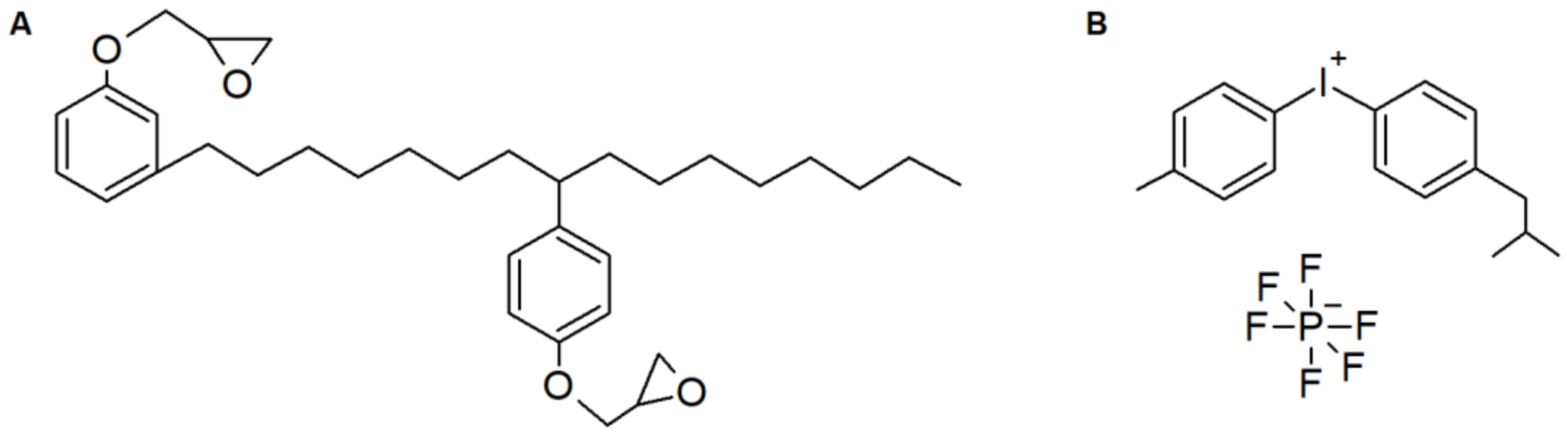
3.1. Morhology and Photocuring Behaviour
In this section, we report the photocuring of a commercial EC resin, with a cationic hexafluorophospate photoinitiator, and of its composites with microfibrillated cellulose. Dry microfibrillated cellulose mats prepared by solvent exchange, but without addition of resin, were also characterized for reference. As detailed below, while the resin containing 5 wt.% PI could reach full conversion within a few minutes of exposure to UV light, for the composites the curing reaction was somewhat hindered, and only by highly increasing the PI amount up to 15 wt.% a full cure could be obtained.
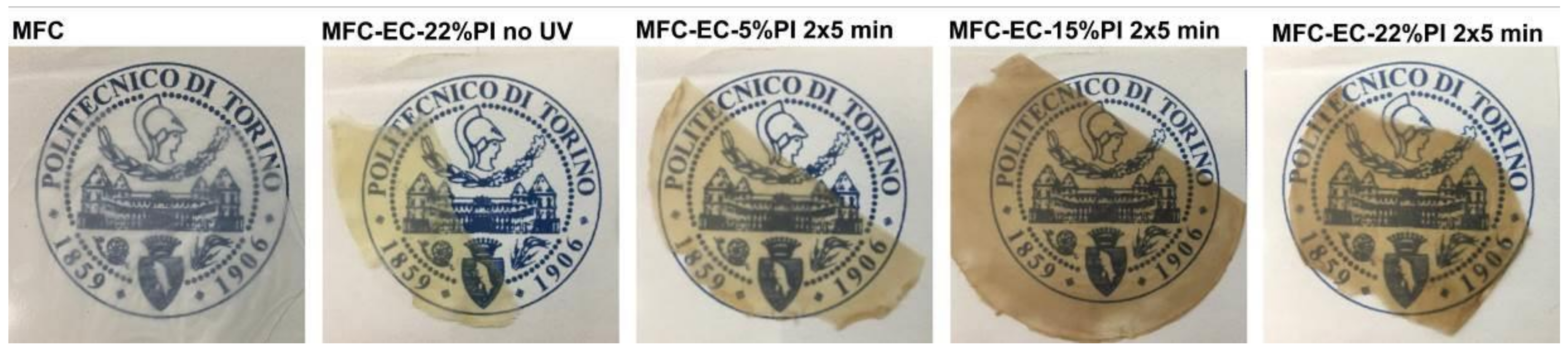
Photos of the dry MFC mat and of the cured composites are shown in
Figure 2. The dry MFC mat obtained by solvent exchange was transparent, provided that a slow evaporation rate of acetone was ensured by keeping the Petri dish covered with his lid during drying. Otherwise, with fast evaporation rates a white non-transparent film was obtained (
Figure S1); therefore, one can suggest that the porosity left in the mat upon evaporation depends highly on the solvent evaporation rate. The composites were relatively transparent; when uncured their color was yellowish, due to the color of the resin, and turned brownish upon curing, being darker with increasing PI concentration. Reaction of MFC with PI contributed to the color change, as also MFC impregnated with only PI was found to develop a brownish color upon irradiation (
Figure S2).
The dry MFC mat, and the uncured and cured composites were also observed by optical microscopy. Bright field images in both reflection and transmission mode are shown in
Figure 3 and dark field images in reflection mode are shown in
Figure 4. The homogeneous structure of entangled fibers shown by the dry MFC mat was maintained also in the composites, before and after curing. A few larger fibers were also visible. A rough surface structure, due to the cellulose microfibrils was highlighted by the images taken in reflection mode.
Both the dry MFC mat and the composites were flexible, and it was possible to roll them on a 4.9 mm diameter cylinder, as shown in
Figure 5.
To explore the photo-induced curing of the cardanol resin and its composites, the FT-IR transmission spectra of the EC resin and of the PI, as well as the ATR FT-IR spectrum of the dry MFC mat were recorded (
Figure 6) as reference.
The spectrum of the EC resin showed two intense peaks at 2927 and 2854 cm
−1 corresponding to sp
3 C–H bond vibrations in the aliphatic chain of the epoxidized cardanol [
4,
5,
12]. At higher wavenumber, some small peaks appeared; the one at 3056 cm
−1 can be attributed to the stretching of the C–H bond of the methyl group attached to the epoxide ring [
18,
19]. As expected, the broad peak centered around 3350 cm
−1 corresponding to the phenolic OH group of cardanol before epoxidation [
5,
20] was not present in the spectrum of EC, confirming that the hydroxyl groups are quantitatively replaced by epoxide groups. Also, the peak at about 3007–3009 cm
−1 characteristic of C=C bonds in the aliphatic chain of cardanol [
5,
20] was not detectable in EC, indicating that the double bond content in this epoxidized cardanol is rather low [
10]. The vibrations of the C=C aromatic bond were seen in the 1550–1650 cm
−1 and 1400–1510 cm
−1 regions [
4,
5,
20]. A shoulder peak at 1258 cm
−1 indicated the presence of the phenolic ether linkage [
14,
20]. The characteristic vibrations of the epoxide ring were found at 911 cm
−1 [
4,
5,
11,
14,
20], 860 cm
−1 [
4,
12] and 776 cm
−1 [
4]. Among these, the most suitable to be followed to assess the degree of curing of the EC was the 911 cm
−1 signal of the epoxide group [
14], as the signals at 860 and 776 cm
−1 overlapped with peaks characteristic of the meta substituted (670–710, 750–805, 870–900 cm
−1) and para substituted (845 cm
−1 γCH) aromatic rings in the EC molecule [
21,
22,
23], and with some photoinitiator’s signals (845 cm
−1 and 780 cm
−1).
Indeed, the spectrum of the photoinitiator showed peaks at 1790–1800 cm
−1 and at 780 cm
−1, related to the propylene carbonate solvent, an intense peak at 845 cm
−1 (stretching vibrations of the PF
6 anion), with shoulders at 876 cm
−1 and 804 cm
−1, while the δ(PF
6) bending vibrations was observed as a narrow strong band at 558 cm
−1 [
24].
The spectrum of the MFC mat was characterized by a broad O–H stretching signal in the 3500–3000 cm
−1 region, relatively weak C-H stretching peaks at 3000–2800 cm
−1, and in the fingerprint region intense bands attributed to the C–O stretching of the pyranose ring skeletal vibration in the 1150–1030 cm
−1 range, and to the β-glycosidic bond vibration at 896 cm
−1; a weak and broad peak centered at 1639 cm
−1 reflected the presence of water adsorbed into the cellulose fibrils [
25,
26,
27,
28,
29,
30].
The photocuring of the resin mixtures containing 5%, 15% and 22% photoinitiator was followed; the higher amounts of photoinitiator were used also for the resin as a comparison with the composites, which could not be cured with the lowest concentration. The formulations were exposed to UV radiation at 100 mW cm
−2 with a medium intensity mercury bulb for intervals of 1 min, and the photo-induced reaction of ring opening photopolymerization was followed by FT-IR in transmission mode (
Figure 7). The peak at 911 cm
−1, characteristic of the epoxide ring, decreased with increasing exposure time and eventually completely disappeared meaning the full conversion of the epoxide rings: for the EC-5%PI resin the peak disappeared within 3 min, while for the EC-15%PI and EC-22%PI resins less than 1 min of irradiation was sufficient. The peak at 3056 cm
−1 and the shoulder at 860 cm
−1 decreased as well with irradiation dose, although quantification was not possible due to overlap with other peaks. The peaks characteristic of the photoinitiator, i.e., 1800 cm
−1, 845 cm
−1, 780 cm
−1 also decreased with increasing UV exposure. While the epoxide conversion proceeded, the broad peak centered around 3450 cm
−1 increased, as expected from the formation of hydroxyls after the opening of the epoxide ring. The spectra revealed other interesting features of the reaction: upon irradiation, between 1760 cm
−1 and 1700 cm
−1 some weak peaks appeared, which could be attributed to the autooxidation of the residual double bonds in the aliphatic chain [
5]. The intensity of the peaks at 2927 cm
−1 and 2854 cm
−1, and at 1600 cm
−1 and 1583 cm
−1 did not change; however, the peaks became slightly broader. The FT-IR spectra of the cured resins of this work were similar to those present in the literature [
14].
The high degree of curing of the EC-5%PI resin was confirmed by measuring the insoluble content, which was in the 86–89 % range both in acetone and in toluene.
The photocuring of the composites upon exposure to UV radiation at 100 mW cm
−2 for increasing time was followed by FT-IR analysis in ATR mode, as described in
Section 3. The ATR FT-IR spectra of the composites with EC-5%PI, EC-15%PI and EC-22%PI resins before, and after different times of light exposure are shown in
Figure 8. When the MFC mat was impregnated with the resins, the intensity of the O–H stretching signal diminished, and the characteristic peaks of the resins, as described in
Section 2.1, appeared. When the 5% PI resin composites were irradiated, a moderate decrease of the epoxy signals was initially visible; however, after 2 min the conversion reaction did not seem to proceed further. When the composites with 15% and 22% PI resins were irradiated for increasing time, the same trends as for the resin alone were seen. The peak at 911 cm
−1 diminished and eventually disappeared completely, after irradiating for more than 2 min per side, faster at the higher PI concentration. The photocuring was however slower than for the resin alone. The consumption of the photoinitiator was confirmed by the decrease of the peaks at 1800 cm
−1 and 845 cm
−1, which initial intensity increased with PI concentration in the resin. As the cationic curing is known to proceed also after the end of irradiation, partially cured samples with EC-5%PI and EC-15%PI were kept in the dark, and FT-IR spectra were taken again after 1 month. The MFC-EC-15%PI composite was eventually fully cured, while the MFC-EC-5%PI composite did not show a noticeable increase of the degree of curing, suggesting that for the latter all the available photoinitiator was consumed during the initial irradiation, and no further dark curing occurred.
3.2. Characterization
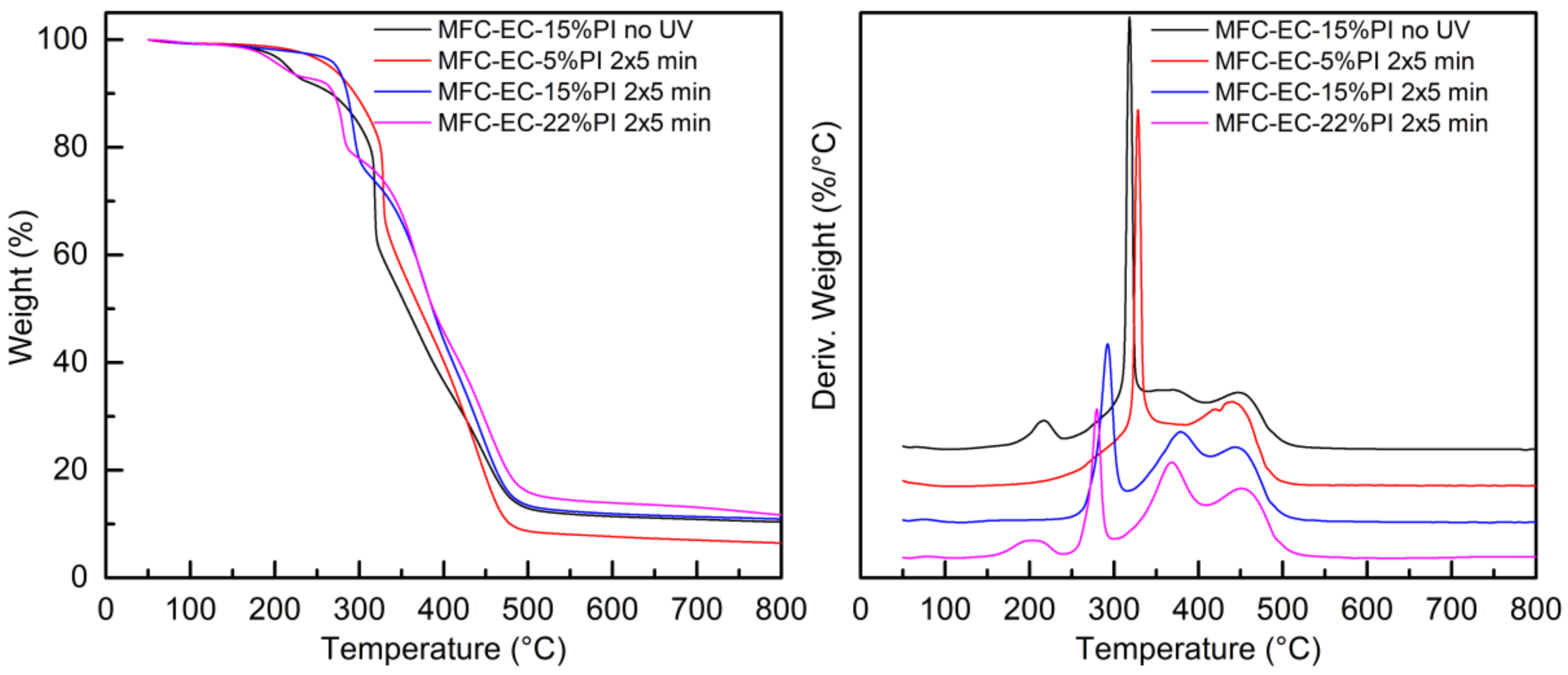
The thermal stability of the dry MFC mat, of the EC monomer and of the fully cured EC-15%PI resin, as well as that of the uncured and cured composites was evaluated by thermogravimetric analysis in an inert atmosphere. The weight-loss curves, and their first derivatives, are reported in
Figure 9 and
Figure 10. The thermogravimetric measurements were performed after 1 month from curing.
The dry MFC mat degraded in one step at around 355 °C, with a residue of about 17% at 750 °C. The initial weight loss below 100 °C can be attributed to adsorbed humidity. The EC presented its first weight loss well above 200 °C; the derivative curve highlighted three different degradation events, with maximum degradation rates at about 335 °C, 365 °C and 440 °C. The residual weight was of about 1%. For the cured EC-15%PI resin only two main degradation steps were present, with maximum rates at 380 °C and 450 °C, and a small degradation step appeared at about 190 °C attributable to the degradation of the photoinitiator. The residue was above 6%, confirming that crosslinking took place. As a comparison, the same epoxidized monomer, crosslinked by thermal curing, the temperature at which a weight loss of 30% had occurred was reported to be in the 350–366 °C range [
10,
31], and its char at 600 °C between 2% and 6.8% [
10]. The thermal stability of our resin cured by UV light is also comparable to that of a different EC resin cured by electron beam with hexafluorophosphate photoinitiator [
12].
The uncured and cured composites showed complex weight profiles, which are reported in
Figure 10, together with the corresponding first derivatives that enable better visualization of the different weight-loss events.
Although a detailed understanding of all the degradation mechanisms involved is beyond the scope of this work, some general considerations can be done. The small initial weight loss before 100 °C can be attributed to evaporation of residual solvent, as seen also in the DSC analysis. The second weight loss for the uncured composite, around 200 °C, can be attributed to loss of the photoinitiator; this weight-loss step was not observed for the cured composites with 5% and 15% photoinitiator, while it was present in the cured composite with 22% of photoinitiator, suggesting that only at the highest concentration not all the photoinitiator was consumed even after dark curing. The sharp weight loss present for the uncured composite with maximum rate at about 320 °C was attributed to the degradation of the cellulose microfibrils; for the composite with 5% photoinitiator after exposure to UV light this weight loss was found at about 330 °C. In both cases the degradation temperature is slightly lower than for the dry MFC mat. Remarkably, for the cured composites with 15% and 22% PI a dramatic shift of MFC degradation to lower temperatures (280–290 °C) is detected. This effect is attributed to the action of the cationic photoinitiator, which upon irradiation generates a strong Brønsted acid. In the composites, the acid, which is meant to promote the cationic polymerization of the epoxidized monomer, is supposed to attack also the cellulose microfibrils, hydrolyzing them. Moreover, this mechanism may explain why a higher amount of photoinitiator is required to cure the composites with respect to the neat resin. In the
Supplementary Information (
Figure S3) is reported the thermogravimetric analysis of MFC impregnated with only photoinitiator and irradiated, showing a highly reduced thermal stability. For the uncured composite, the first degradation step characteristic of the uncured resin was present, overlapping with the MFC degradation, and the second and third degradation steps were visible with maximum rates at 370 °C and 445 °C. A similar pattern was shown for the UV irradiated MFC-EC-5%PI composite, demonstrating that the crosslinking reaction did not advance for this composite. For the cured MFC-EC-15%PI and MFC-EC-22%PI composites the first degradation step of the resin was not present, while the second and third steps became more marked; particularly the second step, which appeared at 370–380 °C, showed an increased intensity, as seen for the cured EC-15%PI resin. The residual weights of 7–12% shown by these composites is compatible with a presence of 20–30 wt.% of MFC.
The EC monomer, as well as the EC-5%PI and EC-15%PI fully cured resins, were analyzed by DSC. The DSC thermograms are shown in
Figure 11.
The curve of the uncured EC resin showed an inflection at −49 °C attributable to the glass transition. After curing, the glass transition temperature of the EC-5%PI resin increased to −4 °C; no other transitions could be remarked above this temperature. Furthermore, for both the uncured EC and the EC-5%PI cured resin no difference could be detected between the first and the second heating scan. On the other hand, for the EC-15%PI cured resin, a glass transition temperature of −7 °C was detected in the first heating scan, followed by two exothermic events that may indicate some residual reactivity. In the second heating scan the T
g increased to −3 °C, and the exothermic events were less intense. When this cured resin was tested one month after curing, the T
g was −3 °C both in the first and in the second heating scans; the exothermic events were very mild in the first heating scan, and disappeared in the second heating scan, indicating that the residual reactivity was almost completely suppressed. As a comparison, for EC thermally cured with amine hardeners, glass transition temperatures ranging from 9 °C to 158 °C were reported, depending on the type and amount of hardener used [
10,
31]. Furthermore, the maximum T
g obtained for an EC cured by electron beam with an hexafluorophosphate photoinitiator was −2.9 °C by DSC, while by DMA it was found to be around 13 °C; with an hexafluoroantimonate photoinitiator higher T
g were obtained [
13]. The glass transition temperatures shown by a cardanol epoxy prepolymer cured by UVC with hexafluorophosphate photoinitiators combined with photosensitizers were around 25−30 °C by DSC and around 20 °C by DMA [
14]. These higher results however must take into account the effect of the prepolymerization of the cardanol epoxy prepolymer on T
g.
The DSC thermograms of the composites are shown in
Figure 12. The thermogram of the uncured MFC-EC-5%PI composite showed a step corresponding to the glass transition temperature of the uncured resin at −48 °C. The thermogram of the MFC-EC-5%PI composite irradiated for 5 min showed a T
g at −44 °C, confirming that negligible cure occurred. For the MFC-EC-15%PI and the MFC-EC-22%PI composites irradiated for 5 min the T
g was in the −3 °C to 1 °C range, similarly to what shown by the fully cured resin. The first heating run of all composites, before and after curing, also showed an endothermic peak around 60–75 °C, which disappeared in the second heating run, and which can be attributed to the evaporation of residual solvent.
The storage modulus (E’) and the loss tangent (tan δ) as a function of temperature, measured for the uncured and cured composites, and for the dry MFC mat, are reported in
Figure 13. The dry MFC mat did not show prominent thermal transitions in the explored temperature range, resulting in almost flat E’ and tan δ curves. The E’ was of about 10
9 Pa in the entire temperature range. At around 50 °C, a dip in the E’ curve, which may be due to the evaporation of residual solvent, appeared for the mat dried at 100 °C, but was not present for the mat dried at 180 °C. For the composites, the thermal transitions of the resin were detected during the temperature scan. When no UV-curing was performed on the composites, the E’ before glass transition was around 10
8 Pa, and a first large peak in the tan δ curve, related to the glass transition of the resin, was present with a peak temperature that varied in the −50 °C to −35 °C range, depending on the tested specimen. A smaller peak appeared in some cases at 10 °C. This suggests that although care was taken to shorten the time between specimen preparation and testing, keeping the sample in the dark and refrigerated, some curing of the resin may have happened even without UV irradiation, as also suggested by TGA. When the MFC-EC-15%PI and MFC-EC-22%PI composites were cured for 5 min per side at 100 mW cm
−2 the peak of tan δ associated with the glass transition temperature shifted to about 12 °C. A very small peak was still visible at −52 °C, possibly due to some uncured resin residues. Above the glass transition temperature, the E’ dropped of about two orders of magnitude for uncured composites, while the decrease was smaller for the cured composites. The T
g obtained by DMA for the fully cured composite was similar to that obtained by DMA for the electron beam cured resin reported in [
13], which also presented a similar difference between the T
g detected by DSC and that measured by DMA as that reported here.
As mentioned above, MFC are hydrophilic and sensitive to water. As expected, the contact angle on the dry MFC mat was lower than 10° as soon as the drop touched the surface, while after a few seconds the drop was absorbed by the mat. The wetting by water of the MFC-EC-22%PI cured composites was hindered: the water contact angle was found to be in the 75° to 85° range, at different locations of a same specimen. This may be due to the microfibrils being covered by a thinner or thicker layer of resin at different locations of the surface, as the resin may fill the valleys of the rough MFC mat surface, as suggested also from the optical micrographs. Although the value is lower than 90° and the materials cannot be defined hydrophobic, the wettability of the composite is remarkably poor. In agreement with this result, the water uptake of the cured MFC-EC-15%PI composite after four weeks of immersion was found to be only 8%, so that the composites can be considered water resistant.