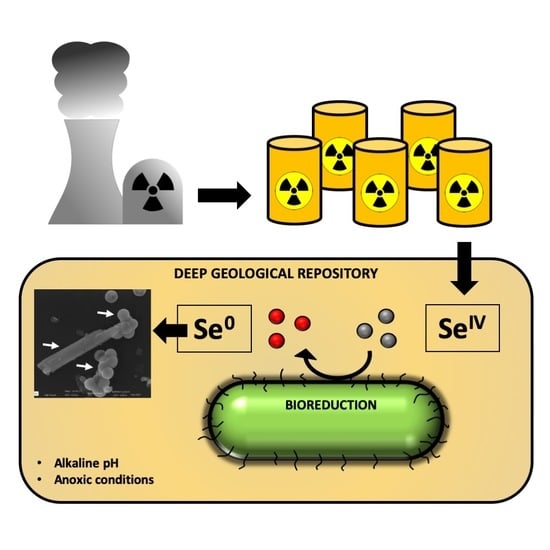
The Bioreduction of Selenite under Anaerobic and Alkaline Conditions Analogous to Those Expected for a Deep Geological Repository System
Abstract
1. Introduction
2. Results and Discussion
2.1. SeIV Reduction under Anaerobic and Neutral pH Conditions
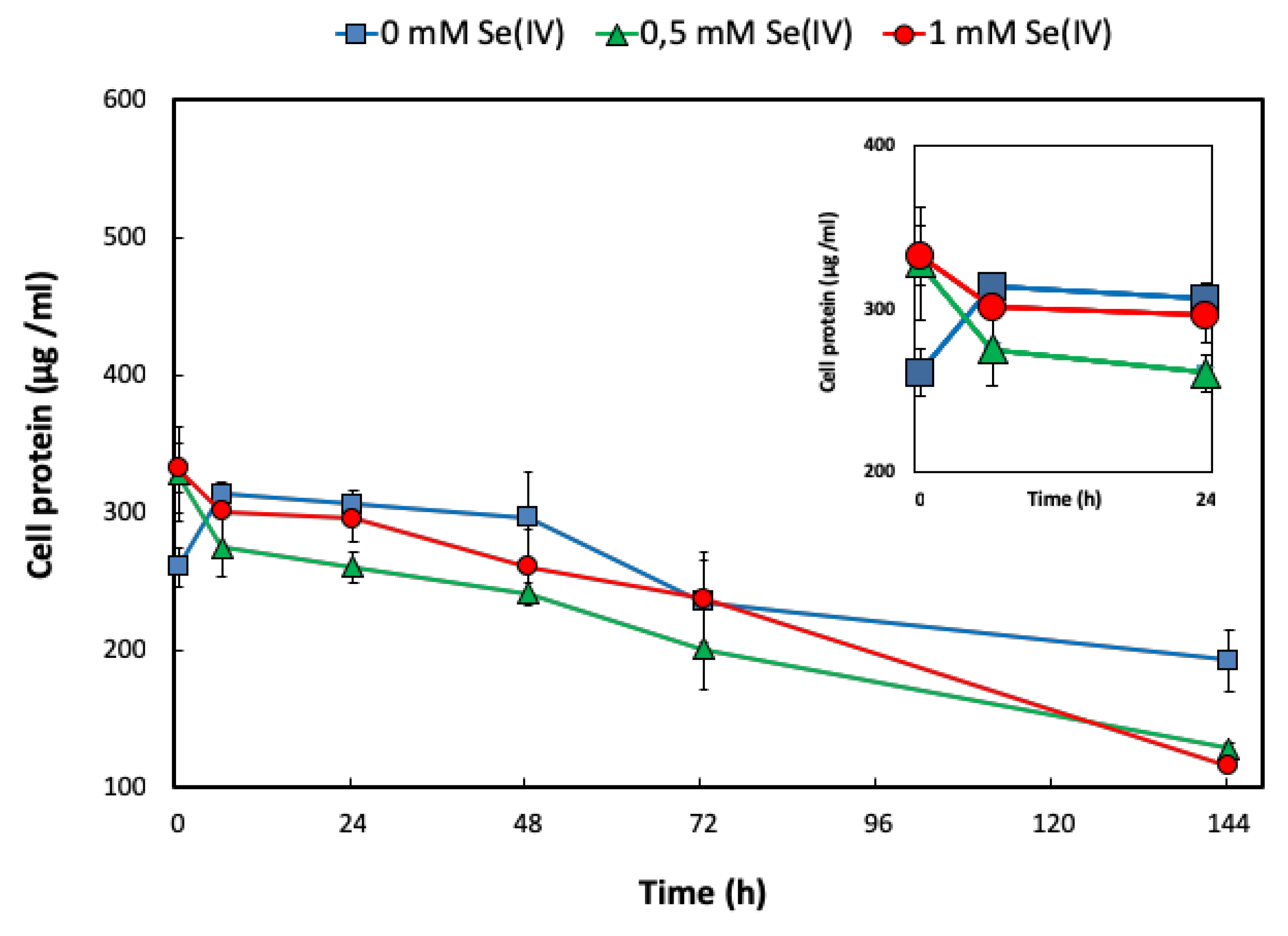
2.1.1. SeIV Reduction and Growth Profile
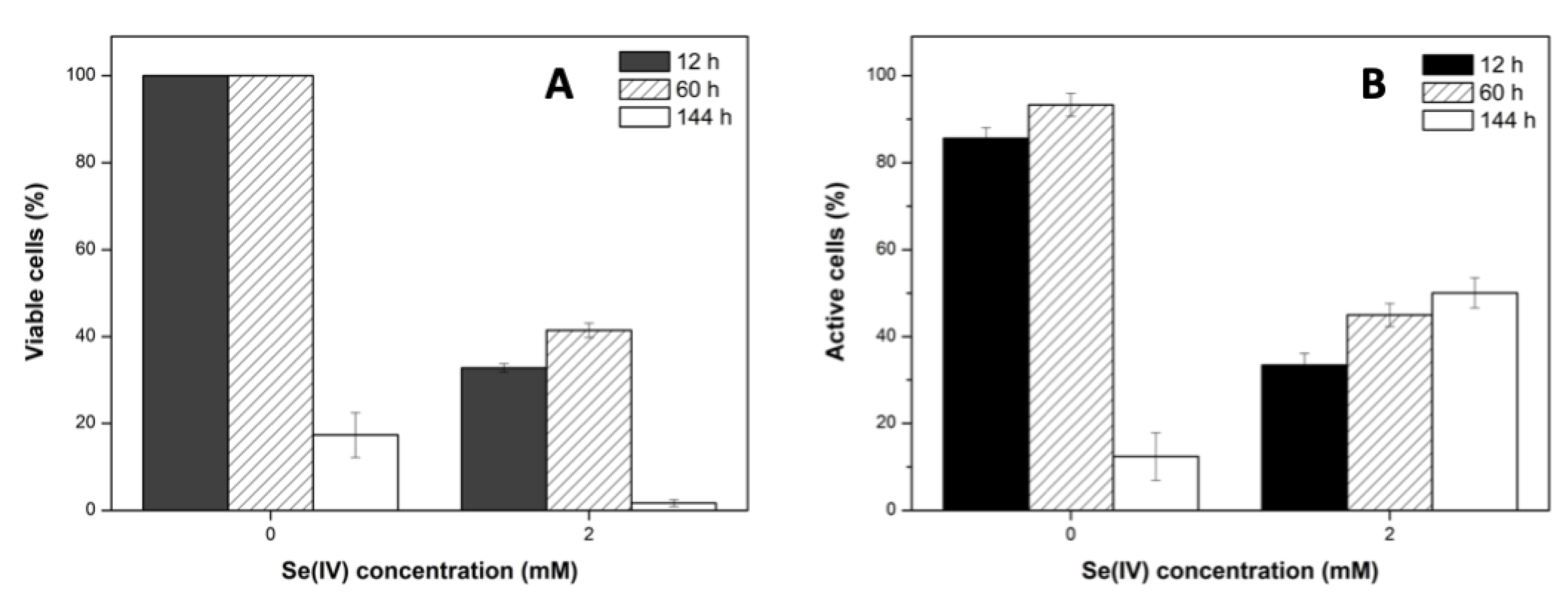
2.1.2. Cell Viability and Metabolic Activity
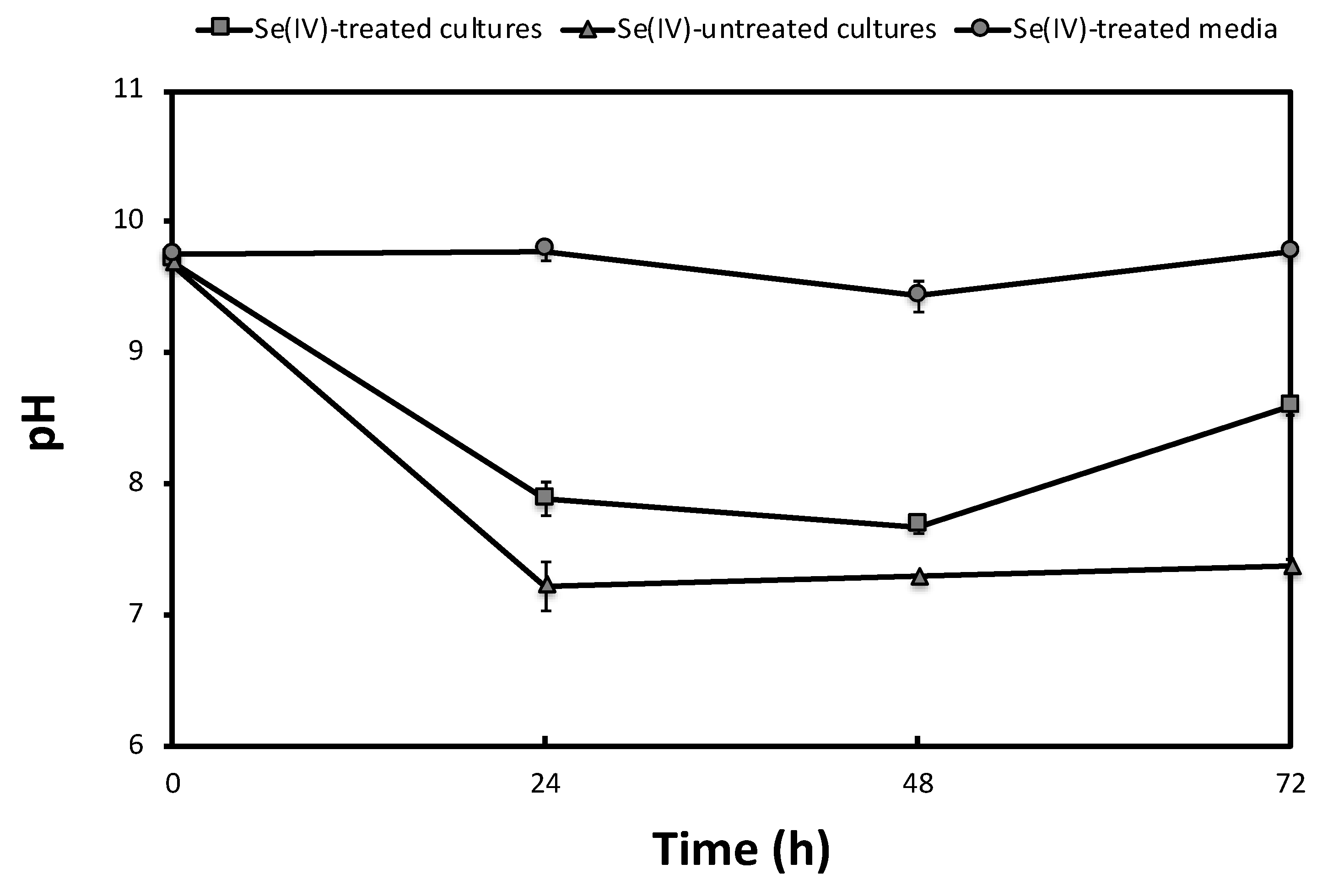
2.2. SeIV Reduction under Anaerobic and Alkaline Conditions
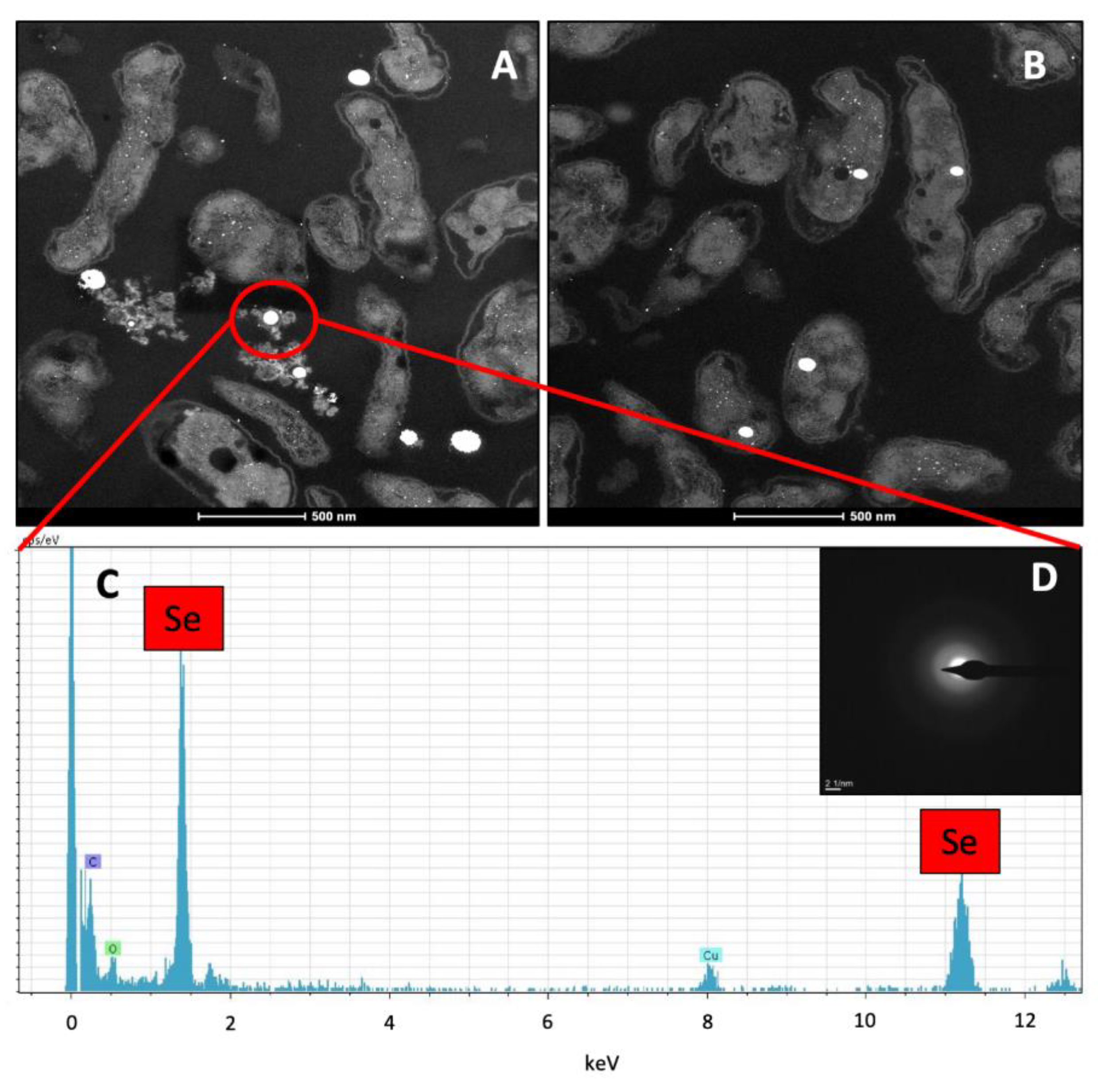
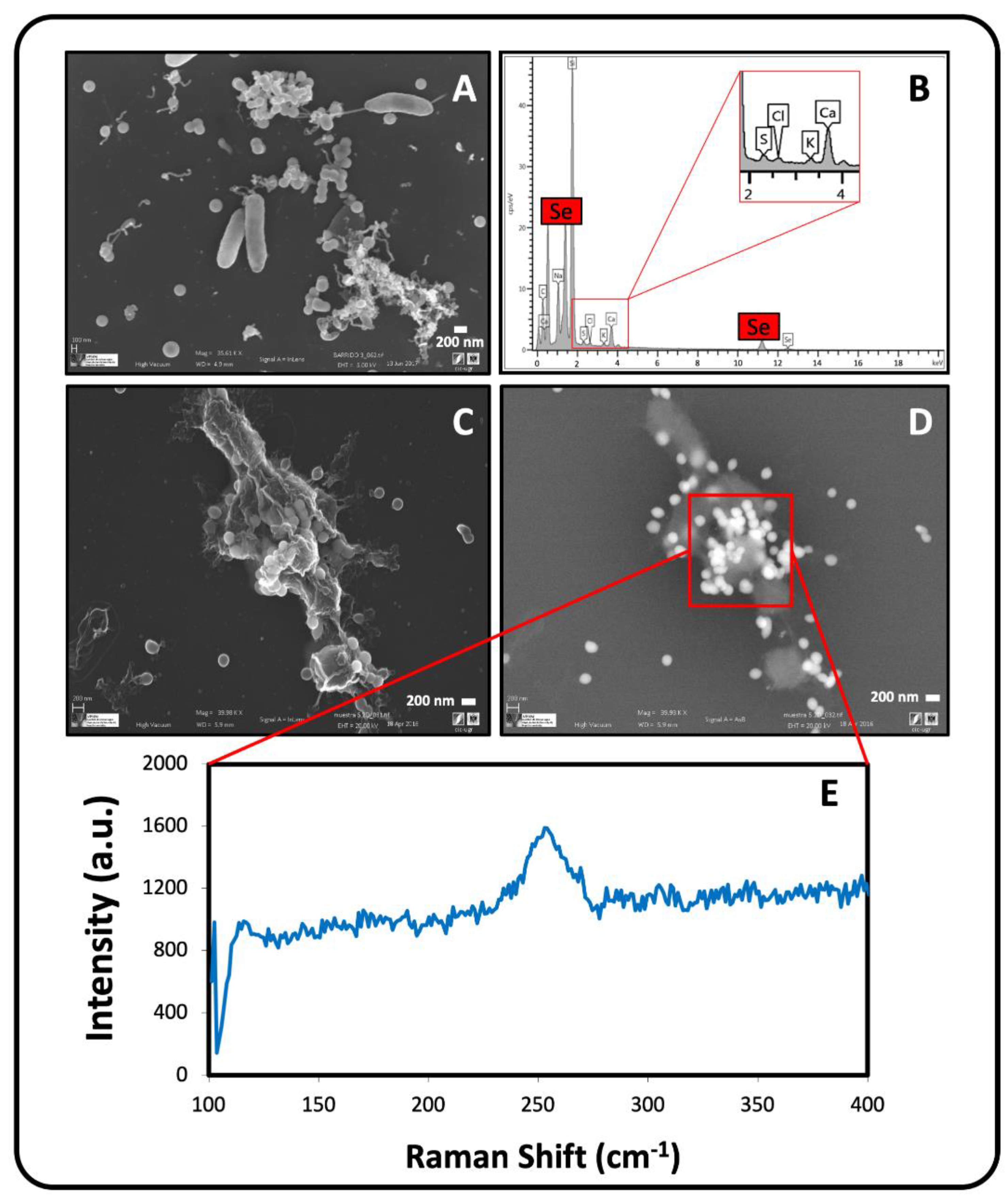
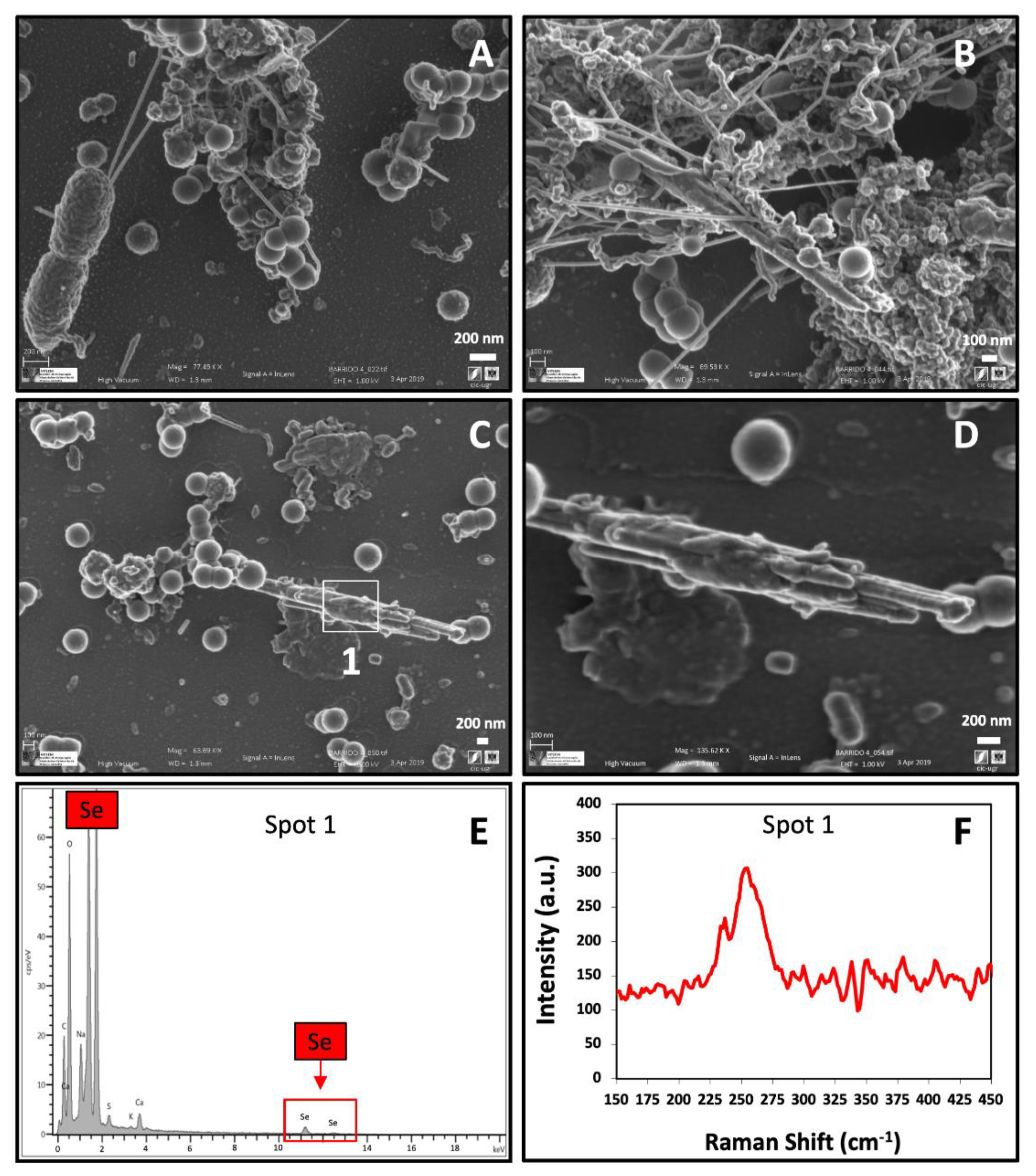
2.3. Electron Microscopic Characterization of SeIV Bioreduction Products
3. Materials and Methods
3.1. Bacterial Strain and Growth Conditions
3.2. Anaerobic Growth under Selenite Stress
3.3. Reduction of SeIV under Anaerobic and Alkaline Conditions
3.4. Flow Cytometry
3.5. Electron Microscopy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Atomic Energy Agency (IAEA). Scientific and Technical Basis for the Geological Disposal of Radioactive Wastes. TR-413, STI/DOC/010/413. Vienna, IAEA 2003. Available online: https://www-pub.iaea.org/MTCD/Publications/PDF/TRS413_web.pdf (accessed on 23 October 2019).
- Delage, P.; Cui, Y.; Tang, A. Clays in radioactive waste disposal. J. Rock Mech. Geotech. Eng. 2010, 2, 111–123. [Google Scholar] [CrossRef]
- Alonso, J.; Becker, D.A.; Storck, R.; Besnus, F.; Pellegrini, D.; Serres, C.; Johnson, L.; Hart, J.; Marivoet, J.; Vieno, T.; et al. Bentonite barriers in integrated performance assessment (Benipa). European Commission, Nuclear Science and Technology, Report EUR 21023 EN. 2004. Available online: http://elibrary.cenn.org/Report/Bentonite%20Barries%20in%20Integrated%20Performance%20Assessment%20Benipa/Part_2.pdf (accessed on 23 October 2019).
- Berner, U. Evolution of pore water chemistry during degradation of cement in a radioactive waste repository environment. Waste Manag. 1992, 12, 201–219. [Google Scholar] [CrossRef]
- Pedersen, K.; Nilsson, E.; Arlinger, J.; Hallbeck, L.; O’Neill, A. Distribution, diversity and activity of microorganisms in the hyper-alkaline spring waters of Maqarin in Jordan. Extremophiles 2004, 8, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, T.L.; Lentini, V.; Gugliandolo, C.; Italiano, F.; Cousin, S.; Stackebrandt, E. Bacterial and archaeal populations at two shallow hydrothermal vents off Panarea Island (Eolian Islands, Italy). Extremophiles 2009, 13, 199–212. [Google Scholar] [CrossRef]
- Lloyd, J.R. Microbial reduction of metals and radionuclides. FEMS Microbiol. Rev. 2003, 27, 411–425. [Google Scholar] [CrossRef]
- Borghese, R.; Baccolini, C.; Francia, F.; Sabatino, P.; Turner, R.J.; Zannoni, D. Reduction of chalcogen oxyanions and generation of nanoprecipitates by the photosynthetic bacterium Rhodobacter capsulatus. J. Hazard. Mater. 2014, 269, 24–30. [Google Scholar] [CrossRef]
- Dardenne, K.; González-Robles, E.; Rothe, J.; Müller, N.; Christill, G.; Lemmer, D.; Praetorius, R.; Kienzler, B.; Metz, V.; Roth, G.; et al. XAS and XRF investigation of an actual HAWC glass fragment obtained from the Karlsruhe vitrification plant (VEK). J. Nucl. Mater. 2015, 460, 209–215. [Google Scholar] [CrossRef]
- Antonioli, P.; Lampis, S.; Chesini, I.; Vallini, G.; Rinalducci, S.; Zolla, L.; Righetti, P.G. Stenotrophomonas maltophilia SeITE02, a new bacterial strain suitable for bioremediation of selenite-contaminated environmental matrices. Appl. Environ. Microbiol. 2007, 73, 6854–6863. [Google Scholar] [CrossRef]
- Lampis, S.; Zonaro, E.; Bertolini, C.; Cecconi, D.; Monti, F.; Micaroni, M.; Turner, R.J.; Butler, C.S.; Vallini, G. Selenite biotransformation and detoxification by Stenotrophomonas maltophilia SeITE02: Novel clues on the route to bacterial biogenesis of selenium nanoparticles. J. Hazard. Mater. 2017, 324, 3–14. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, Y.; Wang, Y.; Xu, D.; Huang, Y.; Wang, D.; Wang, G.; Rensing, C.; Zheng, S. Novel mechanisms of selenate and selenite reduction in the obligate aerobic bacterium Comamonas testosteroni S44. J. Hazard. Mater. 2018, 359, 129–138. [Google Scholar] [CrossRef]
- Kora, A.J. Bacillus cereus, selenite-reducing bacterium from contaminated lake of an industrial area: A renewable nanofactory for the synthesis of selenium nanoparticles. Bioresour. Bioprocess. 2018, 5, 30. [Google Scholar] [CrossRef]
- Fernández-Llamosas, H.; Castro, L.; Blázquez, M.L.; Díaz, E.; Carmona, M. Biosynthesis of selenium nanoparticles by Azoarcus sp. CIB. Microb. Cell Factories 2016, 15, 109. [Google Scholar] [CrossRef] [PubMed]
- Dungan, R.S.; Yates, S.R.; Frankenberger, W.T. Transformations of selenate and selenite by Stenotrophomonas maltophilia isolated from a seleniferous agricultural drainage pond sediment. Environ. Microbiol. 2003, 5, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-B.; Cheng, Y.-Y.; Wu, C.; Li, W.-W.; Li, N.; Yang, Z.-C.; Tong, Z.-H.; Yu, H.-Q. Selenite reduction by Shewanella oneidensis MR-1 is mediated by fumarate reductase in periplasm. Sci. Rep. 2014, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fresneda, M.A.R.; Martín, J.D.; Bolívar, J.G.; Cantos, M.V.F.; Bosch-Estévez, G.; Moreno, M.F.M.; Merroun, M.L. Green synthesis and biotransformation of amorphous Se nanospheres to trigonal 1D Se nanostructures: Impact on Se mobility within the concept of radioactive waste disposal. Environ. Sci. Nano 2018, 5, 2103–2116. [Google Scholar] [CrossRef]
- Williamson, A.J.; Morris, K.; Law, G.T.W.; Rizoulis, A.; Charnock, J.M.; Lloyd, J.R. Microbial Reduction of U(VI) under alkaline conditions: implications for radioactive waste geodisposal. Environ. Sci. Technol. 2014, 48, 13549–13556. [Google Scholar] [CrossRef]
- Smith, S.L.; Boothman, C.; Williams, H.A.; Ellis, B.L.; Wragg, J.; West, J.M.; Lloyd, J.R. Microbial impacts on 99mTc migration through sandstone under highly alkaline conditions relevant to radioactive waste disposal. Sci. Total. Environ. 2017, 575, 485–495. [Google Scholar] [CrossRef]
- Villar, M.V.; Pérez del Villar, L.; Martín, P.L.; Pelayo, M.; Fernández, A.M.; Garralon, A.; Cuevas, J.; Leguey, S.; Caballero, E.; Huertas, F.; et al. The study of Spanish clays for their use as sealing materials in nuclear waste repositories: 20 years of progress. J. Iber. Geol. 2006, 32, 15–36. [Google Scholar]
- Pearce, C.I.; Coker, V.S.; Charnock, J.M.; Pattrick, R.A.D.; Mosselmans, J.F.W.; Law, N.; Beveridge, T.J.; Lloyd, J.R. Microbial manufacture of chalcogenide-based nanoparticles via the reduction of selenite usingVeillonella atypica: Anin situEXAFS study. Nanotechnol. 2008, 19, 155603. [Google Scholar] [CrossRef]
- Kessi, J.; Hanselmann, K.W. Similarities between the abiotic reduction of selenite with glutathione and the dissimilatory reaction mediated by rhodospirillum rubrum and escherichia coli. J. Boil. Chem. 2004, 279, 50662–50669. [Google Scholar] [CrossRef]
- Pearce, C.I.; Pattrick, R.A.; Law, N.; Charnock, J.M.; Coker, V.S.; Fellowes, J.W.; Oremland, R.S.; Lloyd, J.R. Investigating different mechanisms for biogenic selenite transformations: Geobacter sulfurreducens, Shewanella oneidensisandVeillonella atypica. Environ. Technol. 2009, 30, 1313–1326. [Google Scholar] [CrossRef] [PubMed]
- Tam, K.; Ho, C.T.; Lee, J.-H.; Lai, M.; Chang, C.H.; Rheem, Y.; Chen, W.; Hur, H.-G.; Myung, N.V. Growth mechanism of amorphous selenium nanoparticles synthesized by Shewanella sp. HN-41. Biosci. Biotechnol. Biochem. 2010, 74, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Klonowska, A.; Heulin, T.; Vermeglio, A. Selenite and tellurite reduction by shewanella oneidensis. Appl. Environ. Microbiol. 2005, 71, 5607–5609. [Google Scholar] [CrossRef] [PubMed]
- Nancharaiah, Y.V.; Lens, P.N.L. Ecology and biotechnology of selenium-respiring bacteria. Microbiol. Mol. Boil. Rev. 2015, 79, 61–80. [Google Scholar] [CrossRef]
- Basaglia, M.; Toffanin, A.; Baldan, E.; Bottegal, M.; Shapleigh, J.P.; Casella, S. Selenite-reducing capacity of the copper-containing nitrite reductase of Rhizobium sullae. FEMS Microbiol. Lett. 2007, 269, 124–130. [Google Scholar] [CrossRef]
- DeMoll-decker, H.; Macy, J.M. The periplasmic nitrite reductase of Thauera selenatis may catalyze the reduction of selenite to elemental selenium. Arch. Microbiol. 1993, 160, 241–247. [Google Scholar]
- Abrahamsen, L.; Arnold, T.; Brinkmann, H.; Leys, N.; Merroun, M.; Mijnendonckx, K.; Moll, H.; Polvika, P.; Sevcu, J.; Small, J.; et al. A review of anthropogenic organic wastes and their degradation behaviour. Microbiology In Nuclear waste Disposal (MIND project) 2015, 97. Available online: https://igdtp.eu/wp-content/uploads/2017/10/MIND-2015-12-D1.1-ReviewOfAnthropogenicOrganicWastes.pdf (accessed on 23 October 2019).
- Bertron, A.; Jacquemet, N.; Erable, B.; Sablayrolles, C.; Escadeillas, G.; Albrecht, A. Reactivity of nitrate and organic acids at the concrete–bitumen interface of a nuclear waste repository cell. Nucl. Eng. Des. 2014, 268, 51–57. [Google Scholar] [CrossRef]
- Walczak, I.; Camaro, S.; Blanchard, J.-M. Quantitative and qualitative analysis of hydrosoluble organic matter in bitumen leachates. Agron. EDP Sci. 2001, 21, 247–257. [Google Scholar] [CrossRef]
- Givan, A.L. Flow Cytometry: An Introduction. In Flow Cytometry Protocols; Hawley, T.S., Hawley, R.G., Eds.; Humana Press: New York, NY, USA, 2011; pp. 1–29. [Google Scholar] [CrossRef]
- Stubberfield, L.; Shaw, P. A comparison of tetrazolium reduction and FDA hydrolysis with other measures of microbial activity. J. Microbiol. Methods 1990, 12, 151–162. [Google Scholar] [CrossRef]
- David, F.; Berger, A.; Hänsch, R.; Rohde, M.; Franco-Lara, E. Single cell analysis applied to antibody fragment production with Bacillus megaterium: Development of advanced physiology and bioprocess state estimation tools. Microb. Cell Factories 2011, 10, 23. [Google Scholar] [CrossRef]
- Sánchez-Castro, I.; Ruiz-Fresneda, M.A.; Bakkali, M.; Kämpfer, P.; Glaeser, S.P.; Busse, H.J.; López-Fernández, M.; Martínez-Rodríguez, P.; Merroun, M.L. Stenotrophomonas bentonitica sp. nov., isolated from bentonite formations. Int. J. Syst. Evol. Microbiol. 2017, 67, 2779–2786. [Google Scholar] [CrossRef] [PubMed]
- Rizoulis, A.; Steele, H.M.; Morris, K.; Lloyd, J.R.; Lloyd, J. The potential impact of anaerobic microbial metabolism during the geological disposal of intermediate-level waste. Miner. Mag. 2012, 76, 3261–3270. [Google Scholar] [CrossRef][Green Version]
- Wang, T.; Yang, L.; Zhang, B.; Liu, J. Extracellular biosynthesis and transformation of selenium nanoparticles and application in H2O2 biosensor. Colloids Surf. B: Biointerfaces 2010, 80, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Kora, A.J.; Rastogi, L. Bacteriogenic synthesis of selenium nanoparticles by Escherichia coli ATCC 35218 and its structural characterisation. IET Nanobiotechnol. 2016, 11, 179–184. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Z.; Liu, H.; Zhang, L.; Gao, P.; Li, D. Biosynthesis and structural characteristics of selenium nanoparticles by Pseudomonas alcaliphila. Colloids Surf. B: Biointerfaces 2011, 88, 196–201. [Google Scholar] [CrossRef]
- Husen, A.; Siddiqi, K. Plants and microbes assisted selenium nanoparticles: Characterization and application. J. Nanobiotechnol. 2014, 12, 28. [Google Scholar] [CrossRef]
- Jóvári, P.; Delaplane, R.G.; Pusztai, L. Structural models of amorphous selenium. Phys. Rev. B 2003, 67, 172201. [Google Scholar] [CrossRef]
- Fernández-Martínez, A.; Charlet, L. Selenium environmental cycling and bioavailability: A structural chemist point of view. Rev. Environ. Sci. Bio/Technol. 2009, 8, 81–110. [Google Scholar] [CrossRef]
- Cooper, W.C.; Westbury, A. The structure of selenium. In Selenium; Zingaro, R.A., Cooper, W.C., Eds.; Van Nostrand Reinhold Company: New York, NY, USA, 1974; Chapter 3; pp. 148–173. [Google Scholar]
- Popov, A. Correlation between the molecular structure and properties of amorphous selenium. J. Phys. C Solid State Phys. 1976, 9, 675. [Google Scholar] [CrossRef]
- Chen, H.; Shin, D.-W.; Nam, J.-G.; Kwon, K.-W.; Yoo, J.-B. Selenium nanowires and nanotubes synthesized via a facile template-free solution method. Mater. Res. Bull. 2010, 45, 699–704. [Google Scholar] [CrossRef]
- Benkő, I.; Nagy, G.; Tanczos, B.; Ungvari, E.; Sztrik, A.; Eszenyi, P.; Prokisch, J.; Banfalvi, G. Subacute toxicity of nano-selenium compared to other selenium species in mice. Environ. Toxicol. Chem. 2012, 31, 2812–2820. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Krishnani, K.K.; Singh, N.P. Comparative study of selenium and selenium nanoparticles with reference to acute toxicity, biochemical attributes, and histopathological response in fish. Environ. Sci. Pollut. Res. 2018, 25, 8914–8927. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, J.; Wang, T.; Luo, W.; Zhou, Q.; Jiang, G. Elemental selenium particles at nano-size (Nano-Se) are more toxic to Medaka (Oryzias latipes) as a consequence of hyper-accumulation of selenium: A comparison with sodium selenite. Aquat. Toxicol. 2008, 89, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Jordan, N.; Tsushima, S.; Hübner, R.; Weiss, S.; Lens, P.N.L. Shape change of biogenic elemental selenium nanomaterials from nanospheres to nanorods decreases their colloidal stability. Environ. Sci. Nano 2017, 4, 1054–1063. [Google Scholar] [CrossRef]
- Lenz, M.; Van Aelst, A.; Smit, M.; Corvini, P.; Lens, P.N. Biological Production of Selenium Nanoparticles from Waste Waters. Adv. Mater. Res. 2009, 71, 721–724. [Google Scholar] [CrossRef]
- López-Fernández, M.; Fernandez-Sanfrancisco, O.; Moreno-García, A.; Martín-Sánchez, I.; Sánchez-Castro, I.; Merroun, M.L. Microbial communities in bentonite formations and their interactions with uranium. Appl. Geochem. 2014, 49, 77–86. [Google Scholar] [CrossRef]
- Nedelkova, M.; Merroun, M.L.; Merroun, M.L.; Rossberg, A.; Hennig, C.; Selenska-Pobell, S. Microbacterium isolates from the vicinity of a radioactive waste depository and their interactionswith uranium. FEMS Microbiol. Ecol. 2007, 59, 694–705. [Google Scholar] [CrossRef]
- Kumar, R.; Nongkhlaw, M.; Acharya, C.; Joshi, S.R. Uranium (U)-tolerant bacterial diversity from U ore deposit of Domiasiat in North-East India and its prospective utilisation in bioremediation. Microbes Environ. 2013, 28, 33–41. [Google Scholar] [CrossRef]
- Dhanjal, S.; Cameotra, S.S. Aerobic biogenesis of selenium nanospheres by Bacillus cereus isolated from coalmine soil. Microb. Cell Factories 2010, 9, 52. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Merroun, M.L.; Raff, J.; Rossberg, A.; Hennig, C.; Reich, T.; Selenska-Pobell, S. Complexation of Uranium by Cells and S-Layer Sheets of Bacillus sphaericus JG-A12. Appl. Environ. Microbiol. 2005, 71, 5532–5543. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Fresneda, M.A.; Gomez-Bolivar, J.; Delgado-Martin, J.; Abad-Ortega, M.d.M.; Guerra-Tschuschke, I.; Merroun, M.L. The Bioreduction of Selenite under Anaerobic and Alkaline Conditions Analogous to Those Expected for a Deep Geological Repository System. Molecules 2019, 24, 3868. https://doi.org/10.3390/molecules24213868
Ruiz-Fresneda MA, Gomez-Bolivar J, Delgado-Martin J, Abad-Ortega MdM, Guerra-Tschuschke I, Merroun ML. The Bioreduction of Selenite under Anaerobic and Alkaline Conditions Analogous to Those Expected for a Deep Geological Repository System. Molecules. 2019; 24(21):3868. https://doi.org/10.3390/molecules24213868
Chicago/Turabian StyleRuiz-Fresneda, Miguel Angel, Jaime Gomez-Bolivar, Josemaria Delgado-Martin, Maria del Mar Abad-Ortega, Isabel Guerra-Tschuschke, and Mohamed Larbi Merroun. 2019. "The Bioreduction of Selenite under Anaerobic and Alkaline Conditions Analogous to Those Expected for a Deep Geological Repository System" Molecules 24, no. 21: 3868. https://doi.org/10.3390/molecules24213868
APA StyleRuiz-Fresneda, M. A., Gomez-Bolivar, J., Delgado-Martin, J., Abad-Ortega, M. d. M., Guerra-Tschuschke, I., & Merroun, M. L. (2019). The Bioreduction of Selenite under Anaerobic and Alkaline Conditions Analogous to Those Expected for a Deep Geological Repository System. Molecules, 24(21), 3868. https://doi.org/10.3390/molecules24213868