Apoptotic Induction and Anti-Migratory Effects of Rhazya Stricta Fruit Extracts on a Human Breast Cancer Cell Line
Abstract
1. Introduction
2. Results
2.1. Cytotoxic Activity of Rhazya stricta Fruit Extracts Against Breast Human Cancer Cell Lines
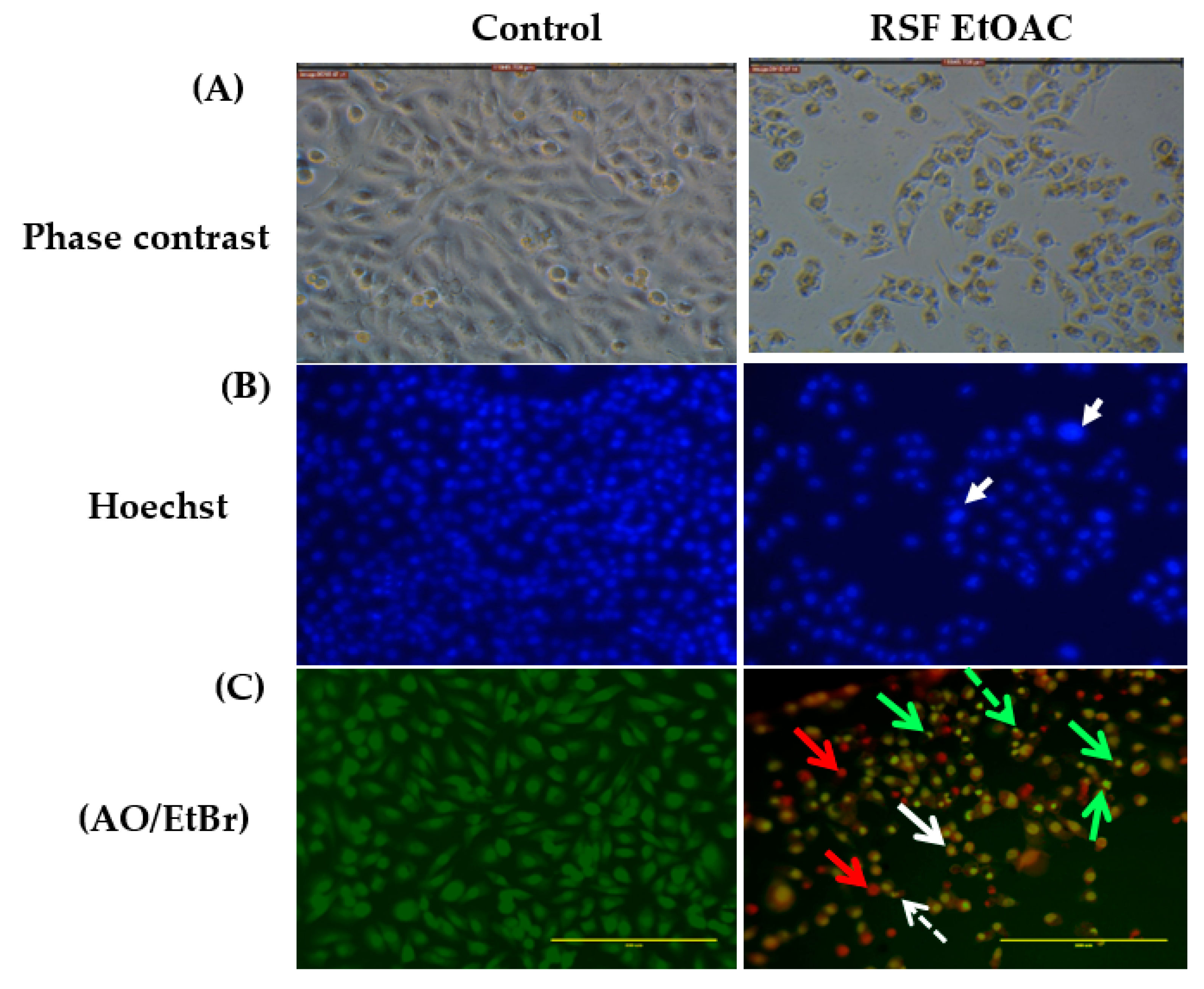
2.2. Apoptotic Activity of RSF EtOAc
2.2.1. Microscopic Studies
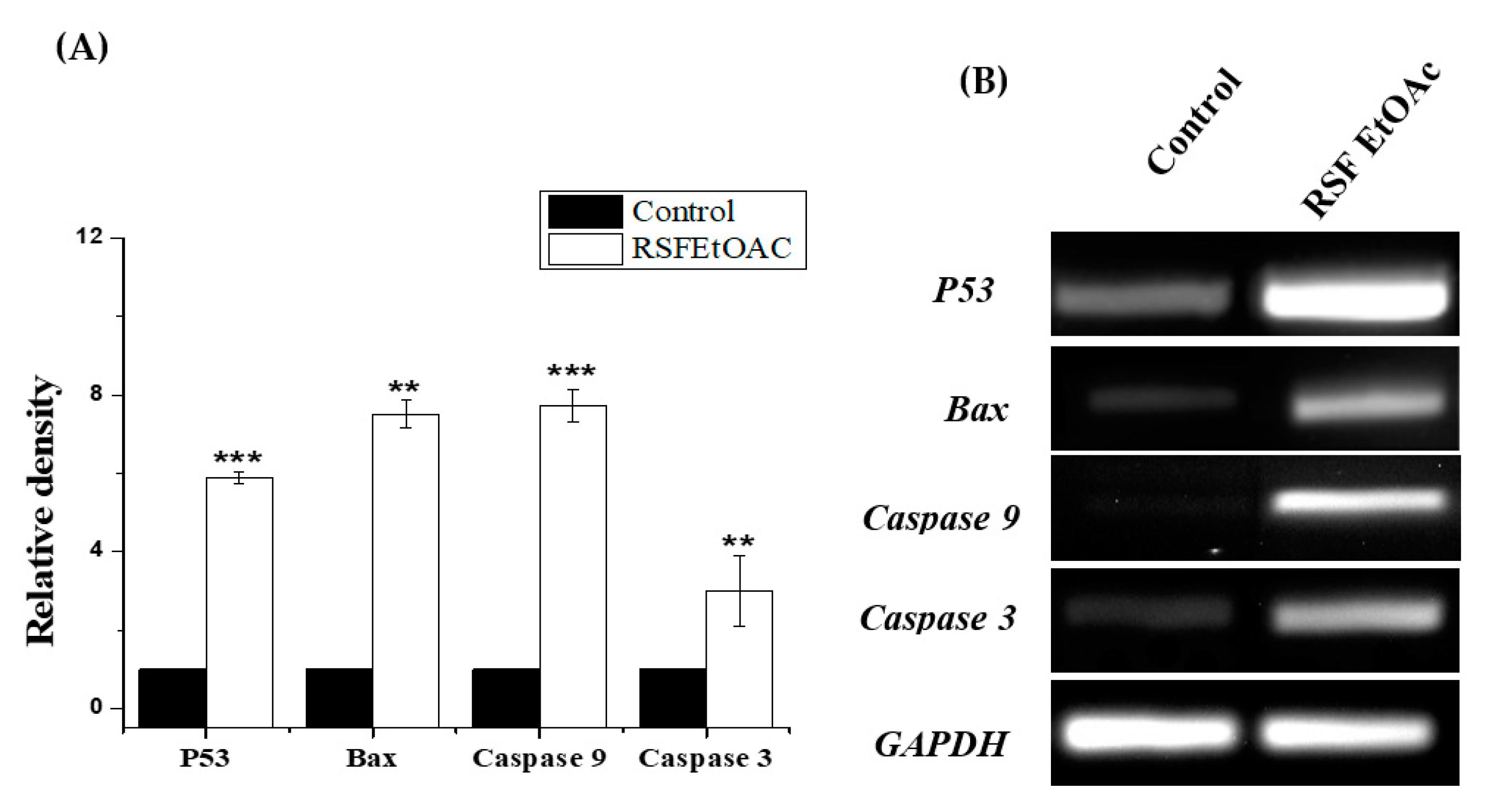
2.2.2. RT-PCR
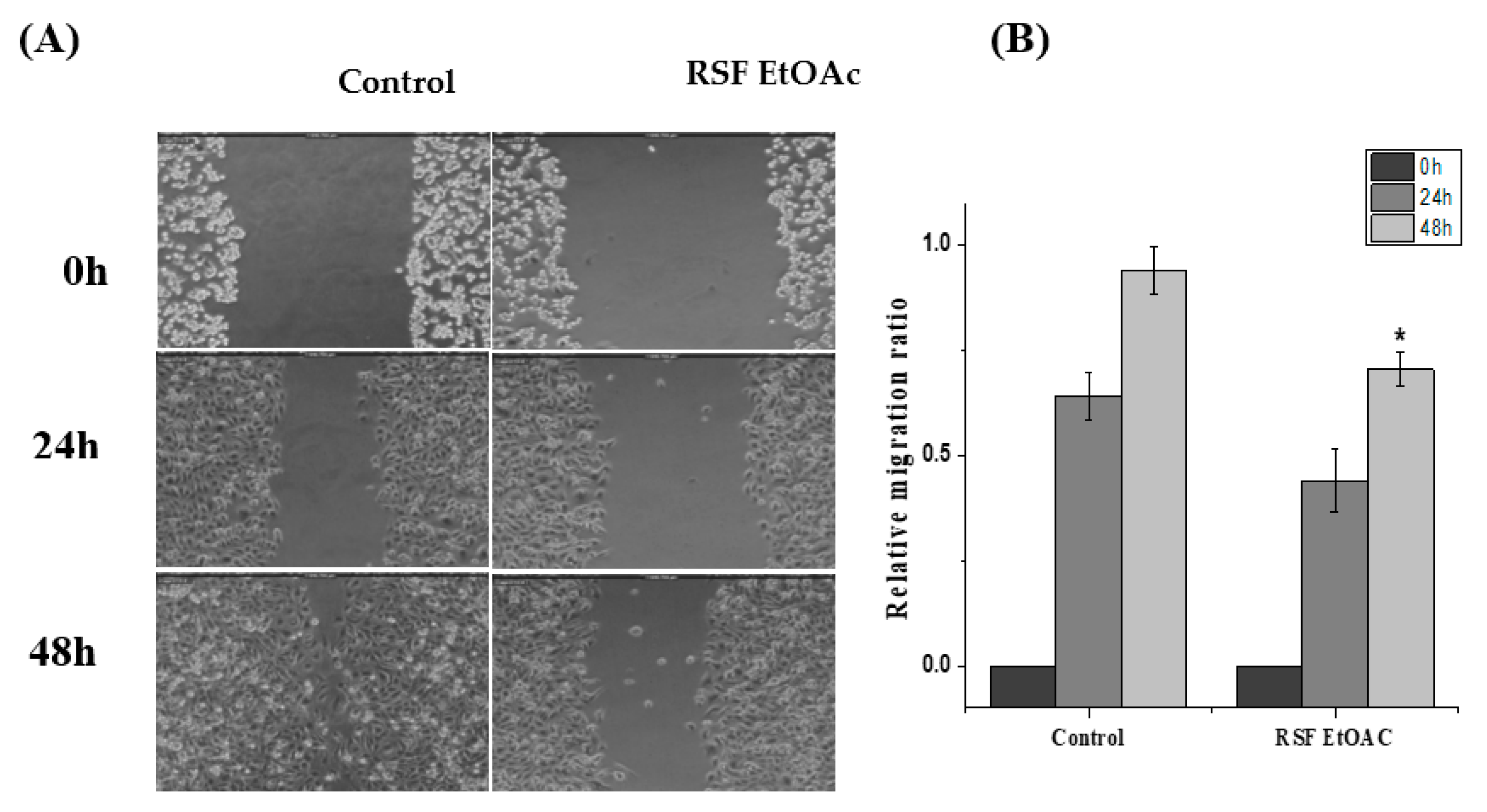
2.3. Effects of RSF EtOAc on Cell Migrations of MDA-MB-231
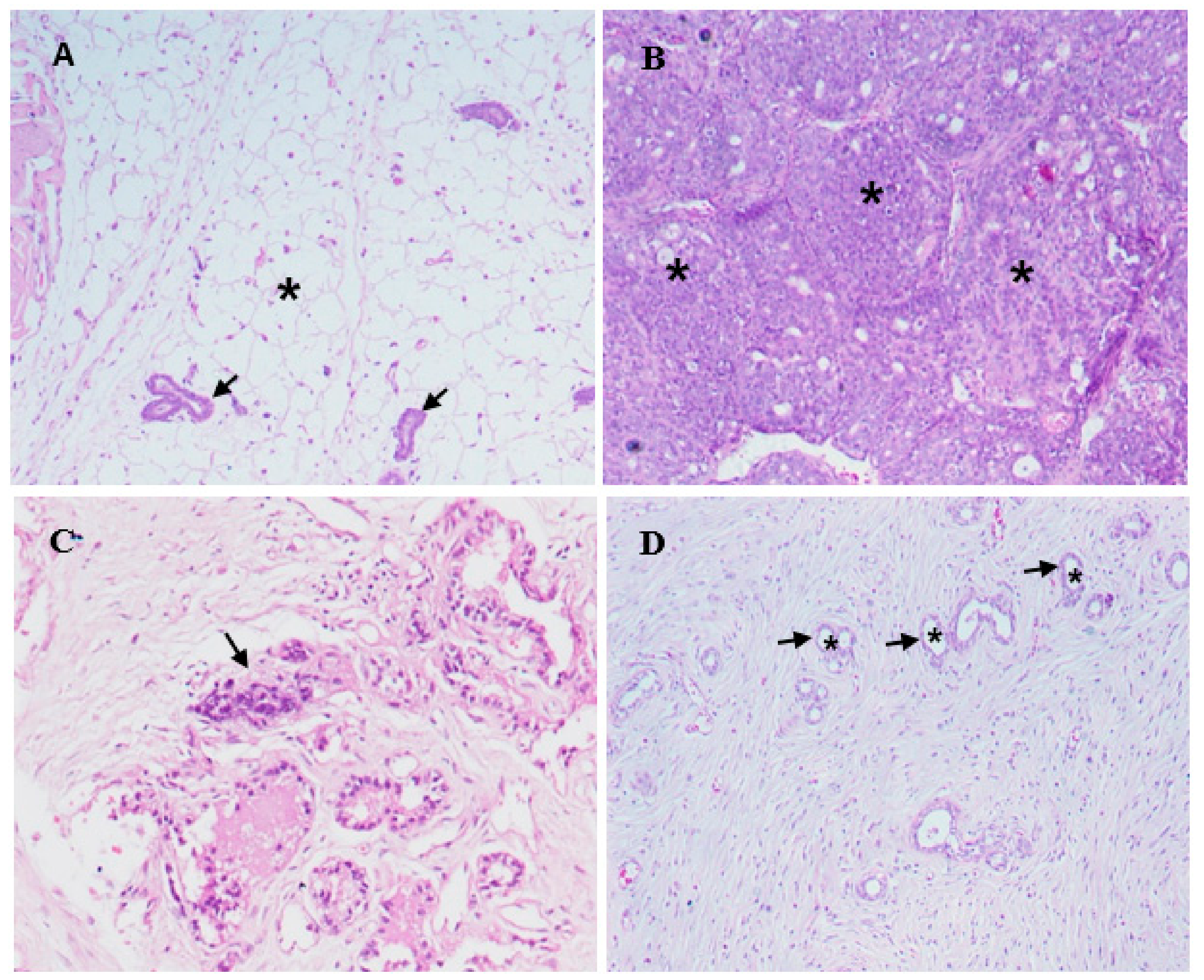
2.4. Histopathology and Morphological Observations
2.5. Quantitative Phytochemical of R. stricta Fruit
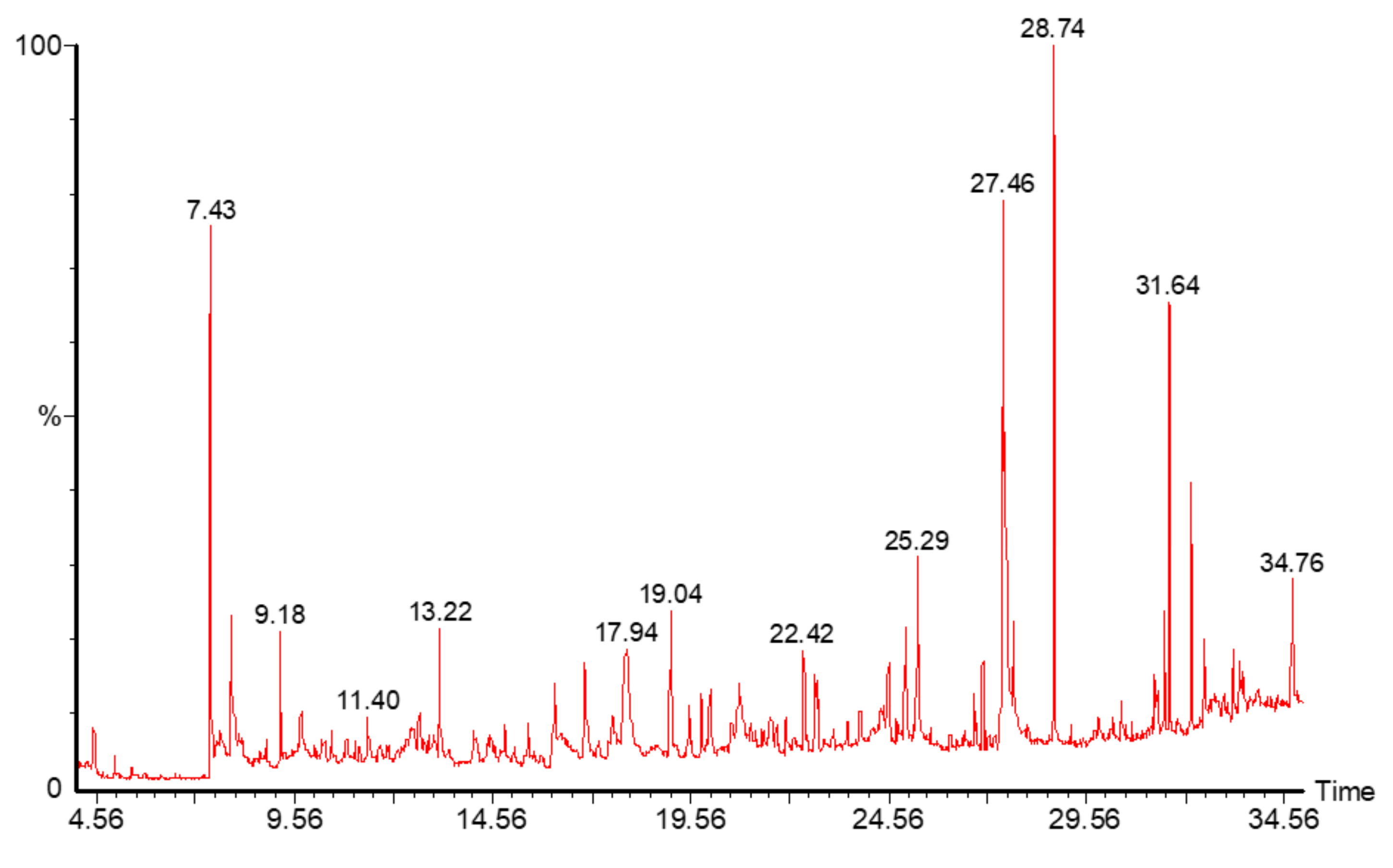
2.6. Gas Chromatography Mass Spectroscopy
3. Discussion
4. Materials and Methods
4.1. Plant Sample Collection and Extract Preparation
4.2. Cytotoxicity Assays
4.2.1. MTT Assay
4.2.2. Lactate Dehydrogenase (LDH) Cytotoxicity Assay
4.3. Assessment of Morphology of Apoptotic Cells by Phase-Contrast Inverted Microscopy, Fluorescent Hoechst 33,258 Staining, and Acridine Orange Ethidium Bromide Dual Staining
4.4. Gene Expression Detection Using RT-PCR
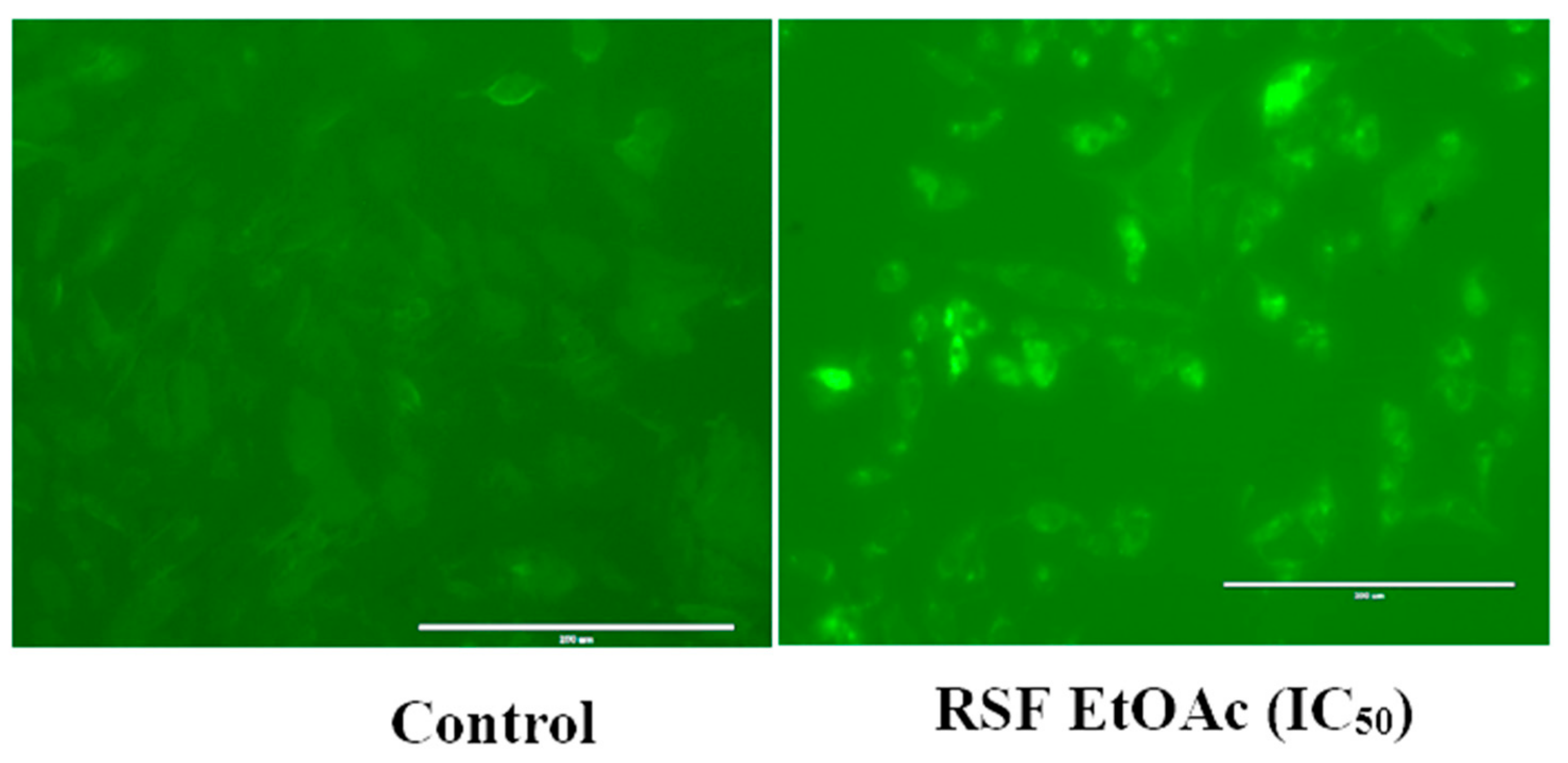
4.5. Caspase 3/7 Green Fluorescence Detection
4.6. Scratch Wound Healing Migration Assay
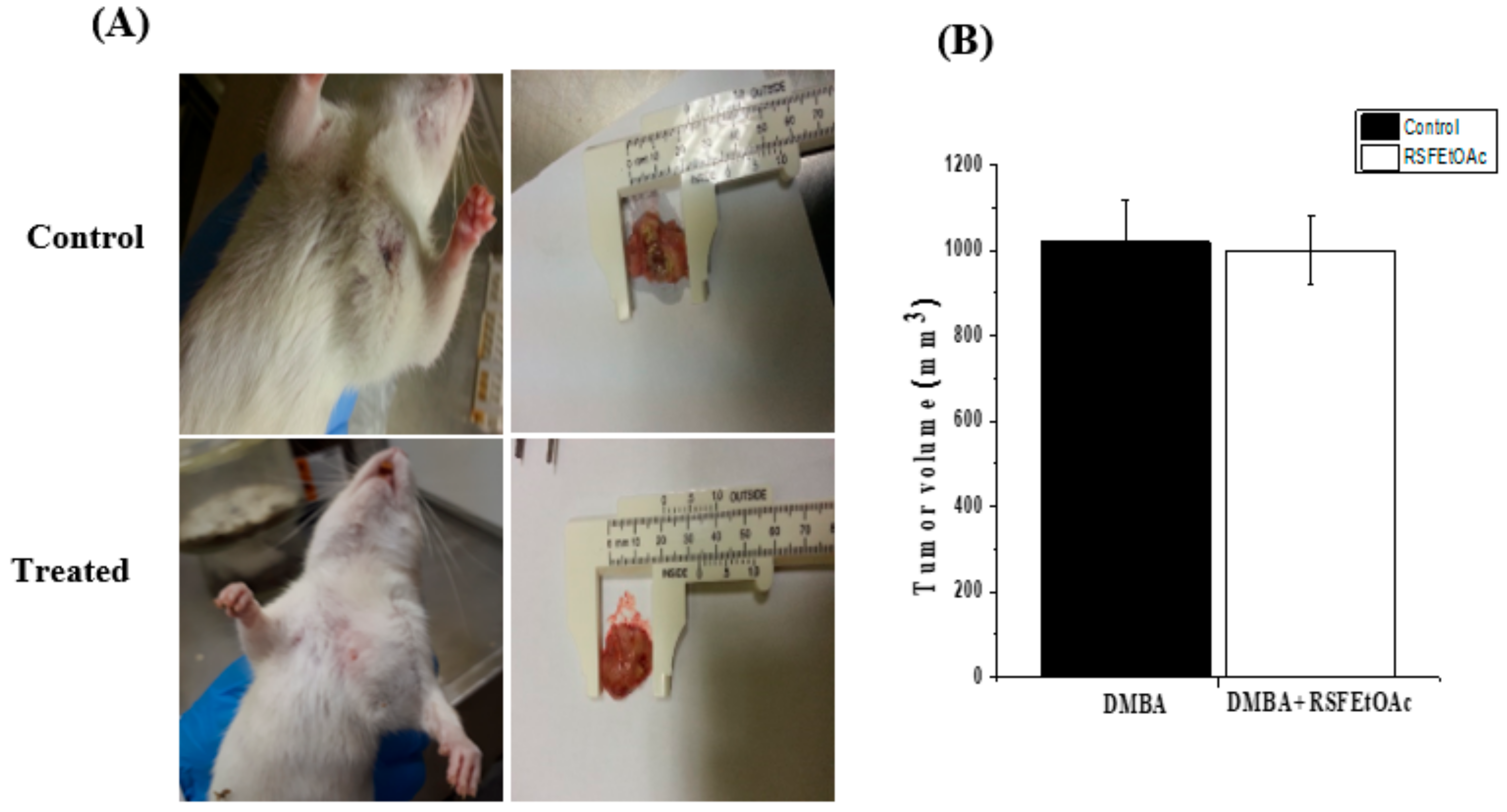
4.7. In Vivo Experimental Studies
Tumor Induction and Plant Extract Treatment
4.8. Determination of Total Phenolic, Flavonoid Contents
4.9. Antioxidant Activity using DPPH Radical Scavenging Method
4.10. Gas Chromatography-Mass Spectroscopy (GC-MS)
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hutchinson, L. Breast cancer: Challenges, controversies, breakthroughs. Nat. Rev. Clin. Oncol. 2010, 7, 669–670. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Al Diab, A.; Qureshi, S.; Al Saleh, K.A.; Al Qahtani, A.H.; Aleem, A.; Algamdi, M. Review on breast cancer in the Kingdom of Saudi Arabia. Middle-East J. Sci. Res. 2013, 14, 532–543. [Google Scholar]
- Al-Madouj, A.N.; Alshahrani, Z.S.; Alrawaji, A.I.; Hayder, M.S.; Al-Shridah, M.M.; Al-Shamrani, T.H. Cancer Incidence Report Saudi Arabia 2013; Saudi Cancer Registry: Riyadh, Saudi Arabia, 2016. [Google Scholar]
- Zahreddine, H.; Borden, K.L. Mechanisms and insights into drug resistance in cancer. Front. Pharmacol. 2013, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Moiseenko, F.; Volkov, N.; Bogdanov, A.; Dubina, M.; Moiseyenko, V. Resistance mechanisms to drug therapy in breast cancer and other solid tumors: An opinion. F1000Research 2017, 6, 288. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Desai, A.G.; Qazi, G.N.; Ganju, R.K.; El-Tamer, M.; Singh, J.; Saxena, A.K.; Bedi, Y.S.; Taneja, S.C.; Bhat, H.K. Medicinal plants and cancer chemoprevention. Curr. Drug Metab. 2008, 9, 581–591. [Google Scholar] [CrossRef]
- Ali, B.H.; Al-Qarawi, A.A.; Bashir, A.K.; Tanira, M.O. Phytochemistry, pharmacology and toxicity of Rhazya stricta Decne: A review. Phytother. Res. 2000, 14, 229–234. [Google Scholar] [CrossRef]
- Baeshen, N.A.; Elkady, A.I.; Abuzinadah, O.A.; Mutwakil, M.H. Potential anticancer activity of the medicinal herb, Rhazya stricta, against human breast cancer. Afr. J. Biotechnol. 2012, 11, 8960–8972. [Google Scholar]
- Elkady, A.I. Crude alkaloid extract of Rhazya stricta inhibits cell growth and sensitizes human lung cancer cells to cisplatin through induction of apoptosis. Genet. Mol. Biol. 2013, 36, 12–21. [Google Scholar] [CrossRef]
- Elkady, A.I.; Hussein, R.A.; El-Assouli, S.M. Harmal extract induces apoptosis of HCT116 human colon cancer cells, mediated by inhibition of nuclear factor-κB and activator protein-1 signaling pathways and induction of cytoprotective genes. Asian Pac. J. Cancer Prev. 2016, 17, 1947–1959. [Google Scholar] [CrossRef] [PubMed]
- Atale, N.; Gupta, S.; Yadav, U.C.; Rani, V. Cell-death assessment by fluorescent and nonfluorescent cytosolic and nuclear staining techniques. J. Microsc. 2014, 255, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Itharat, A.; Houghton, P.J.; Eno-Amooquaye, E.; Burke, P.J.; Sampson, J.H.; Raman, A. In vitro cytotoxic activity of Thai medicinal plants used traditionally to treat cancer. J. Ethnopharmacol. 2004, 90, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Van Cruchten, S.; Van Den Broeck, W. Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat. Histol. Embryol. 2002, 31, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Oren, M. Regulation of the p53 tumor suppressor protein. J. Biol. Chem. 1999, 274, 36031–36034. [Google Scholar] [CrossRef]
- Gasco, M.; Crook, T. p53 family members and chemoresistance in cancer: What we know and what we need to know. Drug Resist. Update 2003, 6, 323–328. [Google Scholar] [CrossRef]
- Haupt, S.; Berger, M.; Goldberg, Z.; Haupt, Y. Apoptosis—The p53 network. J. Cell Sci. 2003, 116, 4077–4085. [Google Scholar] [CrossRef]
- Hussain, S.P.; Harris, C.C. p53 biological network: At the crossroads of the cellular-stress response pathway and molecular carcinogenesis. J. Nippon Med. Sch. 2006, 73, 54–64. [Google Scholar] [CrossRef]
- Steeg, P.S. Tumor metastasis: Mechanistic insights and clinical challenges. Nat. Med. 2006, 12, 895–904. [Google Scholar] [CrossRef]
- Van Zijl, F.; Krupitza, G.; Mikulits, W. Initial steps of metastasis: Cell invasion and endothelial transmigration. Mutat. Res./Rev. Mutat. Res. 2011, 728, 23–34. [Google Scholar] [CrossRef]
- Rowe, R.G.; Weiss, S.J. Navigating ECM barriers at the invasive front: The cancer cell-stroma interface. Annu. Rev. Cell Dev. Biol. 2009, 25, 567–595. [Google Scholar] [CrossRef]
- Abba, M.; Patil, N.; Allgayer, H. MicroRNAs in the Regulation of MMPs and Metastasis. Cancers 2014, 6, 625–645. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Huang, C.Y.; Tsai, C.Y.; Lu, S.Y.; Chiu, C.C.; Fang, K. The aqueous extract of Prunella vulgaris suppresses cell invasion and migration in human liver cancer cells by attenuating matrix metalloproteinases. Am. J. Chin. Med. 2012, 40, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Kamijima, S.; Tobe, T.; Suyama, T.; Ueda, T.; Igarashi, T.; Ichikawa, T.; Ito, H. The prognostic value of p53, Ki-67 and matrix metalloproteinases MMP-2 and MMP-9 in transitional cell carcinoma of the renal pelvis and ureter. Int. J. Urol. 2005, 12, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Na, M.H.; Kim, W.K. Alpha-Lipoic acid reduces matrix metalloproteinase activity in MDA-MB-231 human breast cancer cells. Nutr. Res. 2010, 30, 403–409. [Google Scholar] [CrossRef]
- Al Dhaheri, Y.; Attoub, S.; Arafat, K.; Abuqamar, S.; Viallet, J.; Saleh, A.; Al Agha, H.; Eid, A.; Iratni, R. Anti-metastatic and anti-tumor growth effects of Origanum majorana on highly metastatic human breast cancer cells: Inhibition of NFkappaB signaling and reduction of nitric oxide production. PLoS ONE 2013, 8, e68808. [Google Scholar] [CrossRef]
- Do Thi, N.; Hwang, E.S. Effects of laver extracts on adhesion, invasion, and migration in SK-Hep1 human hepatoma cancer cells. Biosci. Biotechnol. Biochem. 2014, 78, 1044–1051. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, Y.M.; Zhan, Y.Z.; Liu, C.X. Momordica cochinchinensis seed extracts suppress migration and invasion of human breast cancer ZR-75-30 cells via down-regulating MMP-2 and MMP-9. Asian Pac. J. Cancer Prev. 2014, 15, 1105–1110. [Google Scholar] [CrossRef]
- Pei, S.; Yang, X.; Wang, H.; Zhang, H.; Zhou, B.; Zhang, D.; Lin, D. Plantamajoside, a potential anti-tumor herbal medicine inhibits breast cancer growth and pulmonary metastasis by decreasing the activity of matrix metalloproteinase-9 and -2. BMC Cancer 2015, 15, 965. [Google Scholar] [CrossRef]
- Russo, J.; Russo, I.H. Experimentally induced mammary tumors in rats. Breast Cancer Res. Treat. 1996, 39, 7–20. [Google Scholar] [CrossRef]
- Al-Saeedi, F.J. Study of the cytotoxicity of asiaticoside on rats and tumour cells. BMC Cancer 2014, 14, 220. [Google Scholar] [CrossRef] [PubMed]
- Shoja, M.H.; Reddy, N.D.; Nayak, P.G.; Biswas, S.; Srinivasan, K.K.; Rao, C.M. In vitro mechanistic and in vivo anti-tumor studies of Glycosmis pentaphylla (Retz.) DC against breast cancer. J. Ethnopharmacol. 2016, 186, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; O’Hare, M.J.; Stein, R. Models of breast cancer: Is merging human and animal models the future? Breast Cancer Res. 2004, 6, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, B.J.; Specht, A.; Brushett, D. The essential oil of Cinnamomum camphora (L.) Nees and Eberm.-variation in oil composition throughout the tree in two chemotypes from Eastern Australia. J. Essent. Oil Res. 2004, 16, 9–14. [Google Scholar] [CrossRef]
- Cote, H.; Boucher, M.A.; Pichette, A.; Legault, J. Anti-Inflammatory, Antioxidant, Antibiotic, and Cytotoxic Activities of Tanacetum vulgare L. Essential Oil and Its Constituents. Medicines 2017, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Vermaak, I.; Viljoen, A. Camphor—A fumigant during the Black Death and a coveted fragrant wood in ancient Egypt and Babylon—A review. Molecules 2013, 18, 5434. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, A.S.; Yarovaya, C.O.; Shernyukov, C.A.; Pokrovsky, C.E.; Pokrovsky, C.A.; Lavrinenko, V.A.; Zarubaev, V.V.; Tretiak, T.S.; Anfimov, P.M.; Kiselev, O.I.; et al. New quaternary ammonium camphor derivatives and their antiviral activity, genotoxic effects and cytotoxicity. Bioorg. Med. Chem. 2013, 21, 6690–6698. [Google Scholar] [CrossRef]
- Noguchi, C.; Kamitori, K.; Hossain, A.; Hoshikawa, H.; Katagi, A.; Dong, Y.; Sui, L.; Tokuda, M.; Yamaguchi, F. D-Allose Inhibits Cancer Cell Growth by Reducing GLUT1 Expression. Tohoku J. Exp. Med. 2016, 238, 131–141. [Google Scholar] [CrossRef]
- El-Demerdash, A.; Moriou, C.; Martin, M.T.; Petek, S.; Debitus, C.; Al-Mourabit, A. Unguiculins A-C: Cytotoxic bis-guanidine alkaloids from the French Polynesian sponge, Monanchora n. sp. Nat. Prod. Res. 2018, 32, 1512–1517. [Google Scholar] [CrossRef]
- Deutsch, H.F.; Evenson, M.A.; Drescher, P.; Sparwasser, C.; Madsen, P.O. Isolation and biological activity of aspidospermine and quebrachamine from an Aspidosperma tree source. J. Pharm. Biomed. Anal. 1994, 12, 1283–1287. [Google Scholar] [CrossRef]
- Luanpitpong, S.; Talbott, S.J.; Rojanasakul, Y.; Nimmannit, U.; Pongrakhananon, V.; Wang, L.; Chanvorachote, P. Regulation of lung cancer cell migration and invasion by reactive oxygen species and caveolin-1. J. Biol. Chem. 2010, 285, 38832–38840. [Google Scholar] [CrossRef] [PubMed]
- Tuncer, E.; Unver-Saraydin, S.; Tepe, B.; Karadayi, S.; Ozer, H.; Sen, M.; Karadayi, K.; Inan, D.; Elagoz, S.; Polat, Z. Antitumor effects of Origanum acutidens extracts on human breast cancer. J. Balk. Union Oncol. 2013, 18, 77–85. [Google Scholar]
- Abdelmajidzyad, Z.; Morizet, J.; Legres, L.; Benard, J.; Chouaib, S. In vivo effect of the combination of TNF and adriamycin against a human breast cell line expressing the MDR-phenotype. Int. J. Oncol. 1995, 7, 1067–1072. [Google Scholar]
- Ait Mbarek, L.; Ait Mouse, H.; Elabbadi, N.; Bensalah, M.; Gamouh, A.; Aboufatima, R.; Benharref, A.; Chait, A.; Kamal, M.; Dalal, A. Anti-tumor properties of blackseed (Nigella sativa L.) extracts. Braz. J. Med. Biol. Res. 2007, 40, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Derle, A.; Ahire, M.; More, P.; Jagtap, S.; Phadatare, S.D.; Patil, A.B.; Jabgunde, A.M.; Sharma, G.K.; Shinde, V.S.; et al. Phytochemical analysis and free radical scavenging activity of medicinal plants Gnidia glauca and Dioscorea bulbifera. PLoS ONE 2013, 8, e82529. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the extracts and fractions are available from the authors. |


| Fraction Abbrev. | Cell Lines and IC50 (µg/mL) | ||
|---|---|---|---|
| MCF-7 | MDA-MB-231 | MCF-10A | |
| RSF Hex | 104 ± 2.1 | 167 ± 1.4 | 168 ± 1.4 |
| RSF CHCL3 | 49 ± 1.1 | 56 ± 0.6 | 49 ± 1.1 |
| RSF EtOAc | 39 ± 0.8 | 27 ± 0.5 | 47 ± 0.9 |
| RSF MeOH | 160 ± 1.5 | 177 ± 2.2 | 193 ± 0.6 |
| Fraction | Total Phenolics (µg/g) | Total Flavonoid (µg/g) | Antioxidant (%) |
|---|---|---|---|
| RSF Hex | 235 ± 2.5 | 55.63 ± 1.1 | 44.25 ± 1.8 |
| RSF CHCL3 | 246.67 ± 3.4 | 47.89 ± 2.3 | 37.66 ± 2.5 |
| RSF EtOAc | 257 ± 1.5 | 75 ± 2.5 | 51.81 ± 1.6 |
| RSF MeOH | 220 ± 1.7 | 40 ± 1.5 | 41.41 ± 2.1 |
| Compound Name | Chemical Formula | Molecular Weight (g/mol) | RT (min) | Area | Area % |
|---|---|---|---|---|---|
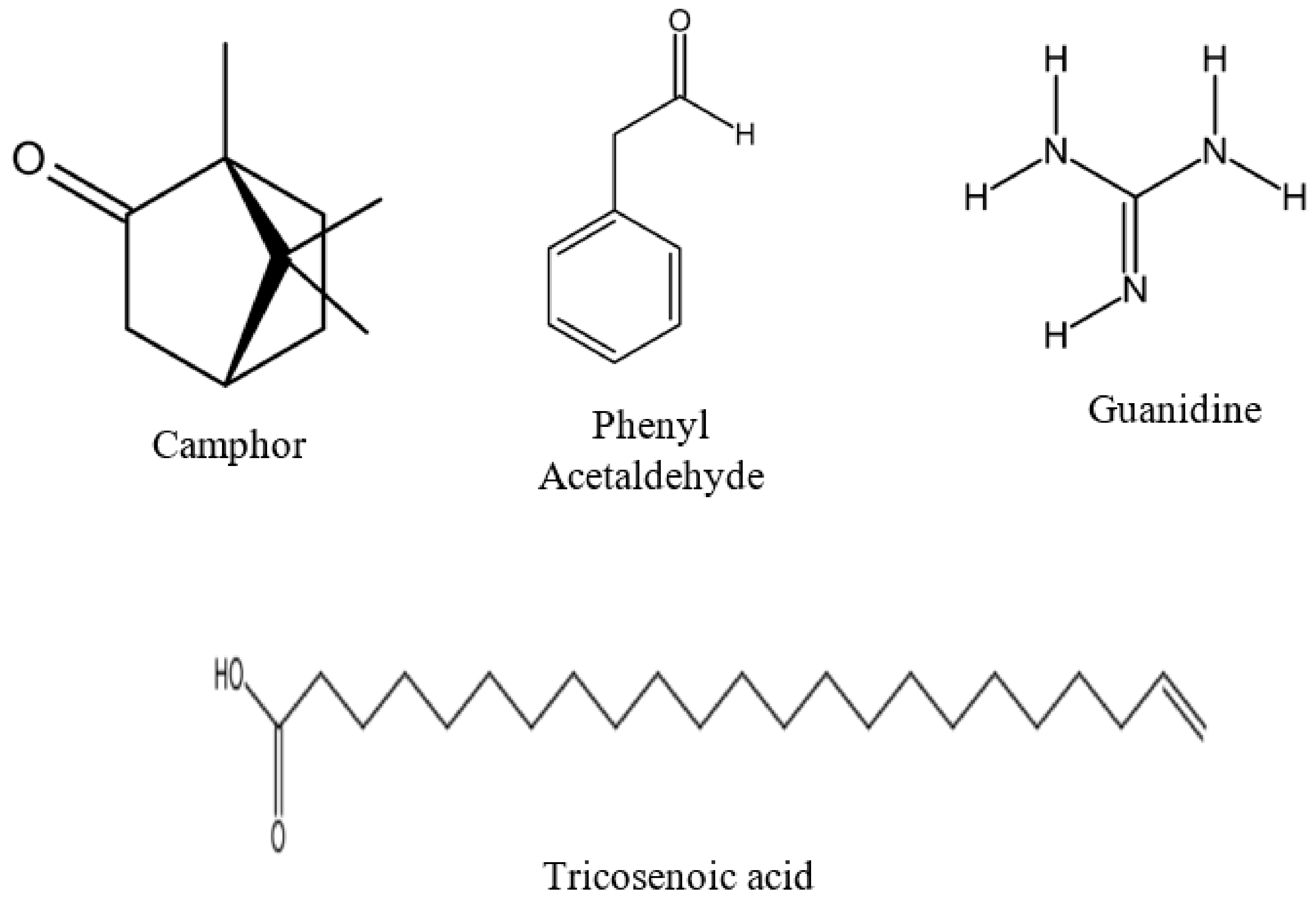
| Phenylacetaldehyde | C8H8O | 120.15 | 9.18 | 257603 | 8.040 |
| (Dimethylamino)methylene malononitrile | C6H7N3 | 121.140 | 9.70 | 37370 | 1.170 |
| trans-2-Undecenal | C11H20O | 168.28 | 13.22 | 103901 | 3.240 |
| Dihydrocitronellal | C10H20O | 156.27 | 14.10 | 64583 | 2.010 |
| Linalyl butyrate | C14H24O2 | 224.344 | 16.90 | 136684 | 4.260 |
| D-Allose | C6H12O6 | 180.156 | 17.95 | 230986 | 7.200 |
| 1-(3,4-Dimethoxyphenyl) ethanone | C10H12O3 | 180.203 | 19.06 | 178135 | 5.560 |
| 2,2-Tricosenoic acid | C23H44O2 | 352.603 | 27.46 | 484030 | 15.100 |
| 9-Octadecenoic acid | C18H34O2 | 282.468 | 27.70 | 64120 | 2.000 |
| Aspidospermine | C22H30N2O2 | 354.494 | 28.74 | 240055 | 7.490 |
| Quebrachamine | C19H26N2 | 282.431 | 31.64 | 211407 | 6.590 |
| Camphor | C10H16O | 152.23 | 32.19 | 675960 | 21.080 |
| Guanidine | CH5N3 | 59.07 | 34.76 | 465365 | 14.520 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Zharani, M.; Nasr, F.A.; Abutaha, N.; Alqahtani, A.S.; Noman, O.M.; Mubarak, M.; Wadaan, M.A. Apoptotic Induction and Anti-Migratory Effects of Rhazya Stricta Fruit Extracts on a Human Breast Cancer Cell Line. Molecules 2019, 24, 3968. https://doi.org/10.3390/molecules24213968
Al-Zharani M, Nasr FA, Abutaha N, Alqahtani AS, Noman OM, Mubarak M, Wadaan MA. Apoptotic Induction and Anti-Migratory Effects of Rhazya Stricta Fruit Extracts on a Human Breast Cancer Cell Line. Molecules. 2019; 24(21):3968. https://doi.org/10.3390/molecules24213968
Chicago/Turabian StyleAl-Zharani, Mohammed, Fahd A. Nasr, Nael Abutaha, Ali S. Alqahtani, Omar M. Noman, Mohammed Mubarak, and Muhammad A. Wadaan. 2019. "Apoptotic Induction and Anti-Migratory Effects of Rhazya Stricta Fruit Extracts on a Human Breast Cancer Cell Line" Molecules 24, no. 21: 3968. https://doi.org/10.3390/molecules24213968
APA StyleAl-Zharani, M., Nasr, F. A., Abutaha, N., Alqahtani, A. S., Noman, O. M., Mubarak, M., & Wadaan, M. A. (2019). Apoptotic Induction and Anti-Migratory Effects of Rhazya Stricta Fruit Extracts on a Human Breast Cancer Cell Line. Molecules, 24(21), 3968. https://doi.org/10.3390/molecules24213968






