Synthetic, Structural, and Anticancer Activity Evaluation Studies on Novel Pyrazolylnucleosides
Abstract
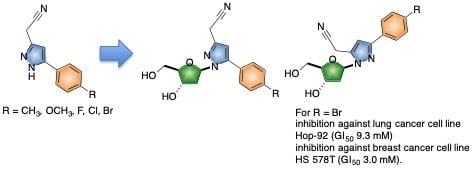
:1. Introduction
2. Results and Discussion
2.1. Chemical Synthesis of the Nucleoside Analogues 5 and 6
2.2. Structural Identification of the Isomeric Pyrazolyl Nucleoside Analogues 3–6
2.3. Anticancer Activity of the Isomeric Pyrazolyl Nucleoside Analogues 5a–e and 6a–e
3. Experimental
3.1. General Information
3.2. Synthesis and Characterization
3.2.1. Synthesis of 2′-Deoxy-1′-(3-cyanomethyl-5-aryl)pyrazolyl-3′,5′-di-O-toluoyl-β-d-ribofuranose (3a–e) and 2′-deoxy-1′-(3-aryl-5-cyanomethyl)pyrazolyl-3′,5′-di-O-toluoyl-β-d-ribofuranose (4a–e)
3.2.2. Synthesis of 2′-Deoxy-1′-(3-cyanomethyl-5-aryl)pyrazolyl-β-D-ribofuranose (5a–e) and 2′-deoxy-1′-(3-aryl-5-cyanomethyl)pyrazolyl-β-d-ribofuranose (6a–e)
3.3. NCI-60 Human Tumor Cell Line Screen
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yates, M.K.; Seley-Radtke, K.L. The evolution of antiviral nucleoside analogues: A review for chemists and non-chemists. Part II: Complex modifications to the nucleoside scaffold. Antivir. Res. 2019, 162, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Bhattarai, D.; Veeraswamy, G.; Choi, Y.; Lee, K. Nucleosides with modified sugar ring: Synthesis and biological activities. Curr. Org. Chem. 2016, 20, 856–897. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, H.; Tao, S.; Ehteshami, M.; Cho, J.H.; McBrayer, T.R.; Tharnish, P.; Whitaker, T.; Amblard, F.; Coats, S.J.; et al. Synthesis and evaluation of 2,6-modified purine 2′-C-methyl ribonucleosides as inhibitors of HCV replication. ACS Med. Chem. Lett. 2016, 7, 17–22. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Milestones in the discovery of antiviral agents: Nucleosides and nucleotides. Acta Pharm. Sin. B. 2012, 2, 535–548. [Google Scholar] [CrossRef]
- Zhou, C.; Chattopadhyaya, J. The synthesis of therapeutic locked nucleos(t)ides. Curr. Opin. Drug. Discov. Devel. 2009, 12, 876–898. [Google Scholar] [CrossRef]
- Wan, J.; Xia, Y.; Liu, Y.; Wang, M.; Rocchi, P.; Yao, J.; Qu, F.; Neyts, J.; Iovanna, J.L.; Peng, L. Discovery of novel arylethynyltriazole ribonucleosides with selective and effective antiviral and antiproloferative activity. J. Med. Chem. 2009, 52, 1144–1155. [Google Scholar] [CrossRef]
- Chu, C.K.; Baker, D.C. (Eds.) Nucleosides and Nucleotides as Antitumor and Antiviral Agents; Plenum Press: New York, NY, USA, 1993; pp. 245–264. [Google Scholar]
- Prakash, T.P. An overview of sugar-modified oligonucleosides for antisense therapeutics. Chem. Biodivers. 2011, 8, 1616–1641. [Google Scholar] [CrossRef]
- Yu, R.Z.; Grundy, J.S.; Geary, R.S. Clinical pharmacokinetics of second generation antisense oligonucleotides. Expert Opin. Drug Metab. Toxicol. 2013, 9, 169–182. [Google Scholar] [CrossRef]
- Watts, J.K. Locked nucleic acid: Tighter is different. Chem. Commun. 2013, 49, 5618–5620. [Google Scholar] [CrossRef]
- Mitsuya, H.; Broder, S. Inhibition of the in vitro infectivity and cytopathic effect of human T-lymphotrophic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV) by 2’,3’-dideoxynucleosides. Proc. Natl. Acad. Sci. USA 1986, 83, 1911–1915. [Google Scholar] [CrossRef]
- Len, C.; Postel, D. Synthesis of 2’,3’-didehydro-2’,3’-dideoxynucleosides via nucleoside route. Curr. Org. Synth. 2006, 3, 261–281. [Google Scholar] [CrossRef]
- Len, C.; Mackenzie, G. Synthesis of 2’,3’-didehydro-2’,3’-dideoxynucleosides having variations at either or both of the 2’- and 3’-positions. Tetrahedron 2006, 62, 9085–9107. [Google Scholar] [CrossRef]
- Len, C.; Mondon, M.; Lebreton, J. Synthesis of cyclonucleosides having a C-C bridge. Tetrahedron 2008, 64, 7453–7475. [Google Scholar] [CrossRef]
- Wengel, J. Synthesis of 3’-C- and 4’-C-branched oligodeoxynucleotides and the development of locked nucleic acid (LNA). Acc. Chem. Res. 1999, 32, 301–310. [Google Scholar] [CrossRef]
- Petersen, M.; Wengel, J. LNA: A versatile tool for therapeutics and genomics. Trends Biotechnol. 2003, 21, 74–81. [Google Scholar] [CrossRef]
- Kalra, N.; Babu, B.R.; Parmar, V.S.; Wengel, J. Conformationally controlled high-affinity targeting of RNA or DNA by novel 2’-amino-DNA/LNA mixmers and pyrenyl-functionalized 2’-amino-DNA. Org. Biomol. Chem. 2004, 2, 2885–2887. [Google Scholar] [CrossRef]
- Babu, B.R.; Hrdlicka, P.J.; McKenzie, C.J.; Wengel, J. Optimizing DNA targeting using N,N-bis(2-pyridylmethyl)-|?-alanyl 2’-amino-LNA. Chem. Commun. 2005, 1705–1707. [Google Scholar] [CrossRef]
- Hrdlicka, P.J.; Jepsen, J.S.; Nielsen, C.; Wengel, J. Synthesis and biological evaluation of nucleobase-modified analogs of the anticancer compounds 3’-C-ethynyluridine (EUrd) and 3’-C-ethynylcytidine (ECyd). Bioorg. Med. Chem. 2005, 13, 1249–1260. [Google Scholar] [CrossRef]
- Prakash, T.P.; Prhavc, M.; Eldrup, A.B.; Cook, P.D.; Carroll, S.S.; Olsen, D.B.; Stahlhut, M.W.; Tomassini, J.E.; MacCoss, M.; Galloway, S.M.; et al. Synthesis and evaluation of S-acyl-2-thioethyl esters of modified nucleoside 5’-monophosphates as inhibitors of hepatitis C virus RNA replication. J. Med. Chem. 2005, 48, 1199–1210. [Google Scholar] [CrossRef]
- Sharma, V.K.; Watts, J.K. Oligonucleotide therapeutics: Chemistry, delivery and clinical progress. Future Med. Chem. 2015, 7, 2221–2242. [Google Scholar] [CrossRef]
- Reid, G.; Wielinga, P.; Zelcer, N.; de Haas, M.; van Deemter, L.; Wijnholds, J.; Balzarini, J.; Borst, P. Characterization of the transport of nucleoside analog drugs by the human multidrug resistance proteins MRP4 and MRP5. Mol. Pharmacol. 2003, 63, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Parmar, V.S.; Jain, S.C.; Jha, A.; Kumar, N.; Kumar, A.; Vats, A.; Jha, H.N.; Mukherjee, S.; Singh, S.K.; Jennings, K.R.; et al. Synthetic and mass spectral fragmentation studies on trisubstituted 2H-pyran-2-ones and comparative EIMS behavior of biologically active 3,5-disubstituted pyrazoles and isoxazoles. Indian J. Chem. 1997, 36B, 872–879. [Google Scholar] [CrossRef]
- Sharma, V.K.; Sharma, R.K.; Singh, P.K.; Singh, S.K. An engrossing history of azidothymidine. Immun. Endoc. Metab. Agents Med. Chem. 2015, 15, 168–175. [Google Scholar] [CrossRef]
- Sidwell, R.W.; Huffman, J.H.; Khare, G.P.; Allen, L.B.; Witkowski, J.T.; Robins, R.K. Broad-spectrum antiviral activity of virazole: 1-β-D-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science 1972, 177, 705–706. [Google Scholar] [CrossRef] [PubMed]
- Storer, R.; Ashton, C.J.; Baxter, A.D.; Hann, M.M.; Marr, C.L.P.; Mason, A.M.; Mo, C.L.; Myers, P.L.; Noble, S.A.; Penn, C.R.; et al. The synthesis and antiviral activity of 4-fluoro-1-β-D-ribofuranosyl-1H-pyrazole-3-carboxamide. Nucleosides Nucleotides 1999, 18, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Balzarini, J.; Kang, G.J.; Dalal, M.; Herdewijn, P.; De Clercq, E.; Broder, S.; Johns, D.G. The anti-HTLV-III (anti-HIV) and cytotoxic activity of 2’,3’-didehydro-2’,3’-dideoxynucleosides: A comparison with their parental 2’,3’-dideoxyribonucleosides. Mol. Pharmacol. 1987, 32, 162–167. [Google Scholar]
- Hamamoto, Y.; Nakashima, H.; Matsui, T.; Mateuda, A.; Ueda, T.; Yamamoto, N. Inhibitory effect of 2’,3’-didehydro-2’,3’-dideoxynucleosides on infectivity, cytopathic effects, and replication of human immunodeficiency vírus. Antimicrob. Agents Chemother. 1987, 31, 907–910. [Google Scholar] [CrossRef]
- Lin, T.S.; Guo, J.Y.; Schinazi, R.F.; Chu, C.K.; Xiang, J.N.; Prusoff, W.H. Synthesis and antiviral activity of various 3’azido analogues of pyrimidine deoxyribonucleosides against Human Immunodeficiency Virus (HIV-1, HTLV-III/LAV). J. Med. Chem. 1988, 31, 336–340. [Google Scholar] [CrossRef]
- Haneishi, T.; Okazaki, T.; Hata, T.; Tamura, C.; Nomura, M.; Naito, A.; Seki, I.; Arai, M. Oxazinomycin, a new carbon-linked nucleoside antibiotic. J. Antibiot. 1971, 24, 797–799. [Google Scholar] [CrossRef]
- Sweeney, M.J.; Davis, F.A.; Gutowski, G.E.; Hamill, R.L.; Hoffman, D.H.; Poore, G.A. Experimental antitumor activity of pyrazomycin. Cancer Res. 1973, 33, 2619–2623. [Google Scholar]
- Harusawa, S.; Matsuda, C.; Araki, L.; Kurihara, T. Efficient and β-steroselective synthesis of pyrazole C-nucleosides. Synthesis 2006, 793–798. [Google Scholar] [CrossRef]
- Nishimura, H.; Mayama, M.; Komatsu, Y.; Kato, H.; Shimaoka, N.; Tanaka, Y. Showdomycin, a new antibiotic from a Streptomyces Sp. J. Antibiot. Ser. A. 1964, 17, 148–155. [Google Scholar]
- Michalik, D.; Peseke, K. Syntheses of pyrazole iso-C-nucleosides. J. Carbohydr. Chem. 2000, 19, 1049–1057. [Google Scholar] [CrossRef]
- Hori, M.; Ito, E.; Takita, T.; Koyama, G.; Takeuchi, T.; Umezawa, H. A new antibiotics, Formycin. J. Antibiot. Ser. A. 1964, 17, 96–99. [Google Scholar]
- Zhou, J.; Yang, M.; Akdag, A.; Wang, H.; Schneller, S.W. Carbocyclic 4’-epi-formycin. Tetrahedron 2008, 64, 433–438. [Google Scholar] [CrossRef]
- Moriyama, K.; Suzuki, T.; Negishi, K.; Graci, J.D.; Thompson, C.N.; Cameron, C.E.; Watanabe, M. Effects of introduction of hydrophobic group on ribavirin base on mutation induction and anti-RNA viral activity. J. Med. Chem. 2008, 51, 159–166. [Google Scholar] [CrossRef]
- Manfredini, S.; Baraldi, P.G.; Bazzanini, R.; Durini, E.; Vertuani, S.; Pani, A.; Marceddu, T.; Demontis, F.; Vargiu, L.; La Calla, P. Pyrazole related nucleosides 5. Synthesis and biological activity of 2’-deoxy-2’,3’-dideoxy- and acyclo-analogues of 4-iodo-1-β-D-ribofuranosyl-3-carboxymethyl pyrazole (IPCAR). Nucleosides Nucleotides Nucleic Acids 2000, 19, 705–722. [Google Scholar] [CrossRef]
- Rauter, A.P.; Figueiredo, J.A.; Ismael, M.I.; Justino, J. Synthesis of new pseudo-C-nucleosides containing pyrazole rings in their structure. J. Carbohydr. Chem. 2004, 23, 513–528. [Google Scholar] [CrossRef]
- Zhang, J.; Visser, F.; King, K.M.; Baldwin, S.A.; Young, J.D.; Cass, C.E. The role of nucleoside transporters in cancer chemotherapy with nucleoside drugs. Cancer Metastasis Rev. 2007, 26, 85–110. [Google Scholar] [CrossRef]
- Podgorska, M.; Kocbuch, K.; Pawelczyk, T. Recent advances in studies on biochemical and structural properties of equilibrative and concentrative nucleoside transporter. Acta Biochim. Pol. 2005, 52, 749–758. [Google Scholar]
- Uhlmann, E.; Peyman, A. Antisense oligonucleotides: A new therapeutic principle. Chem. Rev. 1990, 90, 544–584. [Google Scholar] [CrossRef]
- Dhimitruka, I.; Santa Lucia, J., Jr. Efficient preparation of 2-deoxy-3,5-di-O-p-toluoyl-α-D-ribofuranosyl chloride. Synlett 2004, 335–337. [Google Scholar] [CrossRef]
- Chenon, M.T.; Coupry, C.; Grant, D.M.; Pugmire, R.J. Carbon-13 magnetic resonance study of solvent stabilized tautomerism in pyrazoles. J. Org. Chem. 1977, 42, 659–661. [Google Scholar] [CrossRef]
- Bergstrom, D.E.; Zhang, P.; Johnson, W.T. Design and synthesis of heterocyclic carboxamides as natural nucleic acid base mimics. Nucleosides Nucleotides 1996, 15, 59–68. [Google Scholar] [CrossRef]
- Schneller, S.W. Carbocyclic nucleosides (Carbanucleosides) as new therapeutic leads. Curr. Top. Med. Chem. 2002, 2, 1087–1092. [Google Scholar] [CrossRef]
- Larsen, J.S.; Zahran, M.A.; Pedersen, E.B.; Nielsen, C. Synthesis of triazenopyrazole derivatives as potential inhibitors of HIV-1. Monatsh. Chem. 1999, 130, 1167–1173. [Google Scholar] [CrossRef]
- Tominaga, Y.; Ushirogochi, A.; Matsuda, Y.; Koboyashi, G. Synthesis and reactions of 6-aryl- and 6-styryl-3-cyano-4-methylthio-2H-pyran-2-ones. Chem. Pharm. Bull. 1984, 32, 3384–3395. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the corresponding author, V.S.P. |



| Compound | C-1′ H Shift in the 1H NMR Spectrum (ppm) | Ar- Bearing C Shifts in the 13C NMR Spectrum (ppm) | –CH2CN Bearing C Shifts in the 13C NMR Spectrum (ppm) | |||
|---|---|---|---|---|---|---|
| Series 3 | Series 5 | Series 3 | Series 5 | Series 3 | Series 5 | |
| a | 6.13 | 5.99 | 144.6 | 145.9 | 141.7 | 142.0 |
| b | 6.10 | 6.02 | 146.2 | 145.8 | 144.0 | 141.8 |
| c | 6.05 | 5.97 | 145.4 | 144.9 | 141.9 | 141.9 |
| d | 6.05 | 6.00 | 145.2 | 144.7 | 143.7 | 141.9 |
| e | 6.05 | 6.00 | 145.6 | 143.9 | 142.3 | 141.1 |
| Series 4 | Series 6 | Series 4 | Series 6 | Series 4 | Series 6 | |
| a | 6.17 | 6.13 | 151.0 | 150.2 | 137.9 | 137.7 |
| b | 6.16 | 6.19 | 150.8 | 150.7 | 133.0 | 133.7 |
| c | 6.17 | 6.16 | 152.5 | 149.8 | 135.8 | 135.8 |
| d | 6.19 | 6.17 | 144.1 | 149.6 | 134.3 | 134.6 |
| e | 6.17 | 6.18 | 149.8 | 149.2 | 133.5 | 134.9 |
| Proton | Chemical Shifts (δ) | Coupling Constants (Hz) | ||||||
|---|---|---|---|---|---|---|---|---|
| 3d | 4d | 5d | 6d | 3d | 4d | 5d | 6d | |
| H1′ | 6.29 | 6.48 | 6.09 | 6.29 | dd 5.7, 6.5 | dd 6.5, 5.2 | t* 6.4 | t* 6.2 |
| H2′α | 2.72 | 2.79 | 2.31 | 2.42 | ddd 14.0, 6.7, 4.1 | ddd 14.0, 4.5, 6.6 | ddd 13.5, 3.7, 6.8 | ddd 13.5, 3.7, 6.7 |
| H2′β | 3.54 | 3.63 | 3.01 | 3.00 | ddd 14.0, 6.8, 5.6 | ddd 14.0, 5.2, 6.7 | dt* 13.5, 6.0 | dt* 13.5, 5.9 |
| H3′ | 5.93 | 5.97 | 4.66 | 4.70 | m | m | m | m |
| H4′ | 4.62 | 4.65 | 4.02 | 4.07 | m | m | m | m |
| H5′α | 4.54 | 4.45 | 3.61 | 3.59 | m | dd 13.2, 6.4 | ddd 11.8, 4.8, 7.7 | ddd 11.9, 4.1, 8.1 |
| H5′β | 4.63 | 4.63 | 3.70 | 3.71 | m | m | dt* 11.9, 4.7 | dt* 11.9,4.1 |
| H4 | 6.50 | 6.82 | 6.46 | 6.83 | brs, 1H | t* 0.8 | t* 0.5 | t 0.8 |
| H6 | 3.90 | 4.29 | 3.96 | 4.31 | brs, 2H | 19.0, 0.8 | d 0.5 | 18.9, 0.8 |
| H2″ | 7.67 | 7.87 | 7.60 | 7.87 | ||||
| H3″ | 7.57 | 7.46 | ||||||
| Chemical Shifts (δ) (Carbon Position) | |||
|---|---|---|---|
| 3d | 5d | 4d | 6d |
| 87.6 (C1′) | 87.4 (C1′) | 87.6 (C1′) | 88.0 (C1′) |
| 37.1 (C2′) | 40.5 (C2′) | 37.0 (C2′) | 41.1 (C2′) |
| 76.6 (C3′) | 73.2 (C3′) | 76.1 (C3′) | 73.0 (C3′) |
| 83.5 (C4′) | 89.8 (C4′) | 83.7 (C4′) | 90.0 (C4′) |
| 65.1 (C5′) | 64.2 (C5′) | 64.7 (C5′) | 63.9 (C5′) |
| 143.8 (C3) | 143.6 (C3) | 136.3 (C3) | 136.1 (C3) |
| 107.2 (C4) | 106.7 (C4) | 105.4 (C4) | 104.9 (C4) |
| 146.0 (C5) | 145.8 (C5) | 150.6 (C5) | 150.5 (C5) |
| 17.6 (C6) | 17.6 (C6) | 15.3 (C6) | 15.2 (C6) |
| 118.1 (CN) | 118.0 (CN) | 117.0 (CN) | 117.1 (CN) |
| 166.4 (C6′′′) | 166.5 (C6′′′) | ||
| 166.5 (C6′′′′) | 166.5 (C6′′′′) | ||
| * | 129.3 (C1′′) | # | 134.2 (C1′′) |
| * | 131.6 (C2′′) | # | 127.9 (C2′′) |
| * | 129.9 (C3′′) | # | 129.7 (C3′′) |
| * | 135.6 (C4′′) | # | 132.6 (C4′′) |
 Blue and red arrows indicate the NOESY and HMBC cross peaks, respectively. | ||||
| 3d | 4d | 5d | 6d | |
| NOESY H1′-H6 | o | x | o | x |
| NOESY H1′-H2′′ | x | o | x | o |
| NOESY H4-H2′′ | x | x | x | x |
| HMBC H1′-C5 | x | o | x | o |
| HMBC H1′-C3 | o | x | o | x |
| Panels/Cell Lines | 6d | 6e | Dasatinib | |||
|---|---|---|---|---|---|---|
| GI50 | LC50 | GI50 | LC50 | GI50 | LC50 | |
| Leukemia | ||||||
| CCRF-CEM | >67.5 | >67.5 | 46.3 | >57.5 | 5.3 | >100 |
| K-562 | >67.5 | >67.5 | 40.0 | >57.5 | 0.01 | >100 |
| MOLT-4 | >67.5 | >67.5 | 30.9 | >57.5 | 4.1 | >100 |
| RPMI-8226 | >67.5 | >67.5 | 34.0 | >57.5 | 4.8 | 99.5 |
| SR | >67.5 | >67.5 | 25.5 | >57.5 | 3.1 | 89.3 |
| Non-Small Cell Lung Cancer | ||||||
| A549/ATCC | 44.8 | >67.5 | 29.3 | >57.5 | 0.05 | 75.5 |
| HOP-92 | >67.5 | >67.5 | 9.3 | >57.5 | 0.01 | >100 |
| NCI-H322M | 29.8 | >67.5 | 30.2 | >57.5 | 0.04 | 27.0 |
| NCI-H522 | >67.5 | >67.5 | 25.7 | >57.5 | 0.06 | 55.1 |
| Colon Cancer | ||||||
| HCT-116 | >67.5 | >67.5 | 47.7 | >57.5 | 11.8 | 69.8 |
| HCT-15 | >67.5 | >67.5 | 45.1 | >57.5 | 0.6 | 71.1 |
| CNS Cancer | ||||||
| SF-268 | 33.5 | >67.5 | 20.5 | >57.5 | 0.07 | 75.5 |
| SF-295 | >67.5 | >67.5 | 24.8 | >57.5 | 1.1 | 46.8 |
| SNB-19 | 24.6 | >67.5 | 18.5 | >57.5 | 11.9 | 76.4 |
| SNB-75 | 53.5 | >67.5 | 10.5 | >57.5 | 0.01 | 46.3 |
| U251 | >67.5 | >67.5 | 36.4 | >57.5 | 3.2 | 52.4 |
| Melanoma | ||||||
| LOX IMVI | >67.5 | >67.5 | 49.7 | >57.5 | 0.01 | 2.8 |
| M14 | >67.5 | >67.5 | 57.1 | >57.5 | 3.1 | 51.5 |
| MDA-MB-435 | >67.5 | >67.5 | 53.0 | >57.5 | 4.1 | 65.5 |
| SK-MEL-2 | >67.5 | >67.5 | 39.6 | >57.5 | 1.1 | 71.8 |
| SK-MEL-5 | 60.9 | >67.5 | 11.8 | >57.5 | 4.5 | 44.3 |
| UACC-62 | 37.8 | >67.5 | 10.5 | >57.5 | 2.8 | 47.0 |
| Ovarian Cancer | ||||||
| IGROV1 | 32.6 | >67.5 | 21.5 | >57.5 | 0.01 | 80.0 |
| OVCAR-3 | >67.5 | >67.5 | 39.1 | >57.5 | 0.2 | 74.6 |
| OVCAR-5 | >67.5 | >67.5 | 44.4 | >57.5 | 0.02 | 86.3 |
| Renal Cancer | ||||||
| A498 | 22.6 | >67.5 | 18.8 | >57.5 | 0.03 | 16.0 |
| ACHN | >67.5 | >67.5 | 45.1 | >57.5 | 0.01 | 520 |
| CAK-1 | 22.9 | >67.5 | 18.8 | >57.5 | 0.01 | 5.1 |
| RXF 393 | 38.0 | >67.5 | 34.6 | >57.5 | 0.03 | 10.4 |
| SN12C | 48.4 | >67.5 | 24.3 | >57.5 | 0.05 | 44.4 |
| UO-31 | 19.4 | >67.5 | 16.6 | >57.5 | 0.01 | 82.0 |
| Prostate Cancer | ||||||
| PC-3 | 37.4 | >67.5 | 20.0 | >57.5 | 0.2 | 92.5 |
| DU-145 | >67.5 | >67.5 | 45.1 | >57.5 | 0.1 | 4.9 |
| Breast Cancer | ||||||
| MCF7 | 48.0 | >67.5 | 32.8 | >57.5 | 12.6 | 71.4 |
| MDA-MB-231ATCC | >67.5 | >67.5 | 55.8 | >57.5 | 0.01 | 38.0 |
| HS 578T | 16.6 | >67.5 | 3.0 | >57.5 | 0.01 | >100 |
| BT-549 | 49.9 | >67.5 | 30.0 | >57.5 | 5.9 | 49.8 |
| T-47D | 26.6 | >67.5 | 19.4 | >57.5 | 0.2 | 91.4 |
| MDA-MB-468 | 31.6 | >67.5 | 13.8 | >57.5 | 0.09 | 8.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, Y.; Sharma, D.; Kaushik, K.; Kumar, V.; Jha, A.; Prasad, A.K.; Len, C.; Malhotra, S.V.; Wengel, J.; Parmar, V.S. Synthetic, Structural, and Anticancer Activity Evaluation Studies on Novel Pyrazolylnucleosides. Molecules 2019, 24, 3922. https://doi.org/10.3390/molecules24213922
Yadav Y, Sharma D, Kaushik K, Kumar V, Jha A, Prasad AK, Len C, Malhotra SV, Wengel J, Parmar VS. Synthetic, Structural, and Anticancer Activity Evaluation Studies on Novel Pyrazolylnucleosides. Molecules. 2019; 24(21):3922. https://doi.org/10.3390/molecules24213922
Chicago/Turabian StyleYadav, Yogesh, Deepti Sharma, Kumar Kaushik, Vineet Kumar, Amitabh Jha, Ashok K. Prasad, Christophe Len, Sanjay V. Malhotra, Jesper Wengel, and Virinder S. Parmar. 2019. "Synthetic, Structural, and Anticancer Activity Evaluation Studies on Novel Pyrazolylnucleosides" Molecules 24, no. 21: 3922. https://doi.org/10.3390/molecules24213922
APA StyleYadav, Y., Sharma, D., Kaushik, K., Kumar, V., Jha, A., Prasad, A. K., Len, C., Malhotra, S. V., Wengel, J., & Parmar, V. S. (2019). Synthetic, Structural, and Anticancer Activity Evaluation Studies on Novel Pyrazolylnucleosides. Molecules, 24(21), 3922. https://doi.org/10.3390/molecules24213922