Molecular Structures and Spectral Properties of Natural Indigo and Indirubin: Experimental and DFT Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Geometry Optimization
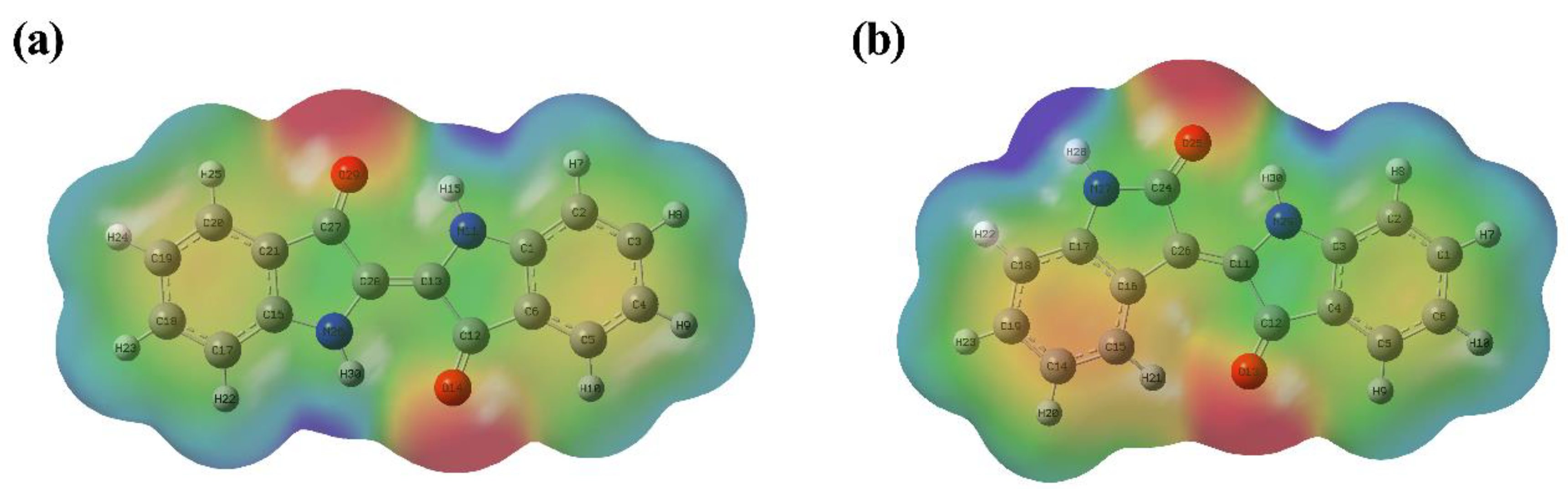
2.2. Analysis of Molecular Electrostatic Potential
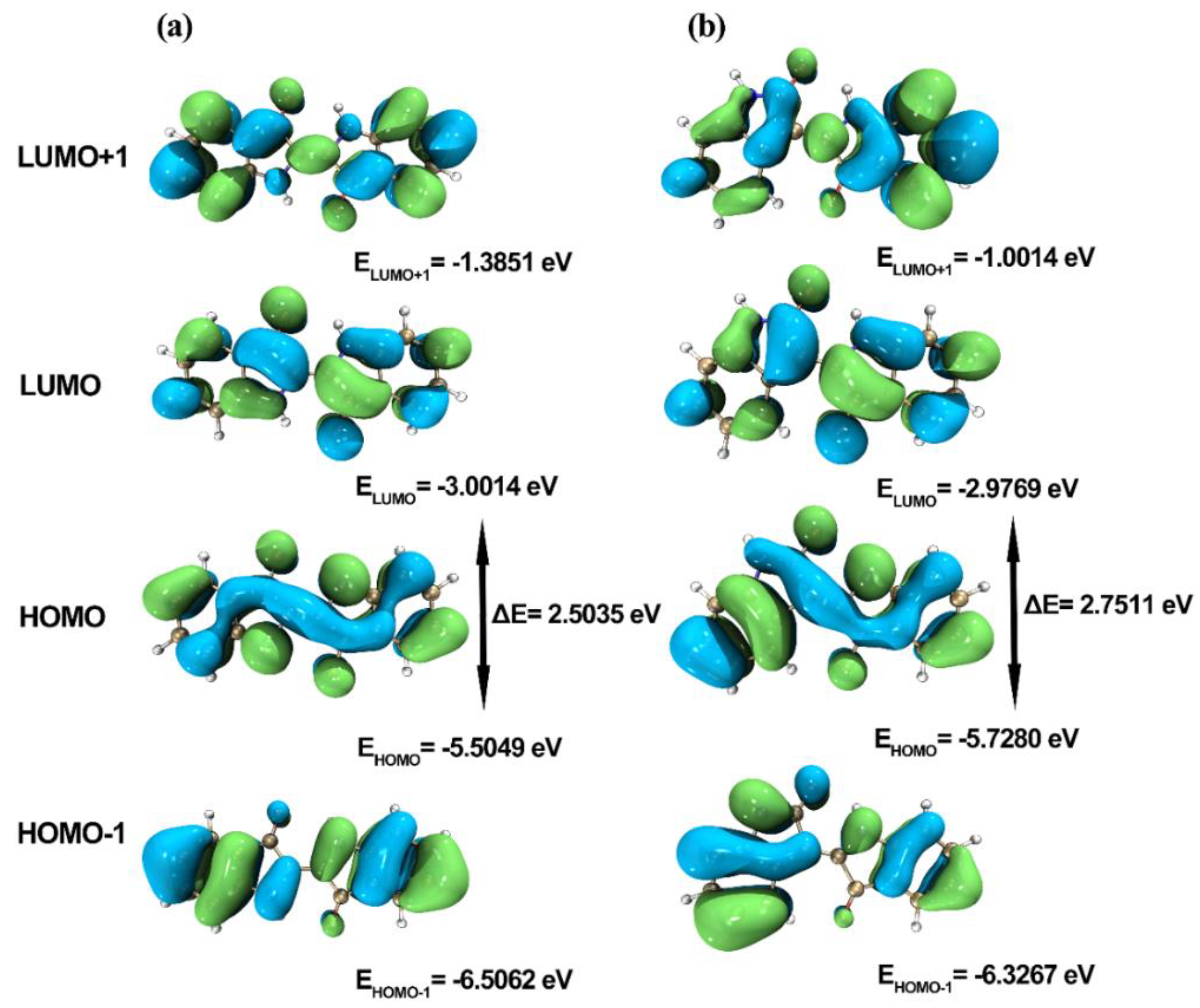
2.3. Frontier Molecular Orbitals
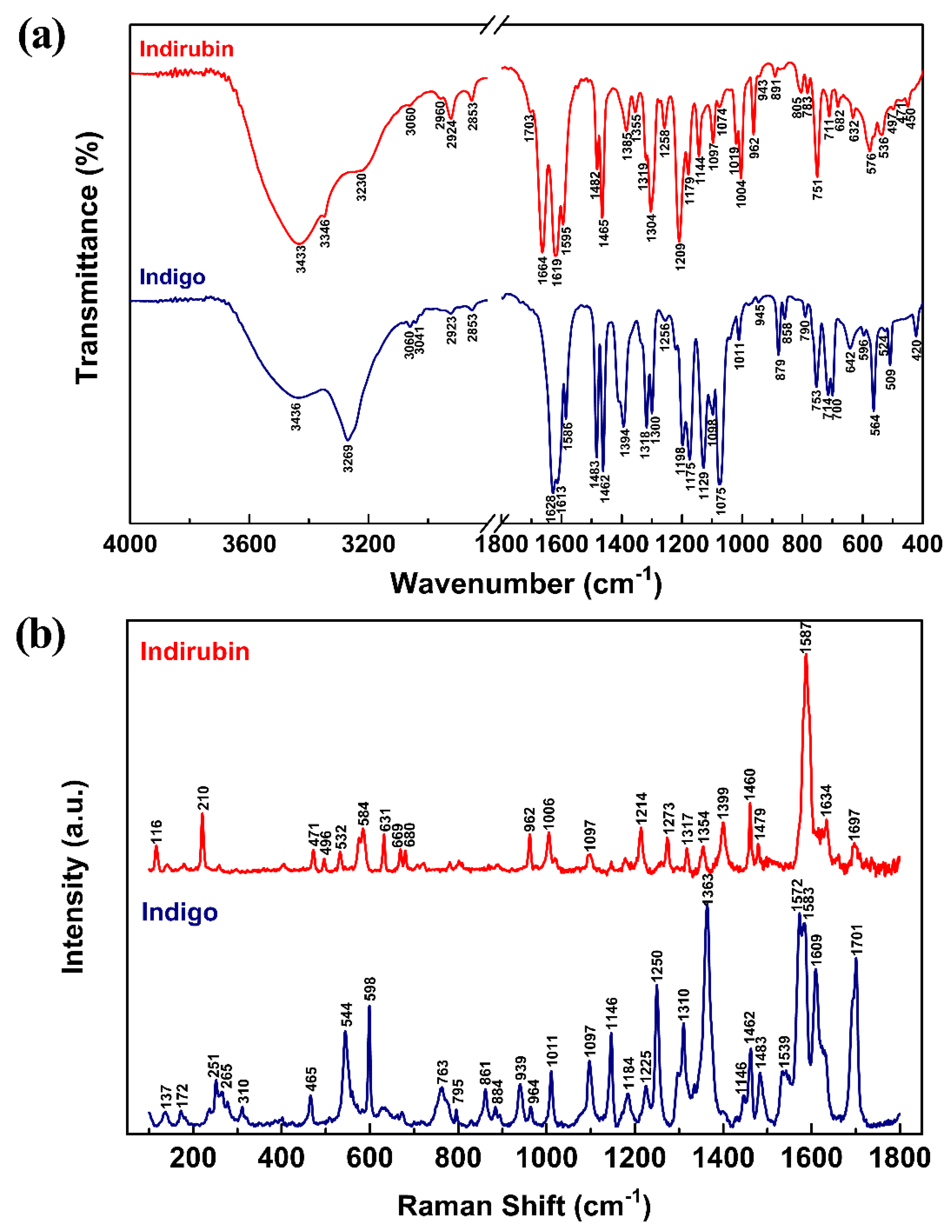
2.4. Vibrational Analysis
2.5. UV-Visible Spectra Analysis
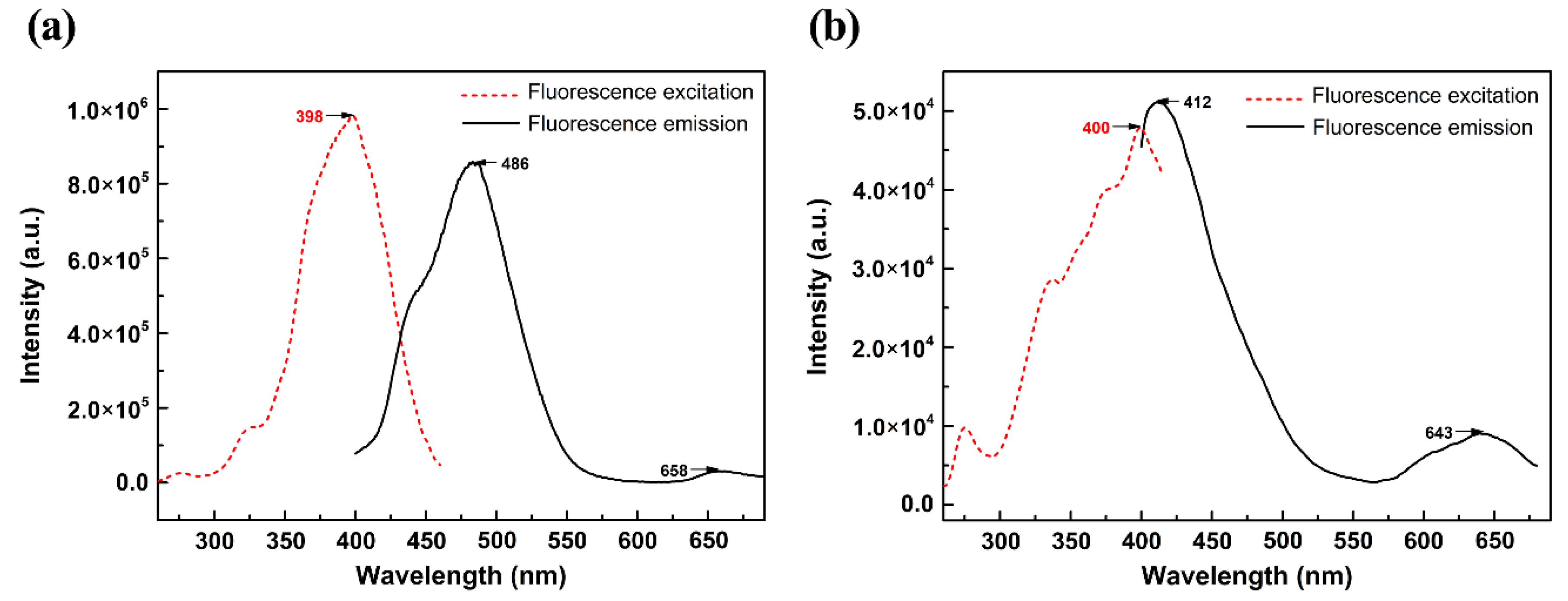
2.6. Fluorescence Spectra Analysis
3. Experimental
3.1. Materials
3.2. Instrumentation
3.2.1. Infrared Spectroscopy
3.2.2. Raman Spectroscopy
3.2.3. UV–Visible Spectroscopy
3.2.4. Fluorescence Spectrometry
3.3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Namgung, S.; Park, H.A.; Kim, J.; Lee, P.G.; Kim, B.G.; Yang, Y.H.; Choi, K.Y. Ecofriendly one-pot biosynthesis of indigo derivative dyes using CYP102G4 and PrnA halogenase. Dyes Pigment. 2019, 162, 80–88. [Google Scholar] [CrossRef]
- He, B.; Pun, A.B.; Zherebetskyy, D.; Liu, Y.; Liu, F.; Klivansky, L.M.; McGough, A.M.; Zhang, B.A.; Lo, K.; Russell, T.P.; et al. New form of an old natural dye: Bay-annulated indigo (BAI) as an excellent electron accepting unit for high performance organic semiconductors. J. Am. Chem. Soc. 2014, 136, 15093–15101. [Google Scholar] [CrossRef] [PubMed]
- Serafini, I.; Lombardi, L.; Fasolato, C.; Sergi, M.; Di, O.F.; Sciubba, F.; Montesano, C.; Guiso, M.; Costanza, R.; Nucci, L. A new multi analytical approach for the identification of synthetic and natural dyes mixtures. The case of orcein-mauveine mixture in a historical dress of a Sicilian noblewoman of nineteenth century. Nat. Prod. Res. 2017, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Süsse, P.; Steins, M.; Kupcik, V. Indigo: Crystal structure refinement based on synchrotron data. Z. Krist.-Cryst. Mater. 1988, 184, 269–273. [Google Scholar] [CrossRef]
- Christie, R.M. Indirubin: The Red Shade of Indigo, 1st ed.; Life in Progress Editions: Roscoff, France, 2006; pp. 103–108. [Google Scholar]
- Lü, H.; Liu, J.; Deng, R.; Song, J. Preparative isolation and purification of indigo and indirubin from folium isatidis by high-speed counter-current chromatography. Phytochem. Anal. 2012, 23, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Meksi, N.; Kechida, M.; Mhenni, F. Cotton dyeing by indigo with the borohydride process: Effect of some experimental conditions on indigo reduction and dyeing quality. Chem. Eng. J. 2007, 131, 187–193. [Google Scholar] [CrossRef]
- Degani, L.; Riedo, C.; Chiantore, O. Identification of natural indigo in historical textiles by GC-MS. Anal. Bioanal. Chem. 2015, 407, 1695–1704. [Google Scholar] [CrossRef]
- Pawlak, K.; Puchalska, M.; Miszczak, A.; Roså‚Oniec, E.; Jarosz, M. Blue natural organic dyestuffs--from textile dyeing to mural painting. Separation and characterization of coloring matters present in elderberry, logwood and indigo. J. Mass Spectrom. 2006, 41, 613–622. [Google Scholar] [CrossRef]
- Irimia-Vladu, M.; Glowacki, E.D.; Troshin, P.A.; Schwabegger, G.; Leonat, L.; Susarova, D.K.; Krystal, O.; Ullah, M.; Kanbur, Y.; Bodea, M.A.; et al. Indigo-a natural pigment for high performance ambipolar organic field effect transistors and circuits. Adv. Mater. 2012, 24, 375–380. [Google Scholar] [CrossRef]
- Tomkinson, J.; Bacci, M.; Picollo, M.; Colognesi, D. The vibrational spectroscopy of indigo: A reassessment. Vib. Spectrosc. 2009, 50, 268–276. [Google Scholar] [CrossRef]
- Xue, Y.; Gong, X.; Xiao, H.; Tian, H. A density functional theory study of indigo and its derivatives. Acta Chim. Sinica 2004, 62, 963–968. [Google Scholar]
- Cervantes-Navarro, F.; Glossman-Mitnik, D. DFT study of the effect of substituents on the absorption and emission spectra of indigo. Chem. Cent. J. 2012, 6, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Jacquemin, D.; Preat, J.; Wathelet, V.; Perpete, E.A. Substitution and chemical environment effects on the absorption spectrum of indigo. J. Chem. Phys. 2006, 124, 74104. [Google Scholar] [CrossRef] [PubMed]
- Tatsch, E.; Schrader, B. Near-infrared fourier transform Raman spectroscopy of indigoids. J. Raman Spectrosc. 1995, 26, 467–473. [Google Scholar] [CrossRef]
- Rio, M.S.D.; Picquart, M.; Haro-Poniatowski, E.; Elslande, E.V.; Uc, V.H. On the Raman spectrum of Maya blue. J. Raman Spectrosc. 2010, 37, 1046–1053. [Google Scholar]
- Ricci, M.; Lofrumento, C.; Becucci, M.; Castellucci, E.M. The Raman and SERS spectra of indigo and indigo-Ag2 complex: DFT calculation and comparison with experiment. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2018, 188, 141–148. [Google Scholar] [CrossRef]
- El-Mansy, M.A.M.; Yahia, I.S.; Alfaify, S. Conformational and vibrational properties of indigo dye: DFT approach. Org. Opto-Elect. 2015, 1, 39–45. [Google Scholar]
- Baran, A.; Fiedler, A.; Schulz, H.; Baranska, M. In situ Raman and IR spectroscopic analysis of indigo dye. Anal. Methods 2010, 2, 1372–1376. [Google Scholar] [CrossRef]
- Rondao, R.; Seixas de Melo, J.; Schaberle, F.A.; Voss, G. Excited state characterization of a polymeric indigo. Phys. Chem. Chem. Phys. 2012, 14, 1778–1783. [Google Scholar] [CrossRef]
- Sele, A.; Bremner, J.B.; Willis, A.C.; Haritakun, R.; Grifth, R.; Keller, P.A. A cascade synthetic route to new bioactive spiroindolinepyrido [1,2-a]indolediones from indirubin. Tetrahedron 2016, 47, 8357–8367. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Yang, M.; Liang, X. Study on the quantum chemistry of indigo and indirubin. J. Sichuan Univ. 2004, 41, 143–147. [Google Scholar]
- Xue, Y.; Peng, Z.; Yin, X. DFT study on structure activity relationship for indirubin and its isomer. Chem. Res. 2005, 16, 85–87. [Google Scholar]
- Perpète, E.A.; Preat, J.; André, J.-M.; Jacquemin, D. An ab initio study of the absorption spectra of indirubin, isoindigo, and related derivatives. J. Phys. Chem. A 2006, 110, 5629–5635. [Google Scholar] [CrossRef] [PubMed]
- Prandraud, H. Structure cristalline de l’indirubine. J. Acta Cryst. 1961, 14, 901–908. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comb. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Amat, A.; Rosi, F.; Miliani, C.; Sgamellotti, A.; Fantacci, S. Theoretical and experimental investigation on the spectroscopic properties of indigo dye. J. Mol. Struct. 2011, 993, 43–51. [Google Scholar] [CrossRef]
- Platania, E.; Lofrumento, C.; Lottini, E.; Azzaro, E.; Ricci, M.; Becucci, M. Tailored micro-extraction method for Raman/SERS detection of indigoids in ancient textiles. Anal. Bioanal. Chem. 2015, 407, 6505–6514. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision, B.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 6; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |



| Structural Parameters | Indigo | Structural Parameters | Indirubin | ||
|---|---|---|---|---|---|
| Internuclear Distance (Å) | Calc. | Exp. [5] | Internuclear Distance (Å) | Calc. | Exp. [25] |
| C(1)-C(2) | 1.393 | 1.393 | C(1)-C(2) | 1.391 | 1.43 |
| C(2)-C(3) | 1.394 | 1.387 | C(2)-C(3) | 1.396 | 1.41 |
| C(3)-C(4) | 1.403 | 1.386 | C(3)-C(4) | 1.401 | 1.41 |
| C(4)-C(5) | 1.390 | 1.392 | C(4)-C(5) | 1.392 | 1.41 |
| C(5)-C(6) | 1.393 | 1.387 | C(5)-C(6) | 1.391 | 1.38 |
| C(1)-C(6) | 1.412 | 1.404 | C(1)-C(6) | 1.405 | 1.39 |
| C(16)-C(17) | 1.393 | 1.393 | C(16)-C(17) | 1.397 | 1.41 |
| C(17)-C(18) | 1.394 | 1.387 | C(17)-C(18) | 1.396 | 1.42 |
| C(18)-C(19) | 1.403 | 1.386 | C(18)-C(19) | 1.395 | 1.41 |
| C(19)-C(20) | 1.390 | 1.392 | C(19)-C(20) | 1.389 | 1.41 |
| C(20)-C(21) | 1.393 | 1.387 | C(20)-C(21) | 1.384 | 1.36 |
| C(21)-C(16) | 1.412 | 1.404 | C(21)-C(16) | 1.417 | 1.40 |
| C(6)-C(12) | 1.468 | 1.424 | C(6)-C(12) | 1.471 | 1.48 |
| C(12)-C(13) | 1.493 | 1.495 | C(12)-C(13) | 1.524 | 1.53 |
| C(21)-C(27) | 1.468 | 1.424 | C(16)-C(28) | 1.456 | 1.51 |
| C(27)-C(28) | 1.493 | 1.495 | C(27)-C(28) | 1.496 | 1.48 |
| N(11)-C(13) | 1.379 | 1.382 | N(11)-C(13) | 1.374 | 1.40 |
| N(26)-C(28) | 1.379 | 1.382 | N(26)-C(27) | 1.377 | 1.38 |
| C(1)-N(11) | 1.387 | 1.380 | C(1)-N(11) | 1.389 | 1.40 |
| C(12)-O(14) | 1.226 | 1.240 | C(12)-O(14) | 1.216 | 1.21 |
| C(13)-C(28) | 1.358 | 1.342 | C(13)-C(28) | 1.368 | 1.31 |
| C(27)-O(29) | 1.226 | 1.240 | C(27)-O(29) | 1.226 | 1.25 |
| Bond angle (°) | Calc. | Exp. [5] | Bond angle (°) | Calc. | Exp. [25] |
| C(1)-N(11)-C(13) | 109.969 | 108.63 | C(1)-N(11)-C(13) | 111.654 | 110 |
| C(6)-C(12)-O(14) | 130.765 | 129.13 | C(6)-C(12)-O(14) | 128.603 | 128 |
| C(1)-N(11)-H(15) | 127.957 | 130.30 | C(1)-N(11)-H(15) | 128.015 | -- |
| C(1)-C(6)-C(12) | 107.676 | 106.03 | C(1)-C(6)-C(12) | 107.884 | 106 |
| C(6)-C(12)-C(13) | 104.199 | 107.23 | C(6)-C(12)-C(13) | 104.402 | 106 |
| C(12)-C(13)-N(11) | 108.355 | 107.23 | C(12)-C(13)-N(11) | 106.487 | 106 |
| C(6)-C(1)-N(11) | 109.800 | 110.83 | C(6)-C(1)-N(11) | 109.573 | 112 |
| C(16)-C(21)-C(27) | 107.676 | 106.03 | C(16)-C(21)-N(26) | 109.065 | 110 |
| C(21)-C(27)-C(28) | 104.199 | 107.23 | N(26)-C(27)-C(28) | 105.995 | 108 |
| C(27)-C(28)-N(26) | 108.355 | 107.23 | C(27)-C(28)-C(16) | 106.622 | 105 |
| C(16)-N(26)-C(28) | 109.969 | 108.63 | C(21)-N(26)-C(27) | 111.544 | 111 |
| C(21)-C(16)-N(26) | 109.800 | 110.83 | C(21)-C(16)-N(28) | 106.774 | 107 |
| N(11)-C(13)-C(28) | 125.977 | 124.93 | N(11)-C(13)-C(28) | 123.244 | 122 |
| C(21)-C(27)-O(29) | 130.765 | 129.13 | N(26)-C(27)-O(29) | 125.564 | 126 |
| C(16)-N(26)-H(30) | 127.957 | 130.30 | C(21)-N(26)-H(30) | 126.055 | -- |
| Thermodynamic Parameters (298 K) | Indigo | Indirubin |
|---|---|---|
| ELUMO+1 (eV) | −1.3851 | −1.0014 |
| ELUMO (eV) | −3.0014 | −2.9769 |
| EHOMO (eV) | −5.5049 | −5.7280 |
| EHOMO−1 (eV) | −6.5062 | −6.3267 |
| ΔELUMO−HOMO (eV) | 2.5035 | 2.7511 |
| Mode | Indigo | Indirubin | ||||||
|---|---|---|---|---|---|---|---|---|
| Experimental | Scaled Computational | Tentative Assignment 1 | Experimental | Scaled Computational | Tentative Assignment 1 | |||
| IR | Raman | IR | Raman | |||||
| 1 | -- | -- | 3487.34 | νs N–H | 3433 | -- | 3530.01 | ν N–H |
| 2 | 3436 | -- | 3486.36 | νas N–H | 3346 | -- | 3398.59 | ν N–H |
| 3 | -- | -- | 3091.68 | νs C–H | 3230 | -- | 3124.20 | ν C–H |
| 4 | 3269 | -- | 3091.54 | νas C–H | 3060 | -- | 3091.65 | ν C–H |
| 5 | 3060 | -- | 3082.39 | νas C–H | 2960 | -- | 3084.44 | ν C–H |
| 6 | 3041 | -- | 3082.38 | ν C–H | 2924 | -- | 3083.39 | ν C–H |
| 7 | -- | -- | 3076.08 | νs C–H | -- | -- | 3076.65 | ν C–H |
| 8 | 2923 | -- | 3076.07 | νas C–H | 2853 | -- | 3073.11 | ν C–H |
| 9 | 2853 | -- | 3063.62 | νas C–H | -- | -- | 3064.40 | ν C–H |
| 10 | -- | -- | 3063.60 | νs C–H | -- | -- | 3061.94 | ν C–H |
| 11 | -- | 1701 | 1707.32 | ν C=C + ν C=O | 1703 | -- | 1710.64 | νs C=O + ν C=C |
| 12 | 1628 | -- | 1654.71 | ν C=O + δ N–H | 1664 | 1697 | 1686.27 | νas C=O + δ N–H |
| 13 | -- | 1609 | 1626.18 | ν C=C +ν C=O + ν C–C | -- | -- | 1611.54 | ν C=C + ν C–C + δ C–H |
| 14 | 1613 | -- | 1597.98 | ν C–Cring + δ C–H | 1619 | 1634 | 1603.14 | ν C–C + δ C–H |
| 15 | -- | 1583 | 1590.48 | ν C=C +ν C=O + ν C–C | -- | -- | 1582.29 | ν C–C + δ N–H + δ C–H |
| 16 | -- | 1572 | 1572.14 | ν C–Cring + δ N–H | 1595 | 1587 | 1578.62 | ν C–C + ν C=C + δ N–H |
| 17 | 1586 | -- | 1571.73 | ν C–Cring | -- | -- | 1566.60 | ν C–C + δ C–H |
| 18 | 1483 | -- | 1468.51 | ν C–C + δ C–H + δ N–H | 1482 | 1479 | 1466.72 | ν C–C + δ C–H + ν N–H |
| 19 | -- | 1483 | 1467.51 | ν C–C + δ C–H + δ N–H | -- | -- | 1461.41 | ν C–C + δ C–H + ν N–H |
| 20 | -- | 1462 | 1441.41 | ν C–Cring + δ C–H | 1465 | 1460 | 1447.83 | δ C–H + ν C–C |
| 21 | 1462 | -- | 1439.48 | ν C–Cring + δ C–H | -- | -- | 1442.37 | ν C–C + δ C–H |
| 22 | 1394 | -- | 1399.78 | δ N–H + δ C–H | 1385 | 1399 | 1390.31 | δ N–H + δ C–H + ν C–C |
| 23 | -- | 1363 | 1361.05 | δ N–H + δ C–H | 1355 | 1354 | 1364.94 | δ N–H + δ C–H + ν C-N |
| 24 | -- | 1340.83 | ν C–C + ν C-N + δ C–H | -- | -- | 1324.29 | ν C–C + ν C-N + δ C–H | |
| 25 | 1318 | -- | 1310.37 | ν C–Cring + δ C–H | -- | 1317 | 1308.36 | ν C–C + δ C–H |
| 26 | -- | 1310 | 1299.01 | δ C–H + ν C–Cring | 1319 | -- | 1276.29 | δ C–H + ν C-N + ν C–C |
| 27 | 1300 | -- | 1276.19 | δ C–H | 1304 | 1273 | 1272.39 | δ C–H + δ N–H + ν C–C |
| 28 | 1256 | -- | 1236.96 | ν C-N +ν C–C + δ C–H | -- | -- | 1255.96 | δ C–H + ν C-N + ν C–C |
| 29 | -- | 1250 | 1226.74 | ν C-N +ν C–C + δ C–H | -- | -- | 1225.67 | δ N–H + δ C–H + ν C-N |
| 30 | -- | 1225 | 1201.04 | δ N–H + δ C–H | -- | -- | 1198.25 | δ N–H + δ C–H |
| 31 | 1198 | -- | 1169.72 | ν C–C + δ C–H | 1258 | 1214 | 1173.76 | δ C=C + δ N–H + δ C–H |
| 32 | -- | 1184 | 1168.23 | ν C–C + δ C–H | -- | -- | 1167.49 | δ C–H + δ N–H + ν C–C |
| 33 | 1175 | -- | 1151.86 | δ C–H + δ N–H + ν C-N | 1209 | -- | 1163.41 | δ C–H + δ N–H + ν C-N |
| 34 | -- | 1146 | 1134.32 | δ C–H | -- | -- | 1140.71 | δ C–H + δ N–H |
| 35 | 1129 | -- | 1122.09 | δ C–H + δ N–H + ν C-N | 1179 | -- | 1132.16 | δ C–H + δ N–H |
| 36 | -- | 1097 | 1080.76 | δ C–H | 1144 | -- | 1085.63 | δ C–H + ν C–Cring |
| 37 | 1098 | -- | 1079.58 | δ C–C + δ C–H | 1097 | -- | 1079.63 | δ C–H + ν C–Cring |
| 38 | 1075 | -- | 1051.04 | ν C–C + δ C–C + δ N–H | 1074 | 1097 | 1012.68 | δ C–H + ν C–Cring |
| 39 | -- | 1011 | 1002.59 | δ C–H | 1019 | -- | 1003.28 | δ C–H + ν C–Cring |
| 40 | 1011 | -- | 1000.51 | ν C–Cring + δ C–H | 1004 | 1006 | 979.67 | ν C–C + δ N–H |
| 41 | -- | -- | 958.97 | τ C–H | -- | -- | 966.82 | γ C–H |
| 42 | -- | -- | 958.94 | ω C–H | -- | -- | 960.63 | γ C–H |
| 43 | 945 | -- | 933.35 | ω C–H | 962 | 962 | 944.80 | δ C–C + δ N–H + δ C–H |
| 44 | -- | -- | 933.23 | τ C–H | -- | -- | 934.39 | γ C–H |
| 45 | -- | 939 | 920.76 | δ C–C + δ C–H | 943 | -- | 925.97 | γ C–H |
| 46 | 879 | -- | 856.57 | δ C–C + δ C-N + δ N–H | 891 | -- | 868.21 | δ C–C + δ N–H + δ C–H |
| 47 | -- | 861 | 845.06 | δ C–C + δ N–H + δ C-N | -- | -- | 855.81 | δ C–C + δ N–H + δ C–H |
| 48 | 858 | -- | 841.66 | ω C–H | -- | -- | 844.28 | γ C–H |
| 49 | -- | -- | 840.65 | τ C–H | -- | -- | 842.74 | γ C–H |
| 50 | -- | -- | 790.96 | γ C–H + γ C–C | -- | -- | 794.85 | γ C–C + γ C–H |
| 51 | -- | -- | 781.77 | γ C–H + γ C–C | 805 | -- | 794.81 | ν C–C + δ C–C + δ C–H |
| 52 | 790 | -- | 754.56 | δ C–C + δ C–H | 783 | -- | 764.1 | γ C=O + γ C-N + γ C–H |
| 53 | -- | 763 | 748.02 | δ C=C + δ C-N | -- | -- | 741.01 | γ C–H |
| 54 | 753 | -- | 738.83 | ω C–H | 751 | -- | 739.95 | γ C–H + γ C–Cring |
| 55 | -- | -- | 738.23 | τ C–H | -- | -- | 729.31 | γ C–H + γ C–Cring |
| 56 | -- | -- | 709.24 | τ C–H | -- | -- | 708.19 | δ C–C + δ C–H + δ N-C |
| 57 | 714 | -- | 694.24 | δ C–C + δ C-N | 711 | -- | 705.46 | γ C–H + γ N–H + γ C–C |
| 58 | 700 | -- | 694.01 | γ C–C + γ C–H | 682 | 680 | 669.41 | δ C–C + δ C–H + δ C-N |
| 59 | -- | 598 | 666.65 | δ N–H + δ C–C + δ C–H | -- | 669 | 661.22 | δ C–C + δ C–H + δ N–H |
| 60 | -- | -- | 591.00 | δ N–H + δ C–C + δ C–H | 632 | 631 | 623.64 | δ C–C + δ C–H + δ N–H |
| 61 | 642 | -- | 587.87 | δ C–C +δ N–H +δ C=O | 576 | -- | 609.31 | γ N–H |
| 62 | 596 | -- | 553.11 | δ C–C + δ C–H | -- | 584 | 572.84 | δ C–C + δ N–H + δ C=O |
| 63 | -- | -- | 552.34 | τ N–H + τ C–H | -- | -- | 565.92 | δ C–H + δ C=O + δ N–H |
| 64 | 564 | -- | 551.13 | ω N–H +ω C–H | -- | -- | 556.22 | γ C–H + γ C–C |
| 65 | -- | 544 | 538.73 | δ C–C + δ C–H | -- | -- | 540.03 | γ C–H + γ N–H + γ C–C |
| 66 | 524 | -- | 504.10 | δ N–H +δ C–C | 536 | 532 | 527.13 | δ C–C + δ N–H + δ C–H |
| 67 | -- | -- | 472.33 | τ N–H | 497 | 496 | 494.40 | γ N–H |
| 68 | 509 | -- | 465.70 | ω N–H | 471 | 471 | 483.97 | δ C–H + δ C–C + δ C-N |
| 69 | -- | 465 | 443.70 | γ C=C +τ C–H + τ N–H | 450 | -- | 449.31 | γ N–H + γ C–H + γ C–C |
| 70 | 420 | -- | 418.13 | ω C–H | -- | -- | 424.65 | γ N–H + γ C–H + γ C–C |
| 71 | -- | -- | 395.95 | τ C–H | -- | -- | 398.24 | γ N–H + γ C–H + γ C–C |
| 72 | -- | -- | 372.22 | ω C-N + ω C–C | -- | -- | 359.51 | δ C=O + δ C–H + δ C–C |
| 73 | -- | 310 | 300.02 | δ C–H + δ C=O + δ C–C | -- | -- | 342.35 | γ C–H + γ N–H + γ C–C |
| 74 | -- | -- | 280.89 | δ C–H + δ C=O + δ C–C | -- | -- | 290.95 | δ C=O + δ N–H + δ C–H |
| 75 | -- | 265 | 251.28 | τ C-N + τ C–H + τ C–C | -- | -- | 268.83 | γ C-N + γ C–C + γ C–H |
| 76 | -- | 251 | 242.76 | ρ C–H | -- | 210 | 250.91 | δ C=O + δ C–C |
| 77 | -- | -- | 223.97 | ω C–C +ω C-N | -- | -- | 235.48 | γ C–C + γ C-N + γ C–H |
| 78 | -- | 172 | 222.05 | δ C=O + δ C–H + δ N–H | -- | -- | 215.76 | δ C–H |
| 79 | -- | -- | 155.98 | ω C=O +ω C–H | -- | -- | 144.67 | γ C–H + γ N-C + γ C=O |
| 80 | -- | 137 | 153.90 | τ C=O + τ C–H | -- | 116 | 139.47 | γ C–H + γ N-C + γ C=O |
| 81 | -- | -- | 93.86 | τ C–H + τ ring | -- | -- | 108.76 | γ C–H + γ C=O |
| 82 | -- | -- | 81.45 | τ ring | -- | -- | 103.18 | δ C–H |
| 83 | -- | -- | 67.99 | δ C–H + δ N–H | -- | -- | 61.04 | τ ring |
| 84 | -- | -- | 29.29 | ω ring | -- | -- | 38.09 | ω ring |
| Molecular | Wavelength (nm) | Transition Coefficient | Oscillator Strengths (f) | Assignment 1 | |
|---|---|---|---|---|---|
| Experimental | Computational | ||||
| Indigo | 612 | 579.77 | 0.70811 | 0.3342 | H → L |
| 268 | 272.14 | 0.66336 | 0.5277 | H−1 → L+1 | |
| Indirubin | 547 | 536.36 | 0.70152 | 0.2591 | H → L |
| 293 | 259.85 | 0.52164 | 0.1432 | H−1 → L+1 | |
| Molecular | Wavelength (nm) | Transition Coefficient | Oscillator Strengths (f) | Assignment 1 | |
|---|---|---|---|---|---|
| Experimental | Computational | ||||
| Indigo | 486 (8.57 × 105) | 438.35 | 0.68114 | 0.7352 | L → H |
| Indirubin | 412 (5.10 × 104) | 433.26 | 0.67598 | 0.6871 | L → H |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, Z.; Sun, J.; Liu, Y. Molecular Structures and Spectral Properties of Natural Indigo and Indirubin: Experimental and DFT Studies. Molecules 2019, 24, 3831. https://doi.org/10.3390/molecules24213831
Ju Z, Sun J, Liu Y. Molecular Structures and Spectral Properties of Natural Indigo and Indirubin: Experimental and DFT Studies. Molecules. 2019; 24(21):3831. https://doi.org/10.3390/molecules24213831
Chicago/Turabian StyleJu, Zixin, Jie Sun, and Yanping Liu. 2019. "Molecular Structures and Spectral Properties of Natural Indigo and Indirubin: Experimental and DFT Studies" Molecules 24, no. 21: 3831. https://doi.org/10.3390/molecules24213831
APA StyleJu, Z., Sun, J., & Liu, Y. (2019). Molecular Structures and Spectral Properties of Natural Indigo and Indirubin: Experimental and DFT Studies. Molecules, 24(21), 3831. https://doi.org/10.3390/molecules24213831







