flavanones and Chromanones. J. Am. Chem. Soc. 2007, 129, 3830–3831. [Google Scholar] [CrossRef]
- Enders, D.; Grondal, C.; Hüttl, M.R.M. Asymmetric Organocatalytic Domino Reactions. Angew. Chem. Int. Ed. 2007, 46, 1570–1581. [Google Scholar] [CrossRef] [PubMed]
- Bharate, J.B.; Vishwakarma, R.A.; Bharate, S.B. Metal-free domino one-pot protocols for quinoline synthesis. RSC Adv. 2015, 5, 42020–42053. [Google Scholar] [CrossRef]
- Prajapati, S.M.; Patel, K.D.; Vekariya, R.H.; Panchal, S.N.; Patel, H.D. Recent advances in the synthesis of quinolines: A review. RSC Adv. 2014, 4, 24463–24476. [Google Scholar] [CrossRef]
- Hassanin, H.M.; Ibrahim, M.A.; Gabr, Y.A.; Alnamer, Y.A. Synthesis and Chemical Reactivity of Pyrano[3,2-c]quinolinones. J. Heterocycl. Chem. 2012, 49, 1269–1289. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, C.; Liu, L.; Zhao, J.; Su, L.; Zhu, Q. Sulfuric acid promoted condensation cyclization of 2-(2-(trimethylsilyl) ethynyl)anilines with arylaldehydes in alcoholic solvents: An efficient one-pot synthesis of 4-alkoxy-2-arylquinolines. Tetrahedron Lett. 2009, 50, 2261–2265. [Google Scholar] [CrossRef]
- Sridharan, V.; Avendano, C.; Menendez, J.C. CAN-catalyzed three-component reaction between anilines and alkyl vinyl ethers: Stereoselective synthesis of 2-methyl-1,2,3,4-tetrahydroquinolines and studies on their aromatization. Tetrahedron 2007, 63, 673–681. [Google Scholar] [CrossRef]
- Gharpure, S.J.; Nanda, S.K.; Adate, P.A.; Shelke, Y.G. Lewis Acid Promoted Oxonium Ion Driven Carboamination of Alkynes for the Synthesis of 4-Alkoxy Quinolines. J. Org. Chem. 2017, 82, 2067–2080. [Google Scholar] [CrossRef]
- Mao, X.-F.; Zhu, X.-P.; Li, D.-Y.; Liu, P.-N. Cu-Catalyzed Cascade Annulation of Alkynols with 2-Azidobenzaldehydes: Access to 6H-Isochromeno [4,3-c]quinoline. J. Org. Chem. 2017, 82, 7032–7039. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Xi, C. MeOTf-induced annulation of arylisocyanates and arylalkynes leading to 4-methoxyl-2,3-diarylquinolines. Tetrahedron Lett. 2018, 59, 2440–2442. [Google Scholar] [CrossRef]
- Yang, T.; Ding, H.; Li, R.; Jin, F.; Song, X.-R.; Chen, X.; Bai, J.; Xiao, Q.; Liang, Y.-M. para-TsOH-Promoted Cascade Reaction of ortho-Propynol Phenyl Azides for the Synthesis of 4-Methoxy Quinolines and Propargyl Methyl Ethers: Insight on Mechanism of Propargylic Alcohols. Asian J. Org. Chem. 2019, 8, 391–398. [Google Scholar] [CrossRef]
- Abbiati, G.; Arcadi, A.; Marinelli, F.; Rossi, E. Domino [3 + 2] Cycloaddition/Annulation Reactions of β-(2-Aminophenyl)-α,β-ynones with Nitrile Oxides: Synthesis of Isoxazolo[4,5-c]quinolines. Eur. J. Org. Chem. 2003, 2003, 1423–1427. [Google Scholar] [CrossRef]
- Strekowski, L.; Zegrocka, O.; Windham, C.; Czarny, A. Practical Synthesis of 4-Chloro-2-(2-naphthyl)quinoline, a Precursor to Triple-Helix DNA Intercalators. Org. Process Res. Dev. 1997, 1, 384–386. [Google Scholar] [CrossRef]
- Akila, S.; Selvi, S.; Balasubramanian, K. The Vilsmeier cyclization of 2′-azido and 2′-aminochalcones—A mild one pot synthesis of 2-aryl-4-chloroquinoline and its N-formyl-1,2-dihydro derivatives. Tetrahedron 2001, 57, 3465–3469. [Google Scholar] [CrossRef]
- Shvartsberg, M.S.; Kolodina, E.A. Synthesis of 4-haloquinolines and their fused polycyclic derivatives. Mendeleev Commun. 2008, 18, 109–111. [Google Scholar] [CrossRef]
- O’Neill, P.M.; Bray, P.G.; Hawley, S.R.; Ward, S.A.; Park, B.K. 4-Aminoquinolines—Past, present, and future; A chemical perspective. Pharmacol. Ther. 1998, 77, 29–58. [Google Scholar] [CrossRef]
- Blauer, G.; Akkawi, M.; Fleischhacker, W.; Hiessboeck, R. Synthesis and optical properties of the chloroquine enantiomers and their complexes with ferriprotoporphyrin IX in aqueous solution. Chirality 1998, 10, 556–563. [Google Scholar] [CrossRef]
- Egan, T.J.; Hunter, R.; Kaschula, C.H.; Marques, H.M.; Misplon, A.; Walden, J. Structure−Function Relationships in Aminoquinolines: Effect of Amino and Chloro Groups on Quinoline−Hematin Complex Formation, Inhibition of β-Hematin Formation, and Antiplasmodial Activity. J. Med. Chem. 2000, 43, 283–291. [Google Scholar] [CrossRef]
- Muzart, J. Gold-catalysed reactions of alcohols: Isomerisation, inter- and intramolecular reactions leading to C–C and C–heteroatom bonds. Tetrahedron 2008, 64, 5815–5849. [Google Scholar] [CrossRef]
- Kabalka, G.W.; Yao, M.-L. Direct Propargylic Substitution of Hydroxyl Group in Propargylic Alcohols. Curr. Org. Synth. 2008, 5, 28–32. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, G.; Kumar, R.K.; Bi, X. Coinage-Metal-Catalyzed Reactions of Propargylic Alcohols. Synthesis 2015, 47, 2317–2346. [Google Scholar]
- Zhu, Y.; Sun, L.; Lu, P.; Wang, Y. Recent Advances on the Lewis Acid-Catalyzed Cascade Rearrangements of Propargylic Alcohols and Their Derivatives. ACS Catal. 2014, 4, 1911–1925. [Google Scholar] [CrossRef]
- Song, X.-R.; Qiu, Y.-F.; Liu, X.-Y.; Liang, Y.-M. Recent advances in the tandem reaction of azides with alkynes or alkynols. Org. Biomol. Chem. 2016, 14, 11317–11331. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-P.; Li, X.-S.; Zhu, X.-Y.; Li, M.; Zhou, L.; Song, X.-R.; Liang, Y.-M. Lewis Acid Catalyzed Dehydrogenative Coupling of Tertiary Propargylic Alcohols with Quinoline N-Oxides. J. Org. Chem. 2017, 82, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-P.; Song, X.-R.; Qiu, Y.-F.; Li, X.-S.; Zhang, H.-R.; Zhu, X.-Y.; Liu, X.-Y.; Liang, Y.-M. Lewis Acid Catalyzed Cyclization of Propargylic Alcohols with 2-Vinylphenol. Org. Lett. 2016, 18, 3866–3869. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-P.; Song, X.-R.; Qiu, Y.-F.; Zhang, H.-R.; Li, L.-H.; Jin, D.-P.; Sun, X.-Q.; Liu, X.-Y.; Liang, Y.-M. Lewis Acid Catalyzed [4 + 3] Cycloaddition of Propargylic Alcohols with Azides. Org. Lett. 2016, 18, 940–943. [Google Scholar] [CrossRef]
- Qiu, Y.-F.; Song, X.-R.; Li, M.; Zhu, X.-Y.; Wang, A.-Q.; Yang, F.; Han, Y.-P.; Zhang, H.-R.; Jin, D.-P.; Li, Y.-X.; et al. BF3·OEt2-AgSCF3 Mediated Trifluoromethylthiolation/Cascade Cyclization of Propynols: Synthesis of 4-((Trifluoromethyl)thio)-2H-chromene and 4-((Trifluoromethyl)thio)-1,2-dihydroquinoline Derivatives. Org. Lett. 2016, 18, 1514–1517. [Google Scholar] [CrossRef]
- Li, R.; Jin, F.; Song, X.-R.; Yang, T.; Ding, H.; Yang, R.; Xiao, Q.; Liang, Y.-M. Acid-promoted cyclization of 2-propynolphenols leading to 4-tosyloxy-2H-chromenes. Tetrahedron Lett. 2019, 60, 331–334. [Google Scholar] [CrossRef]
- Yang, T.; Song, X.-R.; Li, R.; Jin, F.; Zhang, Y.; Bai, J.; Yang, R.; Ding, H.; Xiao, Q. Metal-free and efficient approach to 4-thiocyanated 2H-chromenes via TFA-mediated cascade cyclization of 2-propynolphenols. Tetrahedron Lett. 2019, 60, 1248–1253. [Google Scholar] [CrossRef]
- Li, R.; Song, X.-R.; Chen, X.; Ding, H.; Xiao, Q.; Liang, Y.-M. Copper-Catalyzed Cascade Cyclization of 2-Propynolphenols: Access to 4-Phosphorylated 2H-Chromenes. Adv. Synth. Catal. 2017, 359, 3962–3967. [Google Scholar] [CrossRef]
- Song, X.-R.; Li, R.; Yang, T.; Chen, X.; Ding, H.; Xiao, Q.; Liang, Y.-M. Novel and Efficient Access to Flavones under Mild Conditions: Aqueous HI-Mediated Cascade Cyclization/Oxidative Radical Reaction of 2-Propynolphenols. Eur. J. Org. Chem. 2018, 2018, 5548–5552. [Google Scholar] [CrossRef]
- Yang, T.; Kou, P.; Jin, F.; Song, X.-R.; Bai, J.; Ding, H.; Xiao, Q.; Liang, Y.-M. TFA-Promoted Sulfonation/Cascade Cyclization of 2-Propynolphenols with Sodium Sulfinates to 4-Sulfonyl 2H-Chromenes under Metal-free Conditions. Org. Chem. Front. 2019, 6, 3162–3166. [Google Scholar] [CrossRef]
- Song, X.-R.; Li, R.; Ding, H.; Yang, R.; Xiao, Q.; Liang, Y.-M. Highly efficient access to 4-chloro-2H-chromenes and 1,2-dihydroquinolines under mild conditions: TMSCl-mediated cyclization of 2-propynolphenols/anilines. Tetrahedron Lett. 2016, 57, 4519–4524. [Google Scholar] [CrossRef]
- Song, X.-R.; Li, R.; Ding, H.; Chen, X.; Yang, T.; Jiang, B.; Xiao, Q.; Liang, Y.-M. An efficient approach to 4-chloro quinolines via TMSCl-mediated cascade cyclization of ortho-propynol phenyl azides. Org. Chem. Front. 2018, 5, 1537–1541. [Google Scholar] [CrossRef]
- Wada, T.; Iwasaki, M.; Kondoh, A.; Yorimitsu, H.; Hideki Yorimitsu Oshima, K. Palladium-Catalyzed Addition of Silyl-Substituted Chloroalkynes to Terminal Alkynes. Chem. Eur. J. 2010, 16, 10671–10674. [Google Scholar] [CrossRef]
- Gopinath, V.S.; Pinjari, J.; Dere, R.T.; Verma, A.; Vishwakarma, P.; Shivahare, R.; Moger, M.; Goud, P.S.K.; Ramanathan, V.; Bose, P.; et al. Design, synthesis and biological evaluation of 2-substituted quinolines as potential antileishmanial agents. Eur. J. Med. Chem. 2013, 69, 52. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, V.S.; Rao, M.; Shivahare, R.; Vishwakarma, P.; Ghose, S.; Pradhan, A.; Hindupur, R.; Sarma, K.D.; Gupta, S.; Puri, S.K.; et al. Design, synthesis, ADME characterization and antileishmanial evaluation of novel substituted quinoline analogs. Bioorg. Med. Chem. Lett. 2014, 24, 2046–2052. [Google Scholar] [CrossRef]
- Michael, J.P. Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 2001, 18, 543–559. [Google Scholar] [CrossRef]
- Michael, J.P. Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 2003, 20, 476–493. [Google Scholar] [CrossRef]
- Musiol, R.; Serda, M.; Bielowka, S.H.; Polanski, J. Quinoline-Based Antifungals. Curr. Med. Chem. 2010, 17, 1960–1973. [Google Scholar] [CrossRef]
- Solomon, V.R.; Lee, H. Quinoline as a Privileged Scaffold in Cancer Drug Discovery. Curr. Med. Chem. 2011, 18, 1488–1508. [Google Scholar] [CrossRef]
- Zhang, H.; Tanimoto, H.; Morimoto, T.; Nishiyama, Y.; Kakiuchi, K. Regioselective Rapid Synthesis of Fully Substituted 1,2,3-Triazoles Mediated by Propargyl Cations. Org. Lett. 2013, 15, 5222–5225. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yin, G.; Hong, D.; Lu, P.; Wang, Y. Tandem Reaction of Propargylic Alcohol, Sulfonamide, and N-Iodosuccinimide: Synthesis of N-(2-Iodoinden-1-yl)arenesulfonamide. Org. Lett. 2011, 13, 1024–1027. [Google Scholar] [CrossRef] [PubMed]
- Adeloye, A.O.; Mphahlele, M.J. 2,4-Diarylquinolines: Synthesis, absorption and emission properties. J. Chem. Res. 2011, 35, 254–259. [Google Scholar] [CrossRef]
- Abbitati, G.; Arcadi, A.; Marinelli, F.; Rossi, E.; Verdecchia, M. Rh-Catalyzed Sequential Hydroarylation/Hydrovinylation–Heterocyclization of b-(2-Aminophenyl)-a,b-ynones with Organoboron Derivatives: A New Approach to Functionalized Quinolines. Synlett 2006, 19, 3218–3224. [Google Scholar]
- Azizi, K.; Akrami, S.; Madsen, R. Manganese(III) Porphyrin-Catalyzed Dehydrogenation of Alcohols to form Imines, Tertiary Amines and Quinolines. Chem. Eur. J. 2019, 25, 6439–6446. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the final products are available from the authors. |

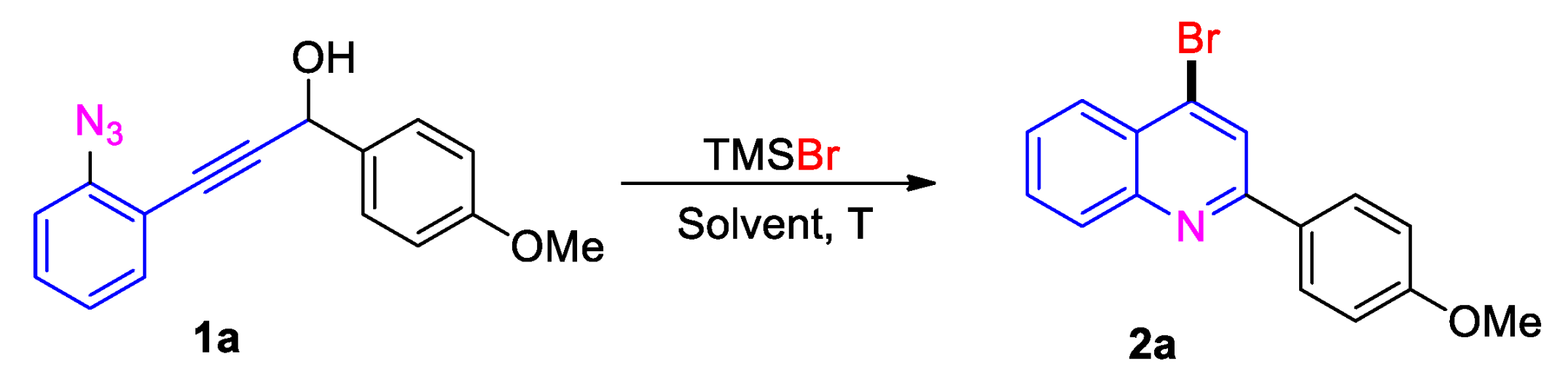
| Entry | Solvent | TMSBr (x Equiv) | T [°C] | Yield [%] |
|---|---|---|---|---|
| 1 | DCE | 2.5 | 60 | 45 |
| 2 | MeCN | 2.5 | 60 | 39 |
| 3 | CH2Cl2 | 2.5 | 40 | 15 |
| 4 | MeNO2 | 2.5 | 60 | 73 |
| 5 | HOAc | 2.5 | 60 | 36 |
| 6 | MeNO2 | 3.5 | 60 | 81 |
| 7 | MeNO2 | 3.0 | 60 | 78 |
| 8 | MeNO2 | 2.0 | 60 | 67 |
| 9 | MeNO2 | 3.5 | 80 | 82 |
| 10 | MeNO2 | 3.5 | rt | 69 |
| 11 b | MeNO2 | 3.5 | 60 | 75 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, F.; Yang, T.; Song, X.-R.; Bai, J.; Yang, R.; Ding, H.; Xiao, Q. TMSBr-Promoted Cascade Cyclization of ortho-Propynol Phenyl Azides for the Synthesis of 4-Bromo Quinolines and Its Applications. Molecules 2019, 24, 3999. https://doi.org/10.3390/molecules24213999
Jin F, Yang T, Song X-R, Bai J, Yang R, Ding H, Xiao Q. TMSBr-Promoted Cascade Cyclization of ortho-Propynol Phenyl Azides for the Synthesis of 4-Bromo Quinolines and Its Applications. Molecules. 2019; 24(21):3999. https://doi.org/10.3390/molecules24213999
Chicago/Turabian StyleJin, Fengyan, Tao Yang, Xian-Rong Song, Jiang Bai, Ruchun Yang, Haixin Ding, and Qiang Xiao. 2019. "TMSBr-Promoted Cascade Cyclization of ortho-Propynol Phenyl Azides for the Synthesis of 4-Bromo Quinolines and Its Applications" Molecules 24, no. 21: 3999. https://doi.org/10.3390/molecules24213999
APA StyleJin, F., Yang, T., Song, X.-R., Bai, J., Yang, R., Ding, H., & Xiao, Q. (2019). TMSBr-Promoted Cascade Cyclization of ortho-Propynol Phenyl Azides for the Synthesis of 4-Bromo Quinolines and Its Applications. Molecules, 24(21), 3999. https://doi.org/10.3390/molecules24213999