New Chemical Constituents from the Bark of Dendropanax morbifera Leveille and Their Evaluation of Antioxidant Activities
Abstract
:1. Introduction
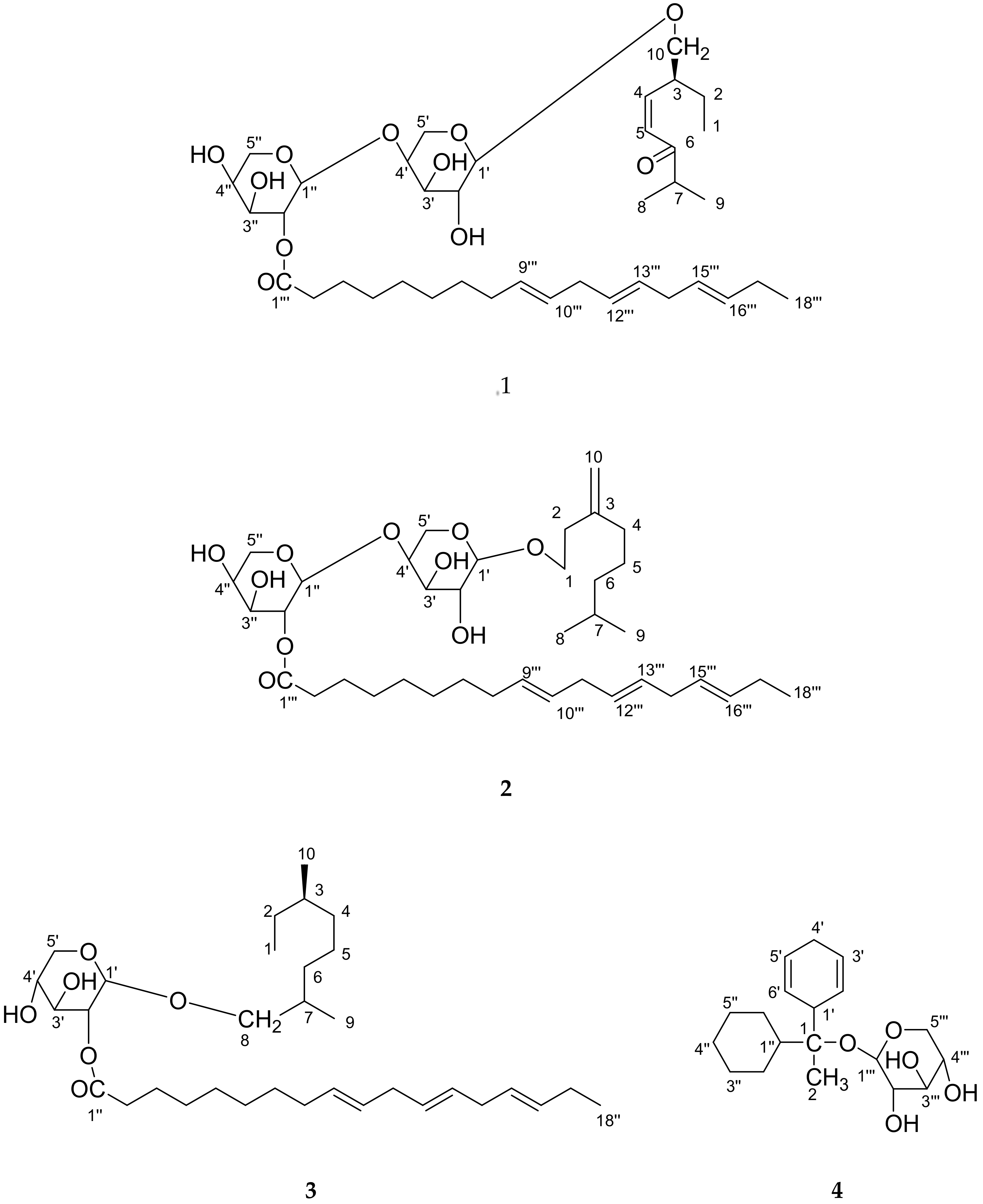
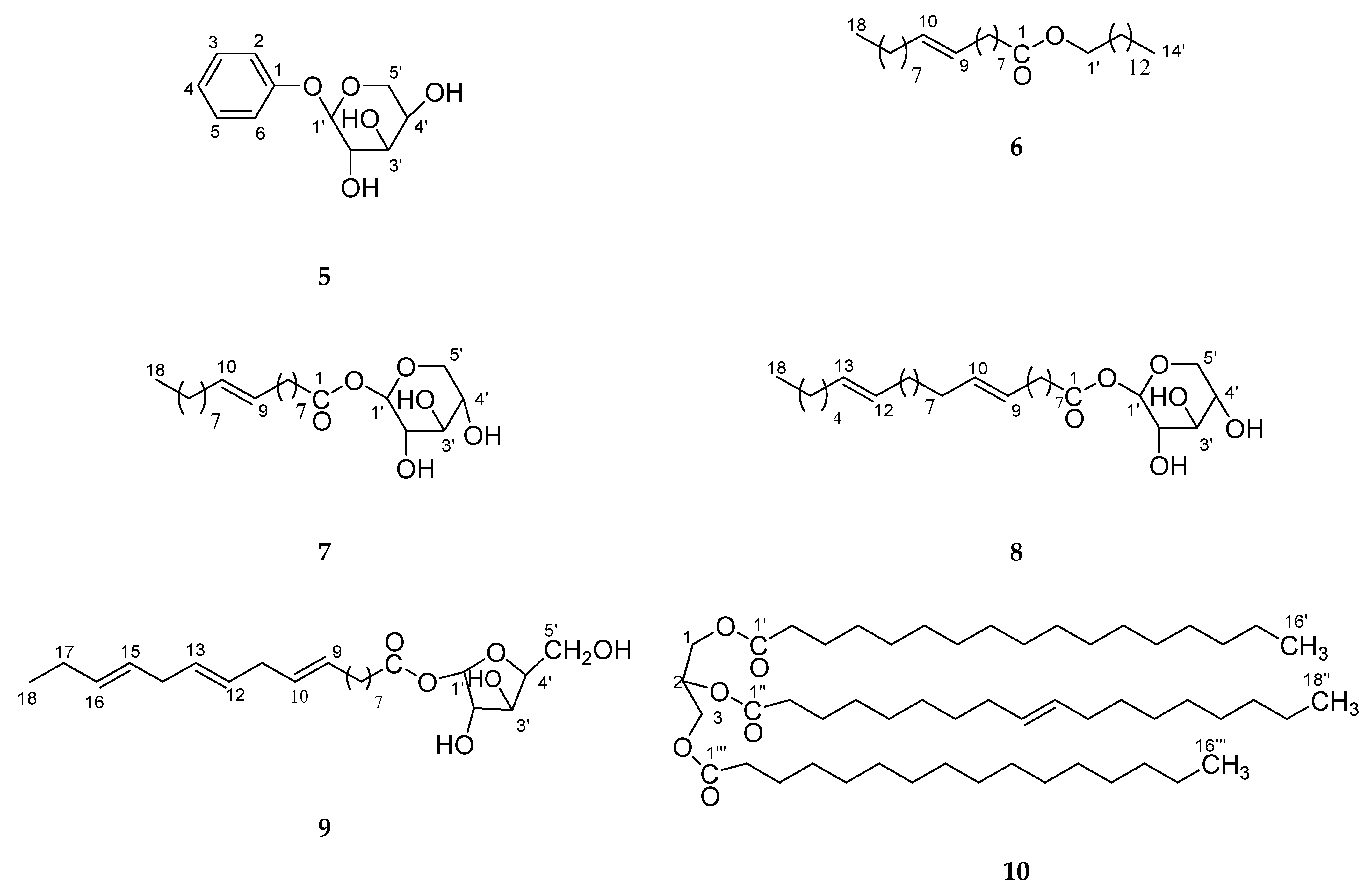
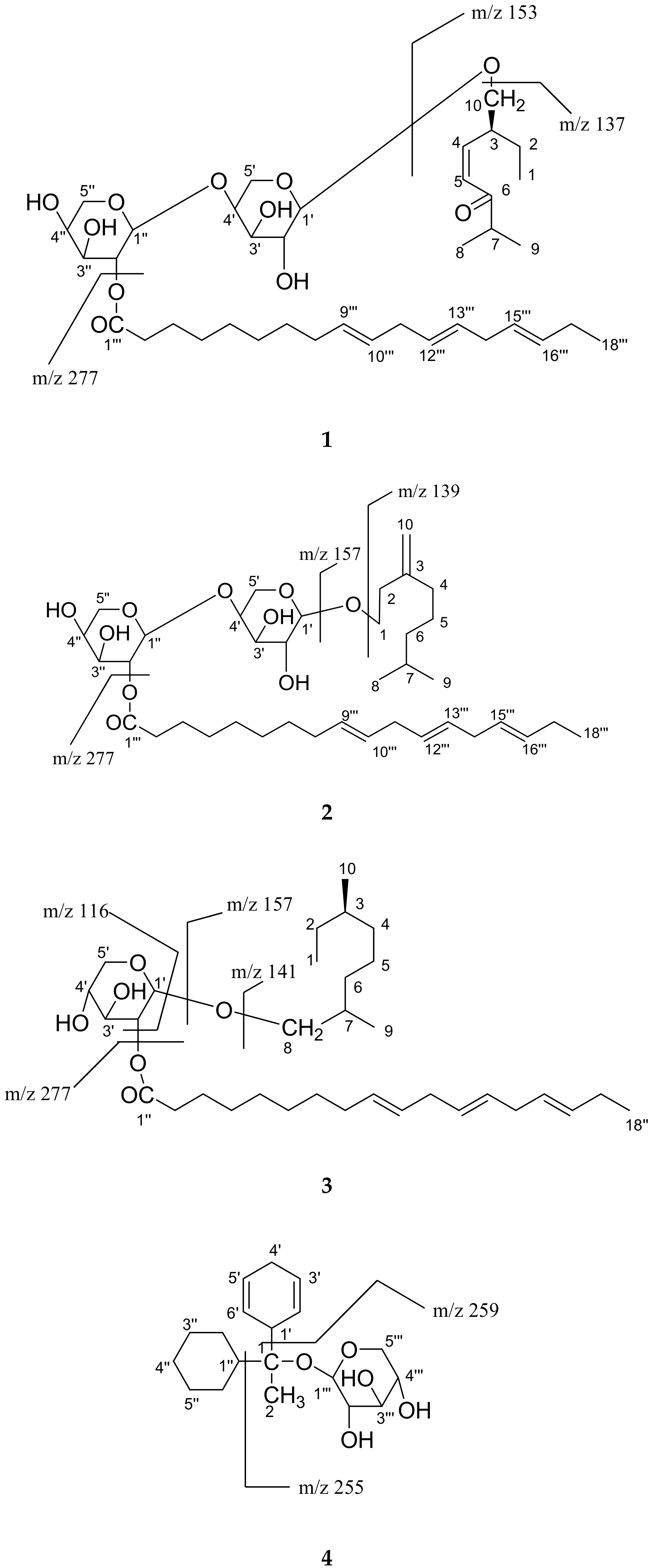
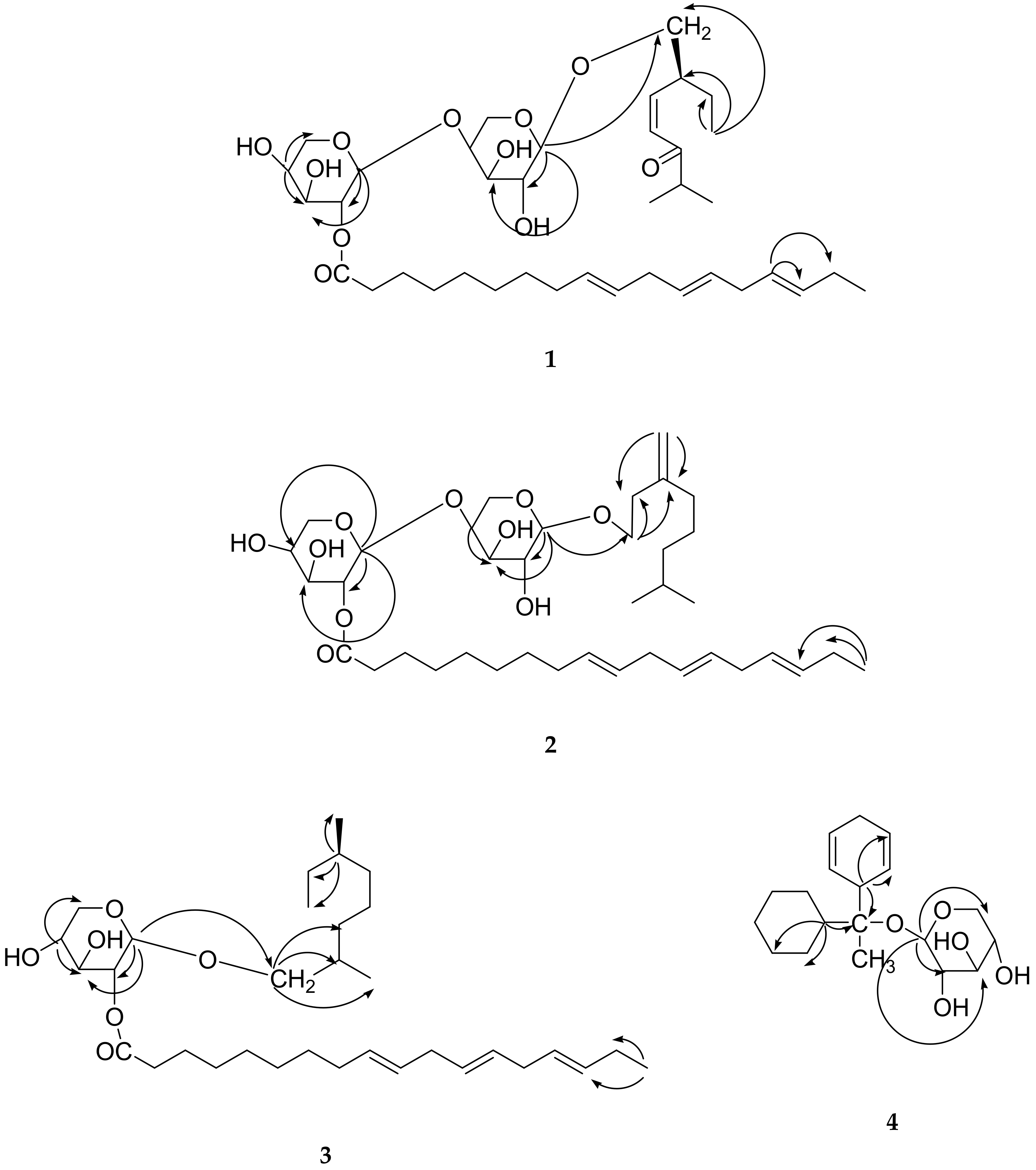
2. Results and Discussion
2.1. Antioxidant Activity
2.1.1. Free Radical Scavenging Activity
2.1.2. Reducing Power
2.1.3. Nitric Oxide Scavenging Activity
2.1.4. Antioxidant Capacity by Phosphomolybdenum Method
3. Materials and Methods
3.1. Chemical and Instruments
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Antioxidant Activity
3.4.1. Chemicals and Instruments in Antioxidant Activity
3.4.2. Free Radical Scavenging Activity
3.4.3. Reducing Power
3.4.4. Nitric Oxide Scavenging Activity
3.4.5. Evaluation of Antioxidant Capacity by Phosphomolybdenum Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Han, S.H.; Jung, Y.H.; Oh, M.H.; Ko, M.H.; Oh, Y.S.; Koh, S.C.; Kim, M.H.; Oh, M.Y. Phytogenetic relationship of the Dendropanax morfera and D. trifidus based on PCR-RAPD. Korean J. Genet. 1998, 20, 173–181. [Google Scholar]
- Bae, K.H. The Medicinal Plants of Korea; Kyo-Hak Publishing Co., Ltd.: Seoul, Korea, 2000. [Google Scholar]
- Park, B.Y.; Min, B.S.; Oh, S.R.; Kim, J.H.; Kim, T.J.; Kim, D.H.; Bae, K.H.; Lee, H.Y. Isolation and anticomplement activity of compounds from Dendropanx morbifera. J. Ethnopharmacol. 2004, 90, 403–408. [Google Scholar] [CrossRef]
- Setzer, W.N.; Green, T.J.; Whitaker, K.W.; Moriarity, D.M.; Yancey, C.A.; Lawton, R.O.; Bates, R.B. A cytotoxic diacetylene from Dendropanax arboreus. Planta Med. 1995, 61, 470–471. [Google Scholar] [CrossRef]
- Bernart, M.W.; Balacschak, M.S.; Alexander, M.R.; Shoemaker, R.H.; Boyd, M.R. Cytotoxic falcarinol oxylipins from Dendropanax arboreus. J. Nat. Prod. 1996, 59, 748–753. [Google Scholar] [CrossRef]
- Kawazu, K.; Noguchi, H.; Fujishita, K.; Iwasa, J. Two new antifungal compounds from Dendropanax trifidus. Tetrahedron Lett. 1973, 33, 3131–3132. [Google Scholar] [CrossRef]
- Oka, K.; Saito, F.; Yasuhara, T.; Sugimoto, A. The allergens of Dendropanax trifidus Makino and Fatsis japonica Decne. Et Planch and evaluation of cross-reactions with other plants of the Araliaceae family. Contact Dermat. 1999, 40, 209–213. [Google Scholar] [CrossRef]
- Oka, K.; Fumio, S.; Tahashi, Y.; Akiko, S. The major allergen of Dendropanax trifidus Makino. Contact Dermat. 1997, 36, 252–255. [Google Scholar] [CrossRef]
- Kobayashi, A.; Tagawa, K.; Yamashita, K. Synthesis of dendrotrifidol and its naturally occurring analogs. Agric. Biol. Chem. 1977, 41, 691–695. [Google Scholar]
- Tori, M.; Tori, T.; Tachibna, K.; Yamada, S.; Tsuyuki, T.; Takahashi, T. The backbone rearrangement of 3β, 4β-epoxyfriedelanr and thje synthesis of dendropanaxide. Bull Chem. Soc. Jpn. 1977, 50, 469–472. [Google Scholar] [CrossRef]
- Gil, R.R.; Ulubelen, A.; Chai, H.B.; Pezzuto, J.M.; Cordell, G.A. Biologically active compounds from the Euphorbiaceae; 2. Two triterpenoids of Euphobia cyparissias. Planta Med. 1994, 60, 594–596. [Google Scholar]
- Chung, I.M.; Kim, Y.C.; Ali, M.; Kim, S.H.; Park, I.; Kim, E.H.; Yang, Y.S.; Park, H.Y.; Son, E.U.; Ahmad, A. Triterpene glycosides from red ginseng marc and their anti-inflammatory activities. Bioorganic Med. Chem. Lett. 2014, 24, 4203–4208. [Google Scholar] [CrossRef]
- Ahmad, A.; Kim, S.H.; Ali, A.; Park, I.; Kim, J.S.; Kim, E.H.; Lim, J.L.; Kim, S.K.; Chung, I.M. New chemical constituents from Oryza sativa and their algicidal activities against blue-green algae. J. Agric. Food Chem. 2013, 61, 8039–8048. [Google Scholar] [CrossRef]
- Chung, I.M.; Song, H.K.; Kim, S.J.; Moon, H. Anticomplement activity of polyacetylenes from leaves of Dendropanax morboifera Leveille. Phytothe. Res. 2011, 25, 784–786. [Google Scholar] [CrossRef]
- Moon, H.I. Antidiabetic effects of dendropanoxide from leaves of Dendropanax morbifera Leveille in normal and streptozotocin-induced diabetic rats. Hum. Exp. Toxicol. 2011, 30. [Google Scholar] [CrossRef]
- Hyun, T.K.; Ko, Y.J.; Kim, E.H.; Chung, I.M.; Kim, J.S. Anti-inflammatory activity and phenolic composition of Dendropanax morbifera leaf extracts. Ind. Crops Prod. 2015, 74, 263–270. [Google Scholar] [CrossRef]
- Choi, J.U.; Kim, D.W.; Park, S.E.; Lee, H.J.; Kim, K.M.; Kim, K.J.; Kim, M.K.; Kim, S.J.; Kim, S. Anti-thrombotic effect of rutin isolated from Dendropanax morbifera Leveille. J. Biosci. Bioeng. 2015, 120, 181–186. [Google Scholar] [CrossRef]
- Katerere, D.R.; Eloff, J.N. Antibacterial and antioxidant activity of Sutherlandia frutescens (Fabaceae), a reputed Anti-HIV/AIDS phytomedicine. Phytother. Res. 2005, 19, 779–781. [Google Scholar] [CrossRef]
- Li, X.M.; Li, X.L.; Zhou, A.G. Evaluation of antioxidant activity of the polysaccharides extracted from Lycium barbarum fruits in vitro. Eur. Polym. J. 2007, 4, 488–497. [Google Scholar] [CrossRef]
- Chang, L.W.; Yen, W.J.; Huang, S.C.; Duh, P.D. Antioxidant activity of sesame coat. Food Chem. 2002, 78, 347–354. [Google Scholar] [CrossRef]
- Chung, Y.C.; Chang, C.T.; Chao, W.W.; Lin, C.F.; Chu, S.T. Antioxidative activity and safety of the 50 ethanolic extract from red bean fermented by Bacillus subtilis IMR-NK1. J. Agric. Food Chem. 2002, 50, 2454–2458. [Google Scholar] [CrossRef]
- Okuda, T.; Kimura, Y.; Yoshida, T.; Hatano, T.; Okuda, H.; Arichi, S. Studies on the activities of tannins and related compounds from medicinal plants and drugs. I Inhibitory effects on lipid peroxidation in mitochondria and microsomes of liver. Chem. Pharm. Bull. 1983, 31, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Kuie, C.W.; Nagashima, Y.; Taguchi, T. Application of antioxidative maillard reaction products from histidine and glucose to sardine products. Nippon Suisan Gakkaishi 1988, 54, 1409–1414. [Google Scholar] [CrossRef]
- Oktay, M.; Culcin, I.; Kufrevioglu, O.I. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. Lebensm. Wiss. Technol. 2003, 36, 263–271. [Google Scholar] [CrossRef]
- Francisco, A.T.B.; Juan, C.E. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar]
- Ahmad, A.; Yoon, J.Y.; Chung, I.M. Chemical constituents from the straw of Oryza sativa. Asian J. Chem. 2013, 25, 9872–9874. [Google Scholar] [CrossRef]
- Hori, R. Studies on carbohydrate derivatives VI. Studies on the surface tension of their aqueous solution. Yakugaku Zasshi 1959, 79, 343–349. [Google Scholar] [CrossRef]
- Priyanka, B.; Vidhu, A.; Mohd, A. New fatty acid glycosides from the seeds of Cicer arietinum. J. Pharmacogn. Phytochem. 2017, 6, 1576–1584. [Google Scholar]
- Yamada, H.; Ra, K.S.; Kiyohara, H.; Cyong, J.C.; Yang, Y.C.; Otsuka, Y. Characterization of anti-complementary neutral polysaccharides from the roots of Bupleurum falcatum. Phytochemistry 1988, 27, 3163–3168. [Google Scholar] [CrossRef]
- Chung, I.M.; Kim, H.J.; Yhn, Y.S.; Ahmad, A. Glycerol derivatives of fatty acids from the fruits of Lycium chinense. Asian J. Chem. 2013, 25, 1083–1085. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on product of browning reaction prepared from glucose amine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Marcocci, L.; Maguire, J.J.; Droy-Lafix, M.T.; Packer, L. The nitric oxide scavenging property of Ginkgo biloba extracts EGb 761. Biochem. Biophys. Res. Commun. 1994, 201, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantification of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Ali, M.; Nagella, P.; Yu, B.R.; Kim, S.H.; Ahmad, A. New polyglucopyranosyl and polyarabinopyranosyl of fatty acid derivatives from the fruits of Lycium chinense and itsantioxidant activity. Food Chem. 2014, 151, 435–443. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–4 are not available from the authors because the samples exhausted in analysis and bioactivity. |
| Parameter | Compound | 10 µg | 25 µg | 50 µg | 100 µg |
|---|---|---|---|---|---|
| Nitric Oxide | C-1 | 17.62 | 18.33 | 38.55 | 40.38 |
| C-2 | 13.52 | 14.28 | 36.82 | 37.64 | |
| C-3 | 9.19 | 10.62 | 20.61 | 28.34 | |
| C-4 | 8.46 | 10.11 | 18.55 | 25.39 | |
| FRAP | C-1 | 22.36 | 25.34 | 78.42 | 79.68 |
| C-2 | 19.55 | 20.39 | 65.43 | 69.77 | |
| C-3 | 10.08 | 10.34 | 42.11 | 54.33 | |
| C-4 | 8.67 | 8.33 | 39.33 | 50.22 | |
| DPPH | C-1 | 11.97 | 13.24 | 65.73 | 69.84 |
| C-2 | 8.72 | 10.02 | 11.32 | 12.62 | |
| C-3 | 4.46 | 5.64 | 22.15 | 28.25 | |
| C-4 | 3.54 | 3.51 | 21.15 | 24.67 |
| Compounds | Antioxidant Capacity (%) as Equivalent to α-Tocopherol (mg/g) |
|---|---|
| C-1 | 15.31 |
| C-2 | 13.20 |
| C-3 | 6.70 |
| C-4 | 5.63 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, I.-M.; Kim, S.-H.; Kwon, C.; Kim, S.-Y.; Yang, Y.-J.; Kim, J.-S.; Ali, M.; Ahmad, A. New Chemical Constituents from the Bark of Dendropanax morbifera Leveille and Their Evaluation of Antioxidant Activities. Molecules 2019, 24, 3967. https://doi.org/10.3390/molecules24213967
Chung I-M, Kim S-H, Kwon C, Kim S-Y, Yang Y-J, Kim J-S, Ali M, Ahmad A. New Chemical Constituents from the Bark of Dendropanax morbifera Leveille and Their Evaluation of Antioxidant Activities. Molecules. 2019; 24(21):3967. https://doi.org/10.3390/molecules24213967
Chicago/Turabian StyleChung, Ill-Min, Seung-Hyun Kim, Chang Kwon, So-Yeon Kim, Yu-Jin Yang, Ju-Sung Kim, Mohd Ali, and Ateeque Ahmad. 2019. "New Chemical Constituents from the Bark of Dendropanax morbifera Leveille and Their Evaluation of Antioxidant Activities" Molecules 24, no. 21: 3967. https://doi.org/10.3390/molecules24213967
APA StyleChung, I.-M., Kim, S.-H., Kwon, C., Kim, S.-Y., Yang, Y.-J., Kim, J.-S., Ali, M., & Ahmad, A. (2019). New Chemical Constituents from the Bark of Dendropanax morbifera Leveille and Their Evaluation of Antioxidant Activities. Molecules, 24(21), 3967. https://doi.org/10.3390/molecules24213967







