A Report on Fungal (1→3)-α-d-glucans: Properties, Functions and Application
Abstract
1. Introduction
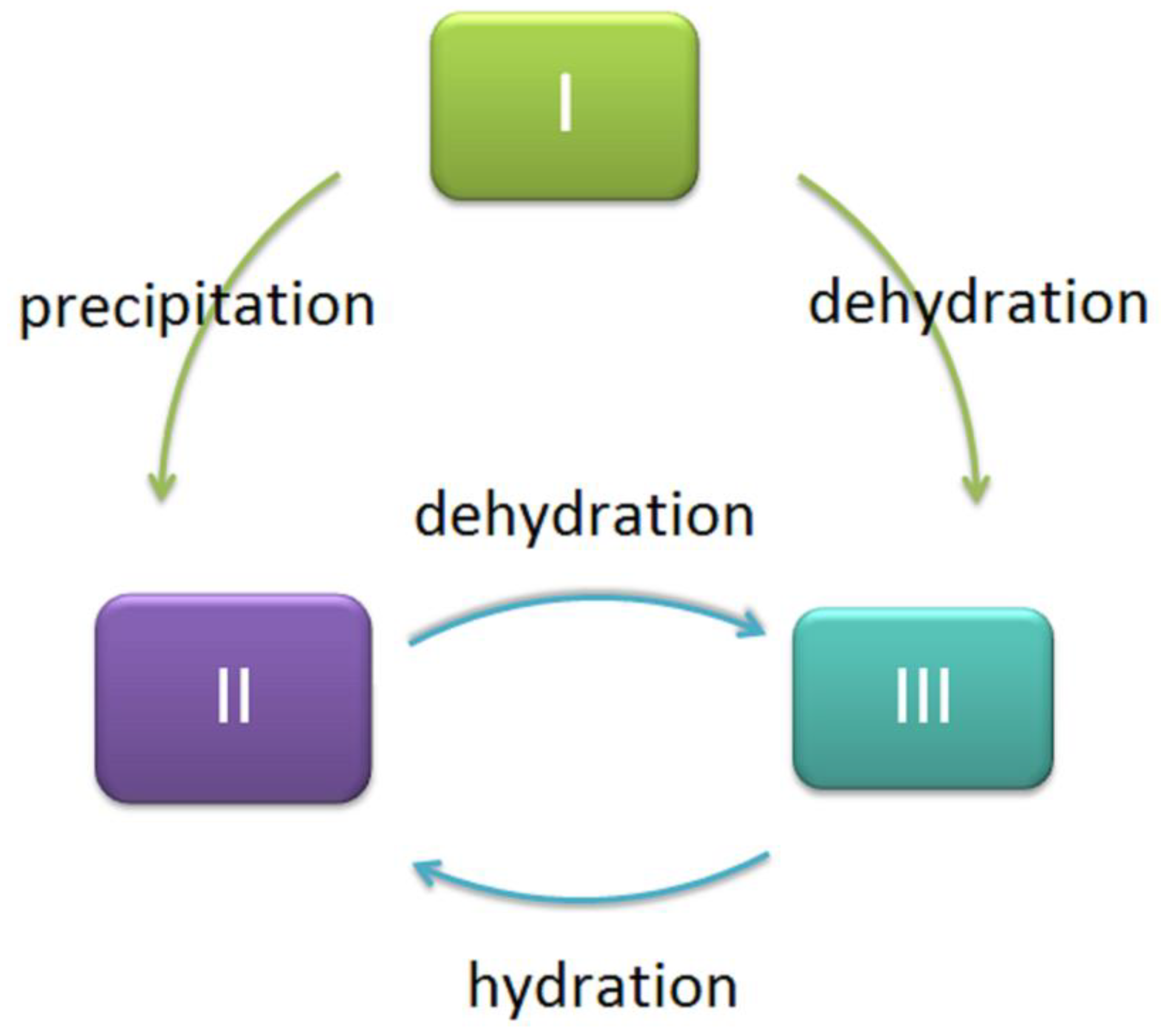
2. Methods for Isolating (1→3)-α-d-Glucans
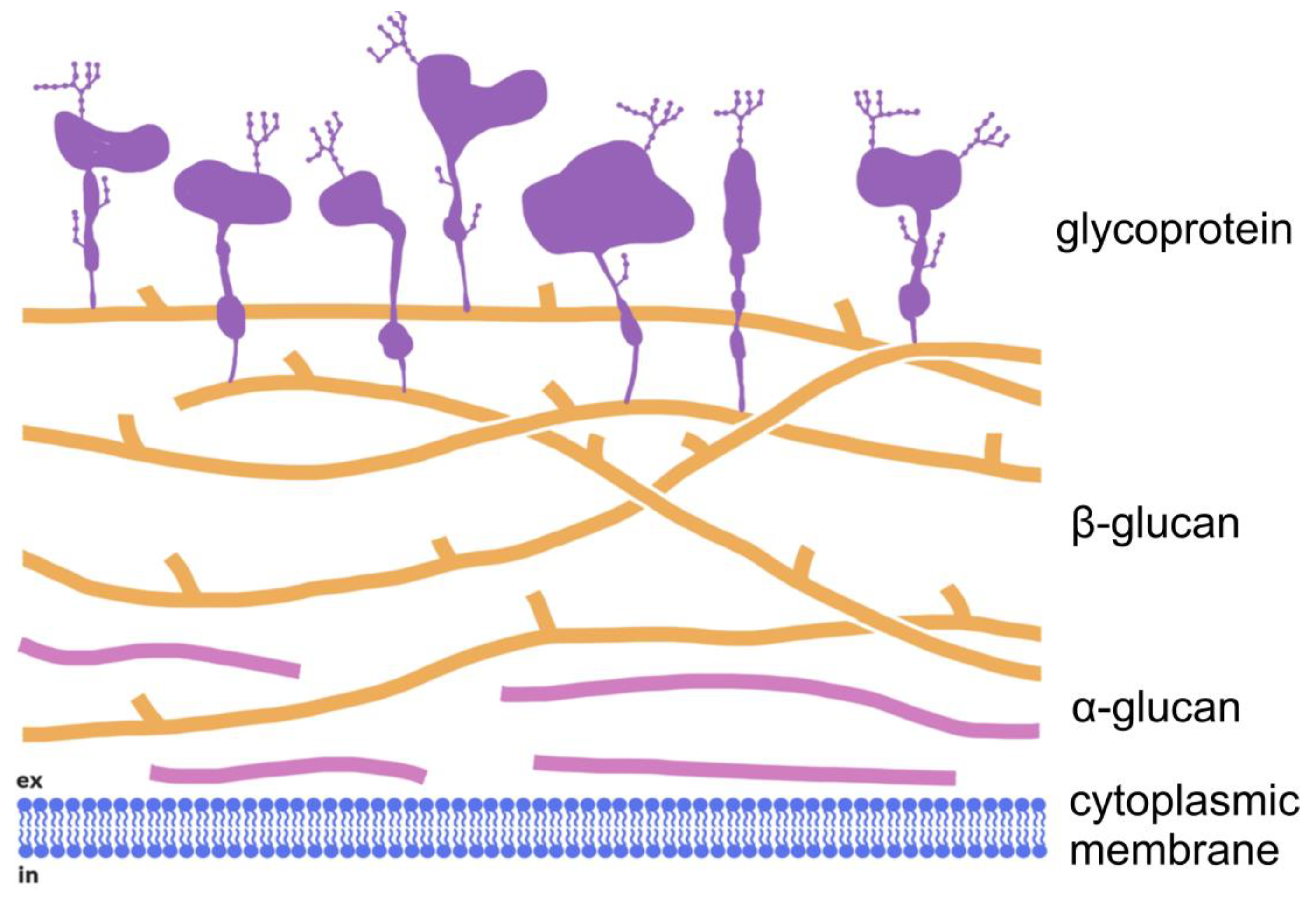
3. Structure and Property of Fungal (1→3)-α-d-Glucans
4. Functions of (1→3)-α-d-Glucans
5. The Biological Role of (1→3)-α-d-GLucans
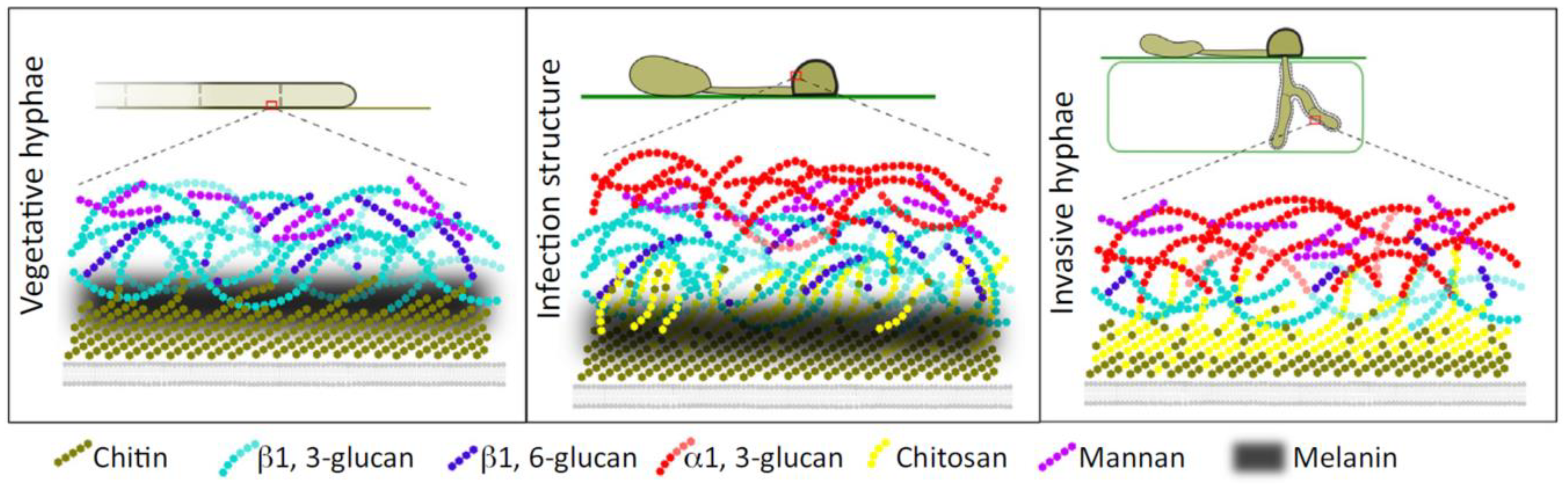
5.1. Fungal (1→3)-α-d-Glucans Is Essential for Successful Plants Infection
5.2. The Role of (1→3)-α-d-Glucans in the Pathogenicity of Aspergillus Fumigatus
6. Applications of (1→3)-α-d-Glucans
6.1. Immunological Activity
6.2. (1→3)-α-d-Glucans as a Mutanase Inducers
6.3. (1→3)-α-d-Glucans as a Prebiotic
6.4. (1→3)-α-d-Glucans as a Support for Enzyme Immobilization
7. Sorption Properties of (1→3)-α-d-Glucans
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Synytsya, A.; Novak, M. Structural analysis of glucans. Ann. Transl. Med. 2014, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Novák, M. Structural diversity of fungal glucans. Carbohydr. Polym. 2013, 92, 792–809. [Google Scholar] [CrossRef] [PubMed]
- Grün, C.H. Structure and Biosynthesis of Fungal α-Glucans. Ph.D. Thesis, University of Utrecht, Utrecht, The Netherlands, 2003. [Google Scholar]
- Wiater, A.; Pleszczynska, M.; Szczodrak, J.; Próchniak, K. α-(1→3)-Glukany œciany komórkowej żółciaka siarkowego–Laetiporus sulphureus (Bull.: Fr.) Murrill–izolacja, charakterystyka i zastosowanie do indukcji syntezy. Biotechnologia 2008, 2, 174–189. [Google Scholar]
- Erwig, L.P.; Gow, N.A.R. Interactions of fungal pathogens with phagocytes. Nat. Rev. Microbiol. 2016, 14, 163–176. [Google Scholar] [CrossRef]
- Beauvais, A.; Fontaine, T.; Aimanianda, V.; Latgé, J.P. Aspergillus cell wall and biofilm. Mycopathologia 2014, 178, 371–377. [Google Scholar] [CrossRef]
- Choma, A.; Wiater, A.; Komaniecka, I.; Paduch, R.; Pleszczyńska, M.; Szczodrak, J. Chemical characterization of a water insoluble (1→3)-α-d-glucan from an alkaline extract of Aspergillus wentii. Carbohydr. Polym. 2013, 91, 603–608. [Google Scholar] [CrossRef]
- Fujikawa, T.; Kuga, Y.; Yano, S.; Yoshimi, A.; Tachiki, T.; Abe, K.; Nishimura, M. Dynamics of cell wall components of Magnaporthe grisea during infectious structure development. Mol. Microbiol. 2009, 73, 553–570. [Google Scholar] [CrossRef]
- Miyazaki, T.; Yamada, M.; Ohno, T. Isolation and Structure of α-1, 3-Linked Glucan from the Hyphal Wall of Phytophthora infestans. Chem. Pharm. Bull. (Tokyo) 1974, 22, 1666–1669. [Google Scholar] [CrossRef][Green Version]
- Rappleye, C.A.; Eissenberg, L.G.; Goldman, W.E. Histoplasma capsulatum α-(1,3)-glucan blocks innate immune recognition by the beta-glucan receptor. Proc. Natl. Acad. Sci. USA 2007, 104, 1366–1370. [Google Scholar] [CrossRef]
- Reese, A.J.; Yoneda, A.; Breger, J.A.; Beauvais, A.; Liu, H.; Griffith, C.L.; Bose, I.; Kim, M.J.; Skau, C.; Yang, S.; et al. Loss of cell wall alpha (1–3) glucan affects Cryptococcus neoformans from ultrastructure to virulence. Mol. Microbiol. 2007, 63, 1385–1398. [Google Scholar] [CrossRef]
- Jelsma, J.; Kreger, D.R. Observations on the cell-wall compositions of the bracket fungi Laetiporus sulphureus and Piptoporus betulinus. Arch. Microbiol. 1978, 119, 249–255. [Google Scholar] [CrossRef]
- Jelsma, J.; Kreger, D.R. Polymorphism in crystalline (1→3)-α-d-glucan from fungal cell-walls. Carbohydr. Res. 1979, 71, 51–64. [Google Scholar] [CrossRef]
- Bobbitt, T.F.; Nordin, J.H.; Roux, M.; Revol, J.F.; Marchessault, R.H. Distribution and conformation of crystalline nigeran in hyphal walls of Aspergillus niger and Aspergillus awamori. J. Bacteriol. 1977, 132, 691–703. [Google Scholar] [PubMed]
- Kanetsuna, F.; Carbonell, L.M.; Gil, F.; Azuma, I. Chemical and ultrastructural studies on the cell walls of the yeastlike and mycelial forms of Histoplasma capsulatum. Mycopathol. Mycol. Appl. 1974, 54, 1–13. [Google Scholar] [CrossRef]
- Wiater, A.; Paduch, R.; Choma, A.; Sylwia, S.; Pleszczynska, M.; Tomczyk, M.; Locatelli, M.; Janusz, S. (1→3)-α-d-Glucans from aspergillus spp.: Structural characterization and biological study on their carboxymethylated derivatives. Curr. Drug Targets 2015, 16, 1488–1494. [Google Scholar] [CrossRef]
- Bhanja, S.K.; Rout, D.; Patra, P.; Sen, I.K.; Nandan, C.K.; Islam, S.S. Water-insoluble glucans from the edible fungus Ramaria botrytis. Bioact. Carbohydrates Diet. Fibre 2014, 3, 52–58. [Google Scholar] [CrossRef]
- Osińska-Jaroszuk, M.; Wiater, A.; Choma, A.; Pleszczyńska, M.; Jaszek, M.; Janusz, G.; Skowronek, M.; Szczodrak, J. (1→3)-α-D-Glucan from fruiting body and mycelium of cerrena unicolor (Bull.) murrill: Structural characterization and use as a novel inducer of mutanase. Int. J. Polym. Sci. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Wiater, A.; Paduch, R.; Choma, A.; Pleszczyńska, M.; Siwulski, M.; Dominik, J.; Janusz, G.; Tomczyk, M.; Szczodrak, J. Biological study on carboxymethylated (1→3)-α-d-glucans from fruiting bodies of Ganoderma lucidum. Int. J. Biol. Macromol. 2012, 51, 1014–1023. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, L.; Cheng, S. Solution properties of an α-(1→3)-d-glucan from Lentinus edodes and its sulfated derivatives. Carbohydr. Res. 2002, 337, 155–160. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, L.; Cheng, S. Effects of urea and sodium hydroxide on the molecular weight and conformation of α-(1→3)-d-glucan from Lentinusedodes in aqueous solution. Carbohydr. Res. 2000, 327, 431–438. [Google Scholar] [CrossRef]
- Wiater, A.; Paduch, R.; Pleszczyńska, M.; Próchniak, K.; Choma, A.; Kandefer-Szerszeń, M.; Szczodrak, J. α-(1→3)-d-Glucans from fruiting bodies of selected macromycetes fungi and the biological activity of their carboxymethylated products. Biotechnol. Lett. 2011, 33, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Deng, L.; Li, S.; Tan, T. Structural characterization of a water-insoluble (1→3)-α-d-glucan isolated from the Penicillium chrysogenum. Carbohydr. Polym. 2007, 67, 133–137. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Agafonova, S.V.; Rokhin, A.V.; Penzina, T.A.; Borovskii, G.B. Branched glucan from the fruiting bodies of Piptoporus betulinus (Bull.:Fr) Karst. Appl. Biochem. Microbiol. 2012, 48, 65–70. [Google Scholar] [CrossRef]
- Wiater, A.; Paduch, R.; Próchniak, K.; Pleszczyńska, M.; Siwulski, M.; Białas, W.; Szczodrak, J. Assessing biological activity of carboxymethylated derivatives of α-(1→3)-glucans isolated from fruiting bodies of cultivated pleurotus species. Żywnosc. Nauka. Technol. Jakosc/Food Sci. Technol. Qual. 2015, 1, 193–206. [Google Scholar] [CrossRef]
- Wiater, A.; Pleszczynska, M.; Szczodrak, J.A. Enzymy rozładające α-(1-3)-glukany. Część I-Źródła mikrobiologiczne, produkcja, właściowości, genetyka. Biotechnologia 2006, 2, 206–220. [Google Scholar]
- Grün, C.H. The structure of cell wall α-glucan from fission yeast. Glycobiology 2005, 15, 245–257. [Google Scholar] [CrossRef]
- Hochstenbach, F.; Klis, F.M.; Van den Ende, H.; Van Donselaar, E.; Peters, P.J.; Klausner, R.D. Identification of a putative alpha-glucan synthase essential for cell wall construction and morphogenesis in fission yeast. Proc. Natl. Acad. Sci. USA 1998, 95, 9161–9166. [Google Scholar] [CrossRef]
- Yoshimi, A.; Sano, M.; Inaba, A.; Kokubun, Y.; Fujioka, T.; Mizutani, O.; Hagiwara, D.; Fujikawa, T.; Nishimura, M.; Yano, S.; et al. Functional analysis of the α-1,3-glucan synthase genes agsa and agsb in aspergillus nidulans: Agsb is the major α-1,3-glucan synthase in this fungus. PLoS ONE 2013, 8, e54893. [Google Scholar] [CrossRef]
- Yoshimi, A.; Miyazawa, K.; Abe, K. Function and biosynthesis of cell wall α-1,3-glucan in fungi. J. Fungi 2017, 3, 63. [Google Scholar] [CrossRef]
- Yano, S.; Yamamoto, S.; Toge, T.; Wakayama, M.; Tachiki, T. Occurrence of a specific protein in basidiomycetelytic enzyme preparation produced by bacillus circulans KA-304 inductively with a cell-wall preparation of Schizophyllum commune. Biosci. Biotechnol. Biochem. 2003, 67, 1976–1982. [Google Scholar] [CrossRef][Green Version]
- Yano, S.; Wakayama, M.; Tachiki, T. Cloning and expression of an α-1,3-glucanase gene from Bacillus circulans KA-304: The enzyme participates in protoplast formation of Schizophyllum commune. Biosci. Biotechnol. Biochem. 2006, 70, 1754–1763. [Google Scholar] [CrossRef] [PubMed]
- Sanz, L.; Montero, M.; Redondo, J.; Llobell, A.; Monte, E. Expression of an α-1,3-glucanase during mycoparasitic interaction of Trichoderma asperellum. FEBS J. 2005, 272, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Pleszczyńska, M.; Wiater, A.; Szczodrak, J. Mutanase from Paenibacillus sp. MP-1 produced inductively by fungal α-1,3-glucan and its potential for the degradation of mutan and Streptococcus mutans biofilm. Biotechnol. Lett. 2010, 32, 1699–1704. [Google Scholar] [CrossRef]
- Pleszczyńska, M.; Wiater, A.; Siwulski, M.; Szczodrak, J. Successful large-scale production of fruiting bodies of Laetiporus sulphureus (Bull.: Fr.) Murrill on an artificial substrate. World J. Microbiol. Biotechnol. 2013, 29, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Nowak, R.; Nowacka-Jechalke, N.; Juda, M.; Malm, A. The preliminary study of prebiotic potential of Polish wild mushroom polysaccharides: The stimulation effect on Lactobacillus strains growth. Eur. J. Nutr. 2018, 57, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.; Wiater, A.; Choma, A.; Wiącek, D.; Bieganowski, A.; Siwulski, M.; Waśko, A. Fungal (1→3)-α-d-glucans as a new kind of biosorbent for heavy metals. Int. J. Biol. Macromol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Choma, A.; Nowak, K.; Komaniecka, I.; Waśko, A.; Pleszczyńska, M.; Siwulski, M.; Wiater, A. Chemical characterization of alkali-soluble polysaccharides isolated from a Boletus edulis (Bull.) fruiting body and their potential for heavy metal biosorption. Food Chem. 2018, 266, 329–334. [Google Scholar] [CrossRef]
- Hasegawa, S.; Nordin, J.H.; Kirkwood, S. Enzymes that hydrolyze fungal cell wall polysaccharides. J. Biol. Chem. 1969, 244, 5460–5470. [Google Scholar]
- Kiho, T.; Yoshida, I.; Katsuragawa, M.; Sakushima, M.; Usui, S.; Ukai, S. Polysaccharides in fungi. XXXIV. A polysaccharide from the fruiting bodies of Amanita muscaria and the antitumor activity of its carboxymethylated product. Biol. Pharm. Bull. 1994, 17, 1460–1462. [Google Scholar] [CrossRef][Green Version]
- Mizuno, K.; Awazu, N.; Tachiki, T. Purification and some properties of p-nitrophenyl-β-d -glucoside-hydrolyzing enzymes in culture filtrate of Bacillus circulans KA-304 grown on cell-wall preparation of Schizophyllum commune. Biosci. Biotechnol. Biochem. 1998, 62, 39–43. [Google Scholar] [CrossRef][Green Version]
- Wiater, A.; Szczodrak, J.; Pleszczyńska, M. Mutanase induction in Trichoderma harzianum by cell wall of Laetiporus sulphureus and its application for mutan removal from oral biofilms. J. Microbiol. Biotechnol. 2008, 18, 1335–1341. [Google Scholar] [PubMed]
- Cardemil, L.; Pincheira, G. Characterization of the carbohydrate component of fraction I in the Neurospora crassa cell wall. J. Bacteriol. 1979, 137, 1067–1072. [Google Scholar] [PubMed]
- Takeda, T.; Nishikawa, Y.; Shibata, S. A new α-glucan from the LIchen Parmelia caperata (L.) Ach. Chem. Pharm. Bull. (Tokyo) 1970, 18, 1074–1075. [Google Scholar] [CrossRef][Green Version]
- Ogawa, K.; Misaki, A.; Oka, S.; Okamura, K. X-Ray diffraction data for (1→3)-α-d-glucan. Carbohydr. Res. 1979, 75, C13–C16. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Xu, X.; Lin, Y.; Cheung, P.C.K.; Kennedy, J.F. Comparison on chain stiffness of a water-insoluble (1→3)-α-d- glucan isolated from Poria cocos mycelia and its sulfated derivative. Carbohydr. Polym. 2005, 59, 257–263. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Nakamura, Y.; Norisuye, T. Viscosity behavior and chain conformation of a (1→3)-α-glucan from Ganoderma lucidum. Polym. Bull. 1998, 41, 471–478. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, J.; Zhang, L.; Nakamura, Y.; Norisuye, T. Chemical structure of the water-insoluble polysaccharide isolated from the fruiting body of Ganoderma lucidum. Polym. J. 1998, 30, 838–842. [Google Scholar] [CrossRef]
- Zonneveld, B. Morphogenesis in Aspergillus nidulans the significance of α-1,3-glucan of the cell wall and α-1,3-glucanase for cleistothecium development. Biochim. Biophys. Acta-Gen. Subj. 1972, 273, 174–187. [Google Scholar] [CrossRef]
- Zonneveld, B.J.M. Inhibitory effect of 2-deoxyglucose on cell wall α-1,3-glucan synthesis and cleistothecium development in Aspergillus nidulans. Dev. Biol. 1973, 34, 1–8. [Google Scholar] [CrossRef]
- Kiho, T.; Yoshida, I.; Nagai, K.; Ukai, S.; Hara, C. (1→3)-α-d-glucan from an alkaline extract of Agrocybe cylindracea, and antitumor activity of its O-(carboxymethyl)ated derivatives. Carbohydr. Res. 1989, 189, 273–279. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Chen, J.; Zhang, F. Solution properties of antitumor carboxymethylated derivatives of α-(1→ 3)-d-Glucan from Ganoderma lucidum. J. Polym. Since 2001, 19, 283–289. [Google Scholar]
- Zhang, L.; Zhang, M.; Zhou, Q.; Chen, J.; Zeng, F. Solution Properties of Antitumor Sulfated Derivative of α-(1→3)-d-Glucan from Ganoderma lucidum. Biosci. Biotechnol. Biochem. 2000, 64, 2172–2178. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Duan, J.; Fang, X.; Fang, J. Chemical modifications of the (1→3)-α-d-glucan from spores of Ganoderma lucidum and investigation of their physicochemical properties and immunological activity. Carbohydr. Res. 2001, 336, 127–140. [Google Scholar] [CrossRef]
- Puanglek, S.; Kimura, S.; Enomoto-Rogers, Y.; Kabe, T.; Yoshida, M.; Wada, M.; Iwata, T. In vitro synthesis of linear α-1,3-glucan and chemical modification to ester derivatives exhibiting outstanding thermal properties. Sci. Rep. 2016, 6, 30479. [Google Scholar] [CrossRef]
- Miyazawa, K.; Yoshimi, A.; Zhang, S.; Sano, M.; Nakayama, M.; Gomi, K.; Abe, K. Increased enzyme production under liquid culture conditions in the industrial fungus Aspergillus oryzae by disruption of the genes encoding cell wall α-1,3-glucan synthase. Biosci. Biotechnol. Biochem. 2016, 80, 1853–1863. [Google Scholar] [CrossRef]
- Beauvais, A.; Maubon, D.; Park, S.; Morelle, W.; Tanguy, M.; Huerre, M.; Perlin, D.S.; Latge, J.P. Two (1-3)-Glucan Synthases with Different Functions in Aspergillus fumigatus. Appl. Environ. Microbiol. 2005, 71, 1531–1538. [Google Scholar] [CrossRef]
- Beauvais, A.; Bozza, S.; Kniemeyer, O.; Formosa, C.; Balloy, V.; Henry, C.; Roberson, R.W.; Dague, E.; Chignard, M.; Brakhage, A.A.; et al. Deletion of the α-(1,3)-glucan synthase genes induces a restructuring of the conidial cell wall responsible for the avirulence of Aspergillus fumigatus. PLoS Pathog. 2013, 9, e1003716. [Google Scholar] [CrossRef]
- Fujikawa, T.; Sakaguchi, A.; Nishizawa, Y.; Kouzai, Y.; Minami, E.; Yano, S.; Koga, H.; Meshi, T.; Nishimura, M. Surface α-1,3-Glucan Facilitates Fungal Stealth Infection by Interfering with Innate Immunity in Plants. PLoS Pathog. 2012, 8, e1002882. [Google Scholar] [CrossRef]
- Fontaine, T.; Beauvais, A.; Loussert, C.; Thevenard, B.; Fulgsang, C.C.; Ohno, N.; Clavaud, C.; Prevost, M.-C.; Latgé, J.-P. Cell wall α1-3glucans induce the aggregation of germinating conidia of Aspergillus fumigatus. Fungal Genet. Biol. 2010, 47, 707–712. [Google Scholar] [CrossRef]
- Wiater, A.; Choma, A.; Szczodrak, J. Insoluble glucans synthesized by cariogenic streptococci: A structural study. J. Basic Microbiol. 1999, 39, 265–273. [Google Scholar] [CrossRef]
- Wiater, A.; Szczodrak, J.; Pleszczyńska, M. Optimization of conditions for the efficient production of mutan in streptococcal cultures and post-culture liquids. Acta Biol. Hung. 2005, 56, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Schilling, K.M.; Bowen, W.H. Glucans synthesized in situ in experimental salivary pellicle function as specific binding sites for Streptococcus mutans. Infect. Immun. 1992, 60, 284–295. [Google Scholar] [PubMed]
- Aires, C.P.; Tenuta, L.M.; Carbonero, E.R.; Sassaki, G.L.; Iacomini, M.; Cury, J.A. Structural characterization of exopolysaccharides from biofilm of a cariogenic streptococci. Carbohydr. Polym. 2011, 84, 1215–1220. [Google Scholar] [CrossRef]
- Davis, H.M.; Hines, H.B.; Edwards, J.R. Structural elucidation of a water-insoluble glucan produced by a cariogenic oral Streptococcus. Carbohydr. Res. 1986, 156, 69–77. [Google Scholar] [CrossRef]
- Hotz, P.; Guggenheim, B.; Schmid, R. Carbohydrates in pooled dental plaque. Caries Res. 1972, 6, 103–121. [Google Scholar] [CrossRef]
- Stuelp-Campelo, P.M.; De Oliveira, M.B.M.; Leão, A.M.A.C.; Carbonero, E.R.; Gorin, P.A.J.; Iacomini, M. Effect of a soluble α-d-glucan from the lichenized fungus ramalina celastri on macrophage activity. Int. Immunopharmacol. 2002, 2, 691–698. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, L.; Cheung, P.C.K.; Tan, X. Evaluation of sulfated α-glucans from Poria cocos mycelia as potential antitumor agent. Carbohydr. Polym. 2006, 64, 337–344. [Google Scholar] [CrossRef]
- Wasser, S. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar] [CrossRef]
- Masuda, Y.; Nakayama, Y.; Tanaka, A.; Naito, K.; Konishi, M. Antitumor activity of orally administered maitake α-glucan by stimulating antitumor immune response in murine tumor. PLoS ONE 2017, 12, 1–15. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, S.W.; Cheung, P.C.K.; Wang, Q. Antitumor polysaccharides from mushrooms: A review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci. Technol. 2007, 18, 4–19. [Google Scholar] [CrossRef]
- Lavi, I.; Levinson, D.; Peri, I.; Tekoah, Y.; Hadar, Y.; Schwartz, B. Chemical characterization, antiproliferative and antiadhesive properties of polysaccharides extracted from Pleurotus pulmonarius mycelium and fruiting bodies. Appl. Microbiol. Biotechnol. 2010, 85, 1977–1990. [Google Scholar] [CrossRef] [PubMed]
- Stephen-Victor, E.; Karnam, A.; Fontaine, T.; Beauvais, A.; Das, M.; Hegde, P.; Prakhar, P.; Holla, S.; Balaji, K.N.; Kaveri, S.V.; et al. Aspergillus fumigatus cell wall α-(1,3)-glucan stimulates regulatory t-cell polarization by inducing PD-L1 expression on human dendritic cells. J. Infect. Dis. 2017, 216, 1281–1294. [Google Scholar] [CrossRef] [PubMed]
- Kakutani, R.; Adachi, Y.; Kajiura, H.; Takata, H.; Kuriki, T.; Ohno, N. Relationship between structure and immunostimulating activity of enzymatically synthesized glycogen. Carbohydr. Res. 2007, 342, 2371–2379. [Google Scholar] [CrossRef] [PubMed]
- Tabarsa, M.; Shin, I.-S.; Lee, J.H.; Surayot, U.; Park, W.; You, S. An immune-enhancing water-soluble α-glucan from Chlorella vulgaris and structural characteristics. Food Sci. Biotechnol. 2015, 24, 1933–1941. [Google Scholar] [CrossRef]
- Pleszczyńska, M.; Wiater, A.; Janczarek, M.; Szczodrak, J. (1→3)-α-d-Glucan hydrolases in dental biofilm prevention and control: A review. Int. J. Biol. Macromol. 2015, 79, 761–778. [Google Scholar] [CrossRef]
- Meyer, M.T.; Phaff, H.J. Purification and properties of (1,3)-α-glucanases from Bacillus circulans WL-12. Microbiol. 1980, 118, 197–208. [Google Scholar] [CrossRef][Green Version]
- Hasegawa, S.; Kirkwood, S.; Nordin, J.H. An alpha-1,3-glucanase from a fungal source. Chem. Ind. 1966, 25, 1033. [Google Scholar] [PubMed]
- Maubon, D.; Park, S.; Tanguy, M.; Huerre, M.; Schmitt, C.; Prévost, M.C.; Perlin, D.S.; Latgé, J.P.; Beauvais, A. AGS3, an α-(1-3)glucan synthase gene family member of Aspergillus fumigatus, modulates mycelium growth in the lung of experimentally infected mice. Fungal Genet. Biol. 2006, 43, 366–375. [Google Scholar] [CrossRef]
- Ghazaei, C. Molecular Insights into Pathogenesis and Infection with Aspergillus fumigatus. Malaysian, J. Med. Sci. 2017, 24, 10–20. [Google Scholar] [CrossRef]
- Latge, J.-P. Aspergillus fumigatus, a saprotrophic pathogenic fungus. Mycologist 2003, 17, 56–61. [Google Scholar] [CrossRef]
- Jahn, B.; Langfelder, K.; Schneider, U.; Schindel, C.; Brakhage, A.A. PKSP-dependent reduction of phagolysosome fusion and intracellular kill of Aspergillus fumigatus conidia by human monocyte-derived macrophages. Cell. Microbiol. 2002, 4, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Komarova, B.S.; Orekhova, M.V.; Tsvetkov, Y.E.; Beau, R.; Aimanianda, V.; Latgé, J.-P.; Nifantiev, N.E. Synthesis of a pentasaccharide and neoglycoconjugates related to fungal α-(1→3)-glucan and their use in the generation of antibodies to trace Aspergillus fumigatus cell wall. Chem. A Eur. J. 2015, 21, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Míčková, K.; Synytsya, A.; Jablonský, I.; Spěváček, J.; Erban, V.; Kováříková, E.; Čopíková, J. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and potential prebiotic activity. Carbohydr. Polym. 2009, 76, 548–556. [Google Scholar] [CrossRef]
- Heath, M.C. Nonhost resistance and nonspecific plant defenses. Curr. Opin. Plant. Biol. 2000, 3, 315–319. [Google Scholar] [CrossRef]
- Partida-Martínez, L.P.; Heil, M. The microbe-free plant: Fact or artifact? Front. Plant. Sci. 2011, 2, 1–16. [Google Scholar] [CrossRef]
- Knogge, W. Plant-Fungal interactions and plant disease. In Subcellular Biochemistry, 1st ed.; Biswas, B.B., Das, H.K., Eds.; Springer: Boston, MA, USA, 1998; Volume 29, pp. 215–251. [Google Scholar] [CrossRef]
- Zeilinger, S.; Gupta, V.K.; Dahms, T.E.S.; Silva, R.N.; Singh, H.B.; Upadhyay, R.S.; Gomes, E.V.; Tsui, C.K.-M.; Nayak, S.C. Friends or foes? Emerging insights from fungal interactions with plants. FEMS Microbiol. Rev. 2016, 40, 182–207. [Google Scholar] [CrossRef]
- Schulz, B.; Römmert, A.-K.; Dammann, U.; Aust, H.-J.; Strack, D. The endophyte-host interaction: A balanced antagonism? Mycol. Res. 1999, 103, 1275–1283. [Google Scholar] [CrossRef]
- Parniske, M. Intracellular accommodation of microbes by plants: A common developmental program for symbiosis and disease? Curr. Opin. Plant. Biol. 2000, 3, 320–328. [Google Scholar] [CrossRef]
- Schulze-Lefert, P. Knocking on the heaven’s wall: Pathogenesis of and resistance to biotrophic fungi at the cell wall. Curr. Opin. Plant. Biol. 2004, 7, 377–383. [Google Scholar] [CrossRef]
- Schulz, B.; Boyle, C. The endophytic continuum. Mycol. Res. 2005, 109, 661–686. [Google Scholar] [CrossRef]
- Grant, M.R.; Jones, J.D.G. Hormone (Dis)harmony moulds plant health and disease. Science 2009, 324, 750–752. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.G. Microbial elicitors and their receptors in plants. Annu. Rev. Phytopathol. 1996, 34, 387–412. [Google Scholar] [CrossRef] [PubMed]
- Ebel, J.; Mithöfer, A. Early events in the elicitation of plant defence. Planta 1998, 206, 335–348. [Google Scholar] [CrossRef]
- Montesano, M.; Brader, G.; Palva, E.T. Pathogen derived elicitors: Searching for receptors in plants. Mol. Plant. Pathol. 2003, 4, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Gordon, S. Immune recognition of fungal β-glucans. Cell. Microbiol. 2005, 7, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Poulain, D.; Jouault, T. Candida albicans cell wall glycans, host receptors and responses: Elements for a decisive crosstalk. Curr. Opin. Microbiol. 2004, 7, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Theis, T.; Stahl, U. Antifungal proteins: Targets, mechanisms and prospective applications. Cell. Mol. Life Sci. 2004, 61, 437–455. [Google Scholar] [CrossRef]
- Altenbach, D.; Robatzek, S. Pattern Recognition Receptors: From the Cell Surface to Intracellular Dynamics. Mol. Plant. Microbe Interact. 2007, 20, 1031–1039. [Google Scholar] [CrossRef]
- De Wit, P.J.G.M. How plants recognize pathogens and defend themselves. Cell. Mol. Life Sci. 2007, 64, 2726–2732. [Google Scholar] [CrossRef]
- Felix, G.; Regenass, M.; Boller, T. Specific perception of subnanomolar concentrations of chitin fragments by tomato cells: Induction of extracellular alkalinization, changes in protein phosphorylation, and establishment of a refractory state. Plant. J. 1993, 4, 307–316. [Google Scholar] [CrossRef]
- Anderson, J.P.; Gleason, C.A.; Foley, R.C.; Thrall, P.H.; Burdon, J.B.; Singh, K.B. Plants versus pathogens: An evolutionary arms race. Funct. Plant. Biol. 2010, 37, 499. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Sohal, B.S. Role of elicitors in inducing resistance in plants against pathogen infection: A review. ISRN Biochem. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Klarzynski, O.; Plesse, B.; Joubert, J.-M.; Yvin, J.-C.; Kopp, M.; Kloareg, B.; Fritig, B. Linear β-1,3 glucans are elicitors of defense responses in tobacco. Plant Physiol. 2000, 124, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Minami, E. Oligosaccharide signalling for defence responses in plant. Physiol. Mol. Plant. Pathol. 2001, 59, 223–233. [Google Scholar] [CrossRef]
- De Ascensao, A.R.F.D.; Dubery, I.A. Soluble and wall-bound phenolics and phenolic polymers in Musa acuminata roots exposed to elicitors from Fusarium oxysporum f.sp. cubense. Phytochemistry 2003, 63, 679–686. [Google Scholar] [CrossRef]
- Miyazawa, K.; Yoshimi, A.; Kasahara, S.; Sugahara, A.; Koizumi, A.; Yano, S.; Kimura, S.; Iwata, T.; Sano, M.; Abe, K. Molecular mass and localization of α-1,3-glucan in cell wall control the degree of hyphal aggregation in liquid culture of aspergillus nidulans. Front. Microbiol. 2018, 9, 2623. [Google Scholar] [CrossRef]
- Schoffelmeer, E.A.M.; Klis, F.M.; Sietsma, J.H.; Cornelissen, B.J.C. The cell wall of fusarium oxysporum. Fungal Genet. Biol. 1999, 27, 275–282. [Google Scholar] [CrossRef]
- Wolski, E.A.; Daleo, G.R.; Andreu, A.B. Induction of pathogenesis related proteins on potato sprouts by an α-1,3-glucan from cell wall of binucleate non-pathogenic Rhizoctonia sp isolate. Phytopathology 2004, 94, S111. [Google Scholar] [CrossRef]
- Wolski, E.A.; Lima, C.; Agusti, R.; Daleo, G.R.; Andreu, A.B.; De Lederkremer, R.M. An α-glucan elicitor from the cell wall of a biocontrol binucleate Rhizoctonia isolate. Carbohydr. Res. 2005, 340, 619–627. [Google Scholar] [CrossRef]
- Wolski, E.; Maldonado, S.; Daleo, G.; Andreu, A. Cell wall α-1,3-glucans from a biocontrol isolate of Rhizoctonia: Immunocytolocalization and relationship with α-glucanase activity from potato sprouts. Mycol. Res. 2007, 111, 976–984. [Google Scholar] [CrossRef]
- Wolski, E.A.; Maldonado, S.; Daleo, G.R.; Andreu, A.B. A novel α-1,3-glucan elicits plant defense responses in potato and induces protection against Rhizoctonia solani AG-3 and Fusarium solani f. sp. eumartii. Physiol. Mol. Plant. Pathol. 2006, 69, 93–103. [Google Scholar] [CrossRef]
- Snarr, B.; Qureshi, S.; Sheppard, D. Immune recognition of fungal polysaccharides. J. Fungi 2017, 3, 47. [Google Scholar] [CrossRef] [PubMed]
- Hamer, J.E.; Talbot, N.J. Infection-related development in the rice blast fungus Magnaporthe grisea. Curr. Opin. Microbiol. 1998, 1, 693–697. [Google Scholar] [CrossRef]
- Otaka, J.; Seo, S.; Nishimura, M. Lutein, a natural carotenoid, induces α-1,3-glucan accumulation on the cell wall surface of fungal plant pathogens. Molecules 2016, 21, 980. [Google Scholar] [CrossRef] [PubMed]
- Geoghegan, I.; Steinberg, G.; Gurr, S. The role of the fungal cell wall in the infection of plants. Trends Microbiol. 2017, 25, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.; Latgé, J.P.; Beauvais, A. α1,3 glucans are dispensable in Aspergillus fumigatus. Eukaryot. Cell 2012, 11, 26–29. [Google Scholar] [CrossRef]
- Dute, R.R.; Weete, J.D.; Rushing, A.E. Ultrastructure of dormant and germinating conidia of Aspergillus Ochraceus. Mycologia 1989, 81, 772–782. [Google Scholar] [CrossRef]
- Gerin, P.A.; Dufrene, Y.; Bellon-Fontaine, M.-N.; Asther, M.; Rouxhetl, P.G. Surface properties of the conidiospores of phanerochaete chrysosporium and their relevance to pellet formation. J. Bacteriol. 1993, 175, 5135–5144. [Google Scholar] [CrossRef][Green Version]
- Grimm, L.H.; Kelly, S.; Hengstler, J.; Göbel, A.; Krull, R.; Hempel, D.C. Kinetic studies on the aggregation of Aspergillus niger conidia. Biotechnol. Bioeng. 2004, 87, 213–218. [Google Scholar] [CrossRef]
- Hobot, J.A.; Gull, K. Changes in the organisation of surface rodlets during germination of Syncephalastrum racemosum sporangiospores. Protoplasma 1981, 107, 339–343. [Google Scholar] [CrossRef]
- Tronchin, G.; Bouchara, J.P.; Ferron, M.; Larcher, G.; Chabasse, D. Cell surface properties of Aspergillus fumigatus conidia: Correlation between adherence, agglutination, and rearrangements of the cell wall. Can. J. Microbiol. 1995, 41, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Lo, T.C.-T.; Hsu, F.-M.; Chang, C.A.; Cheng, J.C.-H. Branched α-(1,4)-Glucans from Lentinula edodes (L10) in combination with radiation enhance cytotoxic effect on human lung adenocarcinoma through the toll-like receptor 4 mediated induction of THP-1 differentiation/activation. J. Agric. Food Chem. 2011, 59, 11997–12005. [Google Scholar] [CrossRef] [PubMed]
- Koppada, R.; Norozian, F.M.; Torbati, D.; Kalomiris, S.; Ramachandran, C.; Totapally, B.R. Physiological effects of a novel immune stimulator drug, (1,4)-α-d-glucan, in rats. Basic Clin. Pharmacol. Toxicol. 2009, 105, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Mendieta, S.; Guillén, D.; Hernández-Pando, R.; Sánchez, S.; Rodríguez-Sanoja, R. Potential of glucans as vaccine adjuvants: A review of the α-glucans case. Carbohydr. Polym. 2017, 165, 103–114. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, L.; Tao, Y.; Zeng, C.; Chen, Y.; Cheung, P.C.K. Solution properties of a water-insoluble (1→3)-α-d-glucan isolated from Poria cocos mycelia. Carbohydr. Polym. 2004, 57, 205–209. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, L.; Cheng, S. Chemical structure and molecular weights of α-(1→3)-glucan from Lentinus edodes. Biosci. Biotechnol. Biochem. 1999, 63, 1197–1202. [Google Scholar] [CrossRef]
- Wei, H.; Scherer, M.; Singh, A.; Liese, R.; Fischer, R. Aspergillus nidulans α-1,3-glucanase (mutanase), mutA, is expressed during sexual development and mobilizes mutan. Fungal Genet. Biol. 2001, 34, 217–227. [Google Scholar] [CrossRef]
- Dekker, N.; Speijer, D.; Grün, C.H.; van den Berg, M.; de Haan, A.; Hochstenbach, F. Role of the α-glucanase Agn1p in fission-yeast cell separation. Mol. Biol. Cell 2004, 15, 3903–3914. [Google Scholar] [CrossRef]
- Dekker, N.; van Rijssel, J.; Distel, B.; Hochstenbach, F. Role of the α-glucanase Agn2p in ascus-wall endolysis following sporulation in fission yeast. Yeast 2007, 24, 279–288. [Google Scholar] [CrossRef]
- Imai, K.; Kikuta, T.; Kobayashi, M.; Matsuda, K. An α-1,3-glucanase from streptomyces sp. KI-8 production and purification. Agric. Biol. Chem. 1977, 41, 1339–1346. [Google Scholar] [CrossRef]
- Nosál’ová, V.; Bobek, P.; Cerná, S.; Galbavý, S.; Stvrtina, S. Effects of pleuran (beta-glucan isolated from Pleurotus ostreatus) on experimental colitis in rats. Physiol. Res. 2001, 50, 575–581. [Google Scholar] [PubMed]
- Wang, T.; Li, H.; Nie, K.; Tan, T. Immobilization of lipase on epoxy activated (1→3)-α-d-glucan isolated from Penicillium chrysongenum. Biosci. Biotechnol. Biochem. 2006, 70, 2883–2888. [Google Scholar] [CrossRef] [PubMed]
- Fishman, A.; Levy, I.; Cogan, U.; Shoseyov, O. Stabilization of horseradish peroxidase in aqueous-organic media by immobilization onto cellulose using a cellulose-binding-domain. J. Mol. Catal. B Enzym. 2002, 18, 121–131. [Google Scholar] [CrossRef]
- Villalonga, R.; Villalonga, M.L.; Gómez, L. Preparation and functional properties of trypsin modified by carboxymethylcellulose. J. Mol. Catal. B Enzym. 2000, 10, 483–490. [Google Scholar] [CrossRef]
- Hertzberg, S.; Kvittingen, L.; Anthonsen, T.; Skjåk-Bræk, G. Alginate as immobilization matrix and stabilizing agent in a two-phase liquid system: Application in lipase-catalysed reactions. Enzyme Microb. Technol. 1992, 14, 42–47. [Google Scholar] [CrossRef]
- Falandysz, J.; Szymczyk, K.; Ichihashi, H.; Bielawski, L.; Gucia, M.; Frankowska, A.; Yamasaki, S.-I. ICP/MS and ICP/AES elemental analysis (38 elements) of edible wild mushrooms growing in Poland. Food Addit. Contam. 2001, 18, 503–513. [Google Scholar] [CrossRef]
- Stijve, T.; Besson, R. Mercury, cadmium, lead and selenium content of mushroom species belonging to the genus Agaricus. Chemosphere 1976, 5, 151–158. [Google Scholar] [CrossRef]
- Falandysz, J.; Borovička, J. Macro and trace mineral constituents and radionuclides in mushrooms: Health benefits and risks. Appl. Microbiol. Biotechnol. 2013, 97, 477–501. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef]
- Malik, A. Metal bioremediation through growing cells. Environ. Int. 2004, 30, 261–278. [Google Scholar] [CrossRef]
- Garnham, G.; Codd, G.; Gadd, G. Kinetics of uptake and intracellular location of cobalt, manganese and zinc in the estuarine green alga Chlorella salina. Appl. Microbiol. Biotechnol. 1992, 37, 270–276. [Google Scholar] [CrossRef]
- Dönmez, G.; Aksu, Z. The effect of copper(II) ions on the growth and bioaccumulation properties of some yeasts. Process. Biochem. 1999, 35, 135–142. [Google Scholar] [CrossRef]
- Vidali, M. Bioremediation. An overview. Pure Appl. Chem. 2001, 73, 1163–1172. [Google Scholar] [CrossRef]
- Megharaj, M.; Ramakrishnan, B.; Venkateswarlu, K.; Sethunathan, N.; Naidu, R. Bioremediation approaches for organic pollutants: A critical perspective. Environ. Int. 2011, 37, 1362–1375. [Google Scholar] [CrossRef] [PubMed]
- Sardar, U.R.; Bhargavi, E.; Devi, I.; Bhunia, B.; Tiwari, O.N. Advances in exopolysaccharides based bioremediation of heavy metals in soil and water: A critical review. Carbohydr. Polym. 2018, 199, 353–364. [Google Scholar] [CrossRef]
| Species of Fungus. | Molecular Mass Weight [kDa] | Viscosity [mPa⋅s] | Optical Rotation [α] [°] | (1→3)-α-d-glucan Content in Fungus (Dry Fungal Mass %) | Structure | Reference |
|---|---|---|---|---|---|---|
| Aspergillus fumigatus | 16.7 | +286 | 12.5 | The backbone chain is formed mainly (91.3–97.8%) of glucose linked by (1→3), while (1→4) linkages are in the minority (1.3–7.2%). There are small amounts of three types of doubly substituted glucose residues, i.e., →2,3)-Glcp-(1→; →3,4)-Glcp-(1→ and →3,6)-Glcp-(1→. | [16] | |
| Aspergillus nidulans | 12.2 | +384 | 9.4 | as above | [16] | |
| Aspergillus niger | 8.8 | +254 | 8.4 | as above | [16] | |
| Aspergillus wentii | 850 | 17.0 | +216 | 6.5 | A linear polymer with 25 subunits; each subunit is constructed of about 200 residues of (1→3)- α-d-glucoses separated by a short spacer of (1→4)-α-d-glucoses. | [7] |
| Cerrena unicolor | 2.12 (Fruiting body) 7.55 (Mycelium) | +206 (Fruiting body) +200 (Mycelium) | 46.1 | About 90% of the (1→3)- linkages; there are also →4)-α-d-Glcp-(1→ (7.4–4.4%) and →3,4)-α-d-Glcp-(1→(3.2–2.5%). | [18] | |
| Ganoderma lucidum | 1.94–1.98 | +25 to +39 | 1.53–3.06 | The backbone chain is formed mainly (74.9–87.9%) of glucose linked by (1→3)-, while (1→4)- linkages are in the minority (6.7–8.7%). There are small amounts of three types of doubly substituted glucose residues, i.e., →2,3)-Glcp-(1→; →3,4)-Glcp-(1→and 3,6)-Glcp-(1→. | [19] | |
| Lentinus edodes | 72.4–521 | 9 | The chain consists mainly of (1→3)-bonds (67%) with a small number of (1→4)-bonds (27.3%) and →3,6)-Glcp-(1→ and →4,6)-Glcp-(1→. | [20,21,22] | ||
| Laetiporus sulphureus | 57 | The chain consists mainly of (1→3)-bonds (91.2%) and a small number of (1→4)-bonds (3%). | [22] | |||
| Penicillium chrysogenum | 180 | 6 | [23] | |||
| Fomitopsis betulina(earlier Piptoporus betulinus) | 270 | 24 | The chain consists mainly of (1→3)-bonds (84.6%) and a small number of (1→4)-bonds (6%). | [22,24] | ||
| Pleurotus citrinopileatus | 4.0 | 91.2% of the (1→3)- linkages | [25] | |||
| Pleurotus djamor | 3.1 | 73.8% of the (1→3)- linkages | [25] | |||
| Pleurotus eryngii | 2.0 | 89.4% of the (1→3)- linkages | [25] | |||
| Pleurotus ostreatus | 6.1 | The chain consists mainly of (1→3)-bonds (82.8%) and a small number of (1→4)-bonds (7.4%). | [22] | |||
| Pleurotus precoce | 2.7 | 84.7% of the (1→3)-linkages | [25] | |||
| Ramaria botrytis | A linear polymer is composed of →3)-α-d-Glcp-(1→ repeating units. | [17] |
| Species of Fungi Used | Decolorization and Removal of the Water-Soluble Fraction | Neutralization Stage | Rinsing Stage | Reference |
|---|---|---|---|---|
| Trichoderma viride | Sodium borohydride, Sodium hydroxide, methanol, methanol water solution | Methanol-acetic acid solution | Methanol-water solution Water, boiled in water Ethanol | [39] |
| Amanita muscaria | Methanol, 0.9% sodium hydroxide, hot water, 5% Na2CO3 1M NaOH solution with sodium borohydride (200 mg) | 1M HCl | Water | [40] |
| Schizophyllum commune | Water 5% KOH activated charcoal | Acetic acid | Water | [41] |
| Laetiporus sulphureus | Water NaOH | HCl | Water | [42] |
| Medicinal Property of (1→3)-α-d-Glucans | Application | Reference |
|---|---|---|
| Anti-tumour properties | Potential anti-cancer drug | [22,25,51,52,53,67,68,69,70,71,72] |
| Immunological activity | Adjuvants in vaccination | [16,25,54,67,68,69,70,71,72,73,74,75] |
| Mutanase inducers | Active ingredient of oral hygiene products (mouthwashes, toothpastes, or chewing gums), | [33,34,42,64,65,66,76,77,78] |
| Role in the pathogenicity of Aspergillus fumigatus | Vaccine and diagnostic test systems | [57,60,79,80,81,82,83] |
| Prebiotic properties | New prebiotic source | [36,84] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Złotko, K.; Wiater, A.; Waśko, A.; Pleszczyńska, M.; Paduch, R.; Jaroszuk-Ściseł, J.; Bieganowski, A. A Report on Fungal (1→3)-α-d-glucans: Properties, Functions and Application. Molecules 2019, 24, 3972. https://doi.org/10.3390/molecules24213972
Złotko K, Wiater A, Waśko A, Pleszczyńska M, Paduch R, Jaroszuk-Ściseł J, Bieganowski A. A Report on Fungal (1→3)-α-d-glucans: Properties, Functions and Application. Molecules. 2019; 24(21):3972. https://doi.org/10.3390/molecules24213972
Chicago/Turabian StyleZłotko, Katarzyna, Adrian Wiater, Adam Waśko, Małgorzata Pleszczyńska, Roman Paduch, Jolanta Jaroszuk-Ściseł, and Andrzej Bieganowski. 2019. "A Report on Fungal (1→3)-α-d-glucans: Properties, Functions and Application" Molecules 24, no. 21: 3972. https://doi.org/10.3390/molecules24213972
APA StyleZłotko, K., Wiater, A., Waśko, A., Pleszczyńska, M., Paduch, R., Jaroszuk-Ściseł, J., & Bieganowski, A. (2019). A Report on Fungal (1→3)-α-d-glucans: Properties, Functions and Application. Molecules, 24(21), 3972. https://doi.org/10.3390/molecules24213972







