Urea Derivative Catalyzed Enantioselective Hydroxyalkylation of Hydroxyindoles with Isatins
Abstract
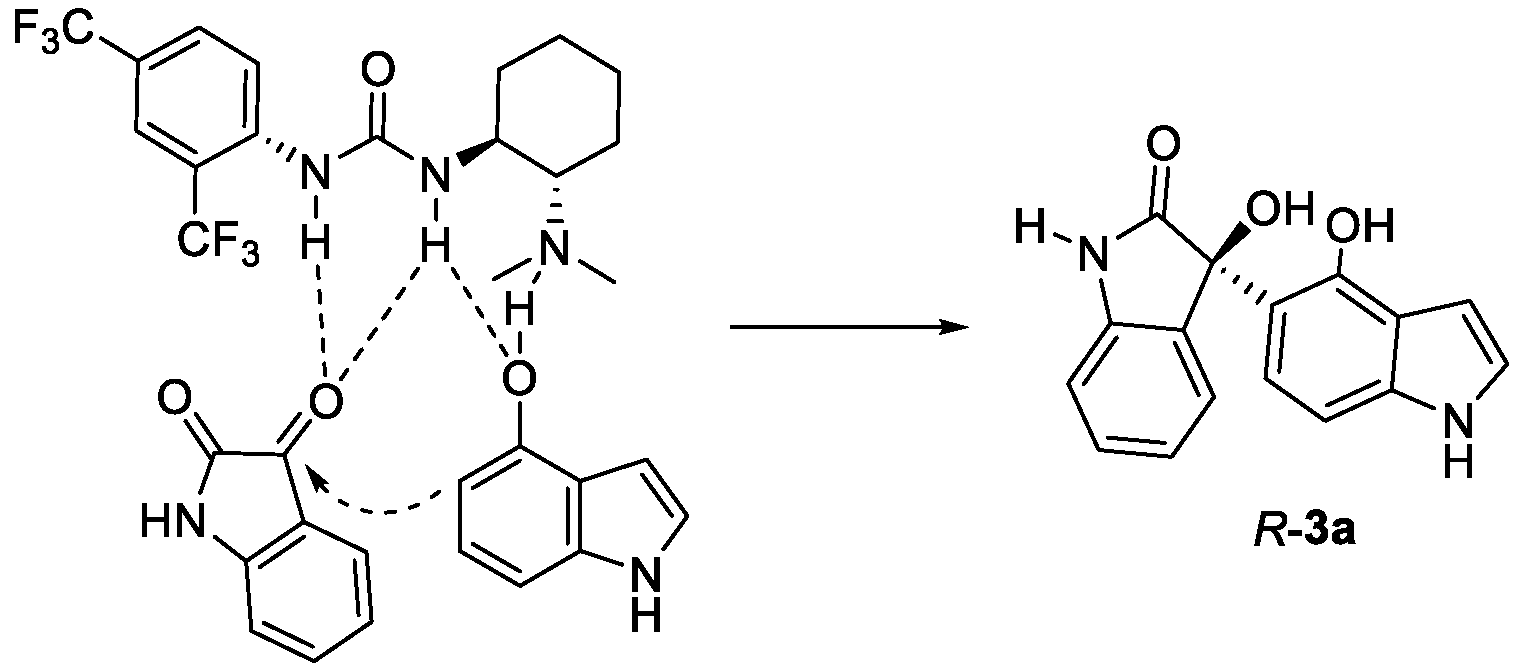
1. Introduction
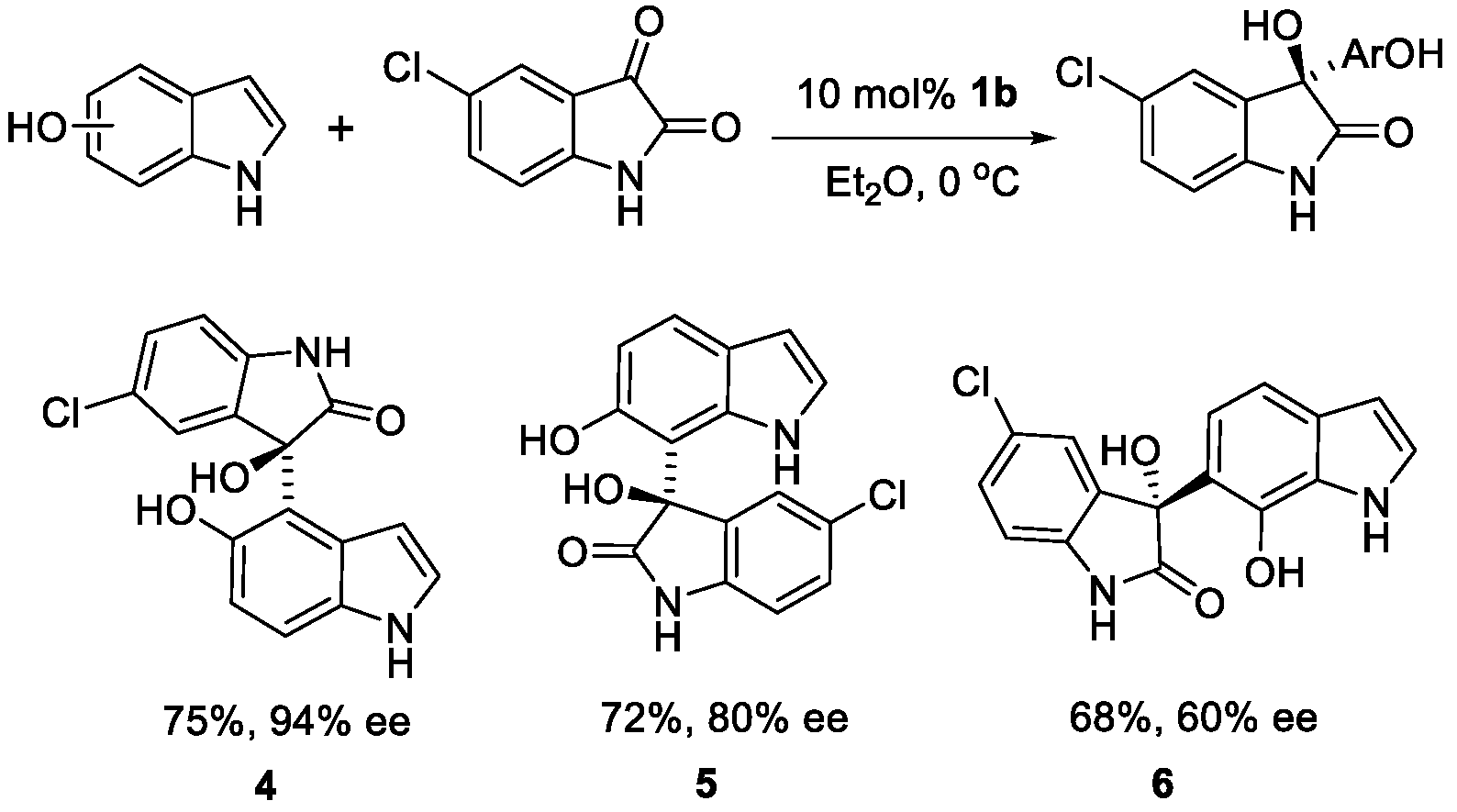
2. Results and Discussion
3. Conclusion
4. Experimental
4.1. Chemistry
4.2. General Procedure for the Enantioselective Friedel-Crafts Reaction of Hydroxyindole and Isatins
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Horton, D.A.; Bourne, G.T.; Smythe, M.L. The Combinatorial Synthesis of Bicyclic Privileged Structures or Privileged Substructures. Chem. Rev. 2003, 103, 893–930. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wu, D.; Snyder, B.; Ptak, R.G.; Kaur, H.; Gochin, M. Development of indole compounds as small molecule fusion inhibitors targeting HIV-1 glycoprotein-41. J. Med. Chem. 2011, 54, 7220–7231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Chen, Q.; Yang, G.-F. A review on recent developments of indole-containing antiviral agents. Eur. J. Med. Chem. 2015, 89, 421–441. [Google Scholar] [CrossRef] [PubMed]
- Sherer, C.; Snape, T. Heterocyclic scaffolds as promising anticancer agents against tumours of the central nervous system: Exploring the scope of indole and carbazole derivatives. Eur. J. Med. Chem. 2015, 97, 552–560. [Google Scholar] [CrossRef]
- Lancianesi, S.; Palmieri, A.; Petrini, M. Synthetic Approaches to 3-(2-Nitroalkyl) Indoles and Their Use to Access Tryptamines and Related Bioactive Compounds. Chem. Rev. 2014, 114, 7108–7149. [Google Scholar] [CrossRef]
- Ishikura, M.; Abe, T.; Choshi, T.; Hibino, S. Simple indole alkaloids and those with a nonrearranged monoterpenoid unit. Nat. Prod. Rep. 2015, 32, 1389–1471. [Google Scholar] [CrossRef]
- Bandini, M.; Umani-Ronchi, A. (Eds.) Catalytic Asymmetric Friedel–Crafts Alkylations||Industrial Friedel–Crafts. Chemistry 2009. [Google Scholar] [CrossRef]
- Jørgensen, K.A. Microwave-Assisted Rapid and Selective Synthesis of cis- and trans-2,4,5-Triarylimidazolines from Aromatic Aldehydes. Synlett 2003, 1117–1120. [Google Scholar] [CrossRef]
- Bandini, M.; Melloni, A.; Umani-Ronchi, A. New Catalytic Approaches in the Stereoselective Friedel–Crafts Alkylation Reaction. Angew. Chem. Int. Ed. 2004, 43, 550–556. [Google Scholar] [CrossRef]
- Poulsen, T.B.; Jørgensen, K.A. Catalytic Asymmetric Friedel-Crafts Alkylation Reactions—Copper Showed the Way. Chem. Rev. 2008, 108, 2903–2915. [Google Scholar] [CrossRef]
- Bandini, M.; Eichholzer, A. Catalytic Functionalization of Indoles in a New Dimension. Angew. Chem. Int. Ed. 2009, 48, 9608–9644. [Google Scholar] [CrossRef] [PubMed]
- You, S.L.; Cai, Q.; Zeng, M. Chiral Bronsted acid catalyzed Friedel-Crafts alkylation reactions. Chem. Soc. Rev. 2009, 38, 2190–2201. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, G.; Bencivenni, G.; Dalpozzo, R. Organocatalytic strategies for the asymmetric functionalization of indoles. Chem. Soc. Rev. 2010, 39, 4449–4465. [Google Scholar] [CrossRef] [PubMed]
- Dalpozzo, R. Strategies for the asymmetric functionalization of indoles: An update. Chem. Soc. Rev. 2015, 44, 742–778. [Google Scholar] [CrossRef]
- Wu, H.; He, Y.P.; Shi, F. Recent Advances in Chiral Phosphoric Acid Catalyzed Asymmetric Reactions for the Synthesis of Enantiopure Indole Derivatives. Synthesis 2015, 47, 1990–2016. [Google Scholar] [CrossRef]
- Cruz, F.A.; Zhu, Y.; Tercenio, Q.D.; Shen, Z.; Dong, Y.M. Alkyne Hydroheteroarylation: Enantioselective Coupling of Indoles and Alkynes via Rh-Hydride Catalysis. J. Am. Chem. Soc. 2017, 139, 10641–10644. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Wang, L.S.; Zhao, J.L. Chiral phosphoric acid catalyzed aza-Friedel-Crafts alkylation of indoles with cyclicaryl αketiminoesters. Tetrahedron Lett. 2017, 58, 213217. [Google Scholar] [CrossRef]
- Zhang, H.H.; Wang, C.S.; Li, C.; Mei, G.J.; Li, Y.; Shi, F. Design and Enantioselective Constructionof Axially Chiral Naphthyl-Indole Skeletons. Angew. Chem. Int. Ed. 2017, 56, 116–121. [Google Scholar] [CrossRef]
- Qi, L.W.; Mao, J.H.; Zhang, J.; Tan, B. Organocatalytic asymmetric arylation of indoles enabled by azo groups. Nat. Chem. 2018, 10, 58–64. [Google Scholar] [CrossRef]
- Nakamura, S.; Furukawa, T.; Hatanaka, T.; Funahashi, Y. Enantioselective aza-Friedel–Crafts reaction of cyclic ketimines with indoles using chiral imidazoline–phosphoric acid catalysts. Chem. Commun. 2018, 54, 3811–3814. [Google Scholar] [CrossRef]
- Vetica, F.; Chauhan, P.; Mahajan, S.; Raabe, G.; Enders, D. Asymmetric Organocatalytic Friedel–Crafts Hydroxyalkylation of Indoles Using Electrophilic Pyrazole-4,5-diones. Synthesis 2018, 50, 1039–1046. [Google Scholar] [CrossRef]
- Lee, J.; Ko, K.M.; Kim, S.G. Ni(ClO4)2− Catalyzed Friedel–Crafts Reaction of Coumarin-Fused Donor–Acceptor Cyclopropanes with Indoles: Stereoselective Synthesis of trans-3,4-Disubstituted-3,4-dihydrocoumarins. Eur. J. Org. Chem. 2018, 4166–4170. [Google Scholar] [CrossRef]
- Evans, D.A.; Fandrick, K.R. Catalytic Enantioselective Pyrrole Alkylations of α,β-Unsaturated 2-Acyl Imidazoles. Org. Lett. 2006, 8, 2249–2252. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.A.; Fandrick, K.R.; Song, H.J.; Scheidt, K.A.; Xu, R. Enantioselective Friedel–Crafts Alkylations Catalyzed by Bis(oxazolinyl)pyridine–Scandium(III) Triflate Complexes. J. Am. Chem. Soc. 2007, 129, 10029–10034. [Google Scholar] [CrossRef]
- Blay, G.; Fernández, I.; Pedro, J.R.; Vila, C. Catalytic enantioselective Friedel–Crafts alkylation at the 2-position of indole with simpleenones. Tetrahedron Lett. 2007, 48, 6731–6734. [Google Scholar] [CrossRef]
- Kang, Q.; Zheng, X.J.; You, S.L. Highly Enantioselective Friedel–Crafts Reaction of 4,7-Dihydroindoles with Imines by Chiral Phosphoric Acids: Facile Access to 2-Indolyl Methanamine Derivatives. Chem. Eur. J. 2008, 14, 3539–3542. [Google Scholar] [CrossRef]
- Hong, L.; Liu, C.; Sun, W.; Wang, L.; Wong, K.; Wang, R. Organocatalytic Enantioselective Friedel–Crafts Alkylation of 4,7-Dihydroindoles with α,β-Unsaturated Aldehydes: An Easy Access to 2-Substituted Indoles. Org. Lett. 2009, 11, 2177–2180. [Google Scholar] [CrossRef]
- Sheng, Y.F.; Li, G.Q.; Kang, Q.; Zhang, A.-J.; You, S.L. Asymmetric Friedel–Crafts Reaction of 4,7-Dihydroindoles with Nitroolefins by Chiral Brønsted Acids under Low Catalyst Loading. Chem. Eur. J. 2009, 15, 3351–3354. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, G.W.; Teng, Y.; Nie, J.; Zheng, Y.; Ma, J.A. Chiral Brønsted Acid-Catalyzed Enantioselective Friedel–Crafts Reaction of 4,7-Dihydroindoles with Trifluoromethyl Ketones. Adv. Synth. Catal. 2010, 352, 2773–2777. [Google Scholar] [CrossRef]
- Blay, G.; Fernández, I.; Muñoz, M.C.; Pedro, J.R.; Recuenco, A.; Vila, C. Enantioselective Synthesis of Tertiary Alcohols through a Zirconium-Catalyzed Friedel–Crafts Alkylation of Pyrroles with α-Ketoesters. J. Org. Chem. 2011, 76, 6286–6294. [Google Scholar] [CrossRef]
- Cui, H.L.; Feng, X.; Peng, J.; Lei, J.; Jiang, K.; Chen, Y.C. Chemoselective Asymmetric N-Allylic Alkylation of Indoles with Morita–Baylis–Hillman Carbonates. Angew. Chem. Int. Ed. 2009, 48, 5737–5740. [Google Scholar] [CrossRef] [PubMed]
- Stanley, L.M.; Hartwig, J.F. Iridium-Catalyzed Regio- and Enantioselective N-Allylation of Indoles. Angew. Chem. Int. Ed. 2009, 48, 7841–7844. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Osipov, M.; Dong, G. Palladium-Catalyzed Dyamic Kinetic Asymmetric Transformations of Vinyl Aziridines with Nitrogen Heterocycles: Rapid Access to Biologically Active Pyrroles and Indoles. J. Am. Chem. Soc. 2010, 132, 15800–15807. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhao, Y.; Qian, B.; Yang, L.; Xia, C.; Huang, H. Enantioselective N–H Functionalization of Indoles with α,β-Unsaturated γ-Lactams Catalyzed by Chiral Brønsted Acids. Angew. Chem. Int. Ed. 2011, 50, 5682–5686. [Google Scholar] [CrossRef]
- Cheng, H.G.; Lu, L.Q.; Wang, T.; Yang, Q.Q.; Liu, X.P.; Li, Y.; Deng, Q.H.; Chen, J.R.; Xiao, W.J. Highly Enantioselective Friedel–Crafts Alkylation/N-Hemiacetalization Cascade Reaction with Indoles. Angew. Chem. Int. Ed. 2013, 52, 3250–3254. [Google Scholar] [CrossRef]
- Sevov, C.S.; Zhou, J.; Hartwig, J.F. Iridium-Catalyzed Intermolecular Hydroamination of Unactivated Alkenes with Indoles. J. Am. Chem. Soc. 2014, 136, 3200–3207. [Google Scholar] [CrossRef]
- Bera, K.; Schneider, C. Brønsted Acid Catalyzed [3+2]-Cycloaddition of Cyclic Enamides with in Situ Generated 2-Methide-2H-indoles: Enantioselective Synthesis of Indolo[1,2-a]indoles. Org. Lett. 2016, 18, 5660–5663. [Google Scholar] [CrossRef]
- Shi, Z.; Yang, Y. Regioselective direct arylation of indoles on the benzenoid moiety. Chem. Commun. 2018, 54, 1676–1685. [Google Scholar] [CrossRef]
- Sandtorv, A.H. Catalyzed Transition Metal-Catalyzed C?H Activation of Indoles. Adv. Synth. Catal. 2015, 357, 2403–2435. [Google Scholar] [CrossRef]
- Lanke, V.; Prabhu, K.R. Regioselective Synthesis of 4-Substituted Indoles via C–H Activation: A Ruthenium Catalyzed Novel Directing Group Strategy. Org. Lett. 2013, 15, 6262–6265. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Q.; Ma, Y.; Jia, Y. Direct Olefination at the C-4 Position of Tryptophan via C–H Activation: Application to Biomimetic Synthesis of Clavicipitic Acid. Org. Lett. 2013, 15, 4528–4531. [Google Scholar] [CrossRef] [PubMed]
- Hartung, C.G.; Fecher, A.; Chapell, B.; Snieckus, V. Directed ortho Metalation Approach to C-7-Substituted Indoles. Suzuki–Miyaura Cross Coupling and the Synthesis of Pyrrolophenanthridone Alkaloids. Org. Lett. 2003, 5, 1899–1902. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Holte, D.; Zoller, J.; Umemiya, S.; Simke, L.R.; Baran, P.S. Total Synthesis of Verruculogen and Fumitremorgin A Enabled by Ligand-Controlled C–H Borylation. J. Am. Chem. Soc. 2015, 137, 10160–10163. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qiu, X.; Zhao, Y.; Mu, Y.; Shi, Z. Palladium-Catalyzed C–H Arylation of Indoles at the C7 Position. J. Am. Chem. Soc. 2016, 138, 495–498. [Google Scholar] [CrossRef]
- Vila, C.; Amr, F.I.; Blay, G.; Muňoz, M.C.; Pedro, J.R. Organocatalytic Enantioselective Synthesis of Pyrazoles Bearing a Quaternary Stereocenter. Chem. Asian J. 2016, 11, 1532–1536. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, S.B.; Zheng, C.; You, S.L. Asymmetric Synthesis of Spiropyrazolones by Rhodium-Catalyzed C (sp2)–H Functionalization/Annulation Reactions. Angew. Chem. Int. Ed. 2017, 56, 4540–4544. [Google Scholar] [CrossRef]
- Xu, Q.L.; Dai, L.X.; You, S.L. Diversity oriented synthesis of indole-based peri-annulated compounds via allylic alkylation reactions. Chem. Sci. 2013, 4, 97–102. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, C.; You, S.L. Catalytic C6 Functionalization of 2,3-Disubstituted Indoles by Scandium Triflate. J. Org. Chem. 2014, 79, 1047–1054. [Google Scholar] [CrossRef]
- Zhou, L.J.; Zhang, Y.C.; Zhao, J.J.; Shi, F.; Tu, S.J. Organocatalytic Arylation of 3-Indolylmethanols via Chemo- and Regiospecific C6-Functionalization of Indoles. J. Org. Chem. 2014, 79, 10390–10398. [Google Scholar] [CrossRef]
- Poulsen, P.H.; Feu, K.S.; Paz, B.M.; Jensen, F.; Jørgensen, K.A. Organocatalytic Asymmetric 1,6-Addition/1,4-Addition Sequence to 2,4-Dienals for the Synthesis of Chiral Chromans. Angew. Chem. Int. Ed. 2015, 54, 8203–8207. [Google Scholar] [CrossRef]
- Montesinos-Magraner, M.; Vila, C.; Rendón-Patiño, A.; Blay, G.; Fernández, I.; Muñoz, M.C.; Pedro, J.R. Organocatalytic Enantioselective Friedel–Crafts Aminoalkylation of Indoles in the Carbocyclic Ring. ACS Catal. 2016, 6, 2689–2693. [Google Scholar] [CrossRef]
- Montesinos-Magraner, M.; Vila, C.; Blay, G.; Fernández, I.; Muñoz, M.C.; Pedro, J.R. Hydroxy-Directed Enantioselective Hydroxyalkylation in the Carbocyclic Ring of Indoles. Org. Lett. 2017, 19, 1546–1549. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Xu, D.; Liang, W.; Wu., W.; Chan, A.S.C.; Zhao, J. Organocatalytic Enantioselective Friedel-Crafts Alkylation/Lactonization Reaction of Hydroxyindoles with Methylene Oxindoles. Adv. Synth. Catal. 2018, 360, 917–924. [Google Scholar] [CrossRef]
- Yang, Z.T.; Yang, W.L.; Chen, L.; Sun, H.; Deng, W.P. Organocatalytic Enantioselective aza-Friedel-Crafts Reactions of Pyrazolinone Ketimines with Hydroxyindoles and Electron-Rich Phenols. Adv. Synth. Catal. 2018, 360, 2049–2205. [Google Scholar] [CrossRef]
- Clark, M.P.; Laughlin, S.K.; Laufersweiler, M.J.; Bookland, R.G.; Brugel, T.A.; Golebiowski, A.; Sabat, M.P.; Townes, J.A.; VanRens, J.C.; Djung, J.F.; et al. Development of Orally Bioavailable Bicyclic Pyrazolones as Inhibitors of Tumor Necrosis Factor-α Production. J. Med. Chem. 2004, 47, 2724–2727. [Google Scholar] [CrossRef] [PubMed]
- Hadi, V.; Koh, Y.H.; Sanchez, T.W.; Barrios, D.; Neamati, N.; Jung, K.W. Development of the next generation of HIV-1 integrase inhibitors: Pyrazolone as a novel inhibitor scaffold. Bioorg. Med. Chem. Lett. 2010, 20, 6854–6857. [Google Scholar] [CrossRef] [PubMed]
- Wright, Z.V.; Wu, N.C.; Kadam, R.U.; Wilson, I.A.; Wolan, D.W. Structure-based optimization and synthesis of antiviral drug Arbidol analogues with significantly improved affinity to influenza hemagglutinin. Bioorg. Med. Chem. Lett. 2017, 27, 3744–3748. [Google Scholar] [CrossRef]
- Okino, T.; Hoashi, Y.; Takemoto, Y. Enantioselective Michael Reaction of Malonates to Nitroolefins Catalyzed by Bifunctional Organocatalysts. J. Am. Chem. Soc. 2003, 125, 12672–12673. [Google Scholar] [CrossRef]
- Okino, T.; Nakamura, S.; Furukawa, T.; Takemoto, Y. Enantioselective aza-Henry Reaction Catalyzed by a Bifunctional Organocatalyst. Org. Lett. 2004, 6, 625–627. [Google Scholar] [CrossRef]
- Hoashi, Y.; Okino, T.; Takemoto, Y. Enantioselective Michael Addition to α, β-unsaturated Imides Catalyzed by a Bifunctional Organocatalyst. Angew. Chem. Int. Ed. Engl. 2005, 44, 4032–4035. [Google Scholar] [CrossRef]
- Zea, A.; Valero, G.; Alba, A.R.; Moyano, A.; Rios, R. Development of Diphenylamine-linked Bis(imidazoline) Ligands and Their Application in Asymmetric Friedel-Crafts Alkylation of Indole Derivatives with Nitroalkenes. Adv. Synth. Catal. 2010, 352, 1102–1106. [Google Scholar] [CrossRef]
- Raimondi, W.; Baslé, O.; Constantieux, T.; Bonne, D.; Rodriguez, J. Activation of 1, 2-Ketoesters with Takemoto’s Catalyst toward Michael Addition to Nitroalkenes. Adv. Synth. Catal. 2012, 354, 563–568. [Google Scholar] [CrossRef]
- Ansari, S.; Raabe, G.; Enders, D. Asymmetric Michael Addition of 1, 3-Bis(phenylthio)propan-2-one to Nitroalkenes Employing Takemoto’s Thiourea Catalyst. Monatsh. Chem. 2013, 144, 641–646. [Google Scholar] [CrossRef]
- Wang, Y.; Mo, M.; Zhu, K.; Zheng, C.; Zhang, H.; Wang, W.; Shao, Z. Asymmetric Synthesis of Syn-propargylamines and Unsaturated β-amino Acids under Brønsted Base Catalysis. Nat. Commun. 2015, 6, 8544–8582. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, H.-F.; Zhao, J.-Z.; Du, Z.-H.; Da, C.-S. Organocatalytic Enantioselective Cross-aldol Reaction of o-Hydroxyarylketones and Trifluoromethyl Ketones. Org. Lett. 2017, 19, 2634–2637. [Google Scholar] [CrossRef]

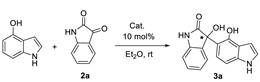
| Entry | Catalyst a | Yield (%) b | %ee c | Configuration d |
|---|---|---|---|---|
| 1 | 1a | 70 | 68 | R |
| 2 | 1b | 88 | 79 | R |
| 3 | 1c | 75 | 72 | S |
| 4 | 1d | 85 | 76 | S |
| 5 | 1e | - | - | - |
| 6 | 1f | 72 | 55 | S |
| 7 | 1g | 65 | 35 | S |
| 8 | 1h | 66 | 46 | S |
| Entry. | Solvent | Temperature | Catalyst. Amount (% mmol) | Yield (%) b | %ee c |
|---|---|---|---|---|---|
| 1 | Et2O | rt | 10 | 88 | 79 |
| 2 | DCM | rt | 10 | 69 | 32 |
| 3 | toluene | rt | 10 | 70 | 28 |
| 4 | THF | rt | 10 | 76 | 69 |
| 5 d | Et2O | rt | 10 | 79 | 74 |
| 6 | Et2O | rt | 5 | 79 | 77 |
| 7 | Et2O | rt | 20 | 91 | 76 |
| 8 | Et2O | 0 °C | 10 | 85 | 83 |
| 9 | Et2O | 0 °C | 20 | 83 | 80 |
| 10 | Et2O | −20 °C | 10 | 72 | 68 |
| 11 | Et2O | −40 °C | 10 | 68 | 73 |
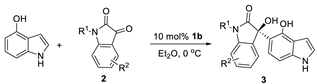
| Entry | R1, R2 | Product | Yield (%) b | %ee c |
|---|---|---|---|---|
| 1 | R1 = H, R2 = H, 2a | 3a | 85(70) d | 83(85) d |
| 2 | R1 = H, R2 = 4-Br, 2b | 3b | 65 | 50 |
| 3 | R1 = H, R2 = 5-Me, 2c | 3c | 85 | 85 |
| 4 | R1 = H, R2 = 5-OMe, 2d | 3d | 78 | 82 |
| 5 | R1 = H, R2 = 5-Cl, 2e | 3e | 92 | 94 |
| 6 | R1 = H, R2 = 5-Br, 2f | 3f | 80(69) d | 90(84) d |
| 7 | R1 = H, R2 = 6-Br, 2g | 3g | 83 | 71 |
| 8 | R1 = H, R2 = 7-F, 2h | 3h | 90 | 92 |
| 9 | R1 = H, R2 = 7-Cl, 2i | 3i | 81 | 84 |
| 10 | R1 = Me, R2 = H, 2j | 3j | 82(77) d | 78(80) d |
| 11 | R1 = Bn, R2 = H, 2k | 3k | 87(91) d | 74(90) d |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Wang, L.; Zhang, J.; Jin, Y. Urea Derivative Catalyzed Enantioselective Hydroxyalkylation of Hydroxyindoles with Isatins. Molecules 2019, 24, 3944. https://doi.org/10.3390/molecules24213944
Wu H, Wang L, Zhang J, Jin Y. Urea Derivative Catalyzed Enantioselective Hydroxyalkylation of Hydroxyindoles with Isatins. Molecules. 2019; 24(21):3944. https://doi.org/10.3390/molecules24213944
Chicago/Turabian StyleWu, Hao, Liming Wang, Junwei Zhang, and Ying Jin. 2019. "Urea Derivative Catalyzed Enantioselective Hydroxyalkylation of Hydroxyindoles with Isatins" Molecules 24, no. 21: 3944. https://doi.org/10.3390/molecules24213944
APA StyleWu, H., Wang, L., Zhang, J., & Jin, Y. (2019). Urea Derivative Catalyzed Enantioselective Hydroxyalkylation of Hydroxyindoles with Isatins. Molecules, 24(21), 3944. https://doi.org/10.3390/molecules24213944





