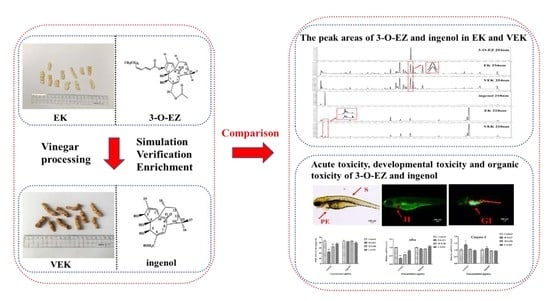
Toxicity Reduction of Euphorbia kansui Stir-Fried with Vinegar Based on Conversion of 3-O-(2′E,4′Z-Decadi-enoyl)-20-O-acetylingenol
Abstract
:1. Introduction
2. Results
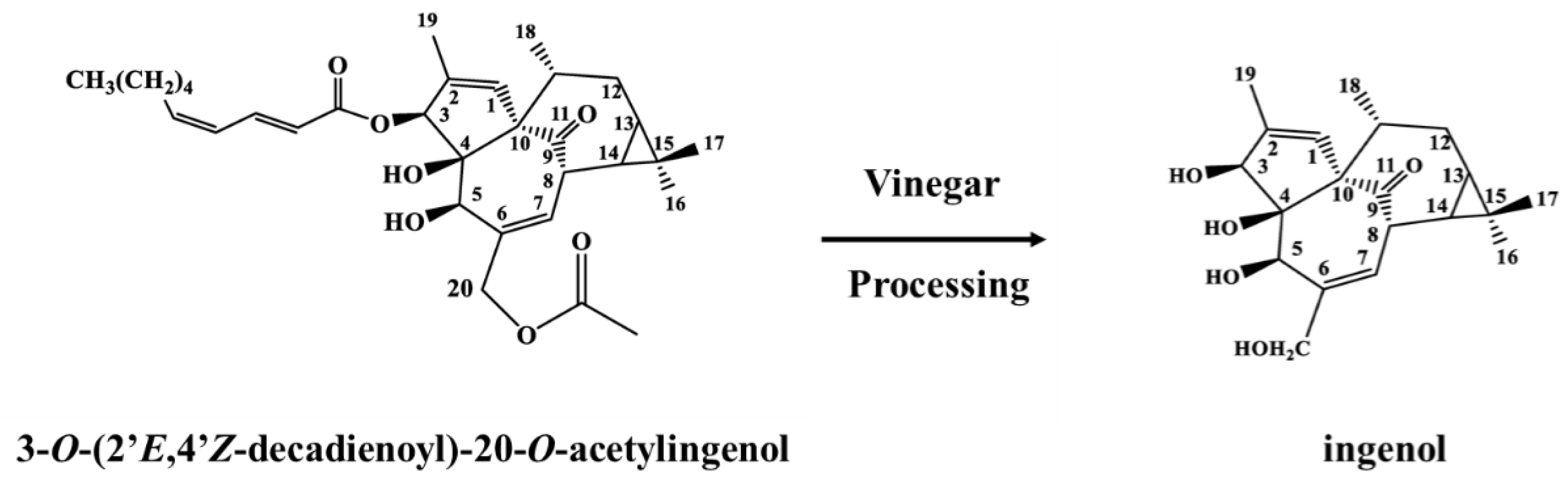
2.1. Identification of Hydrolysate
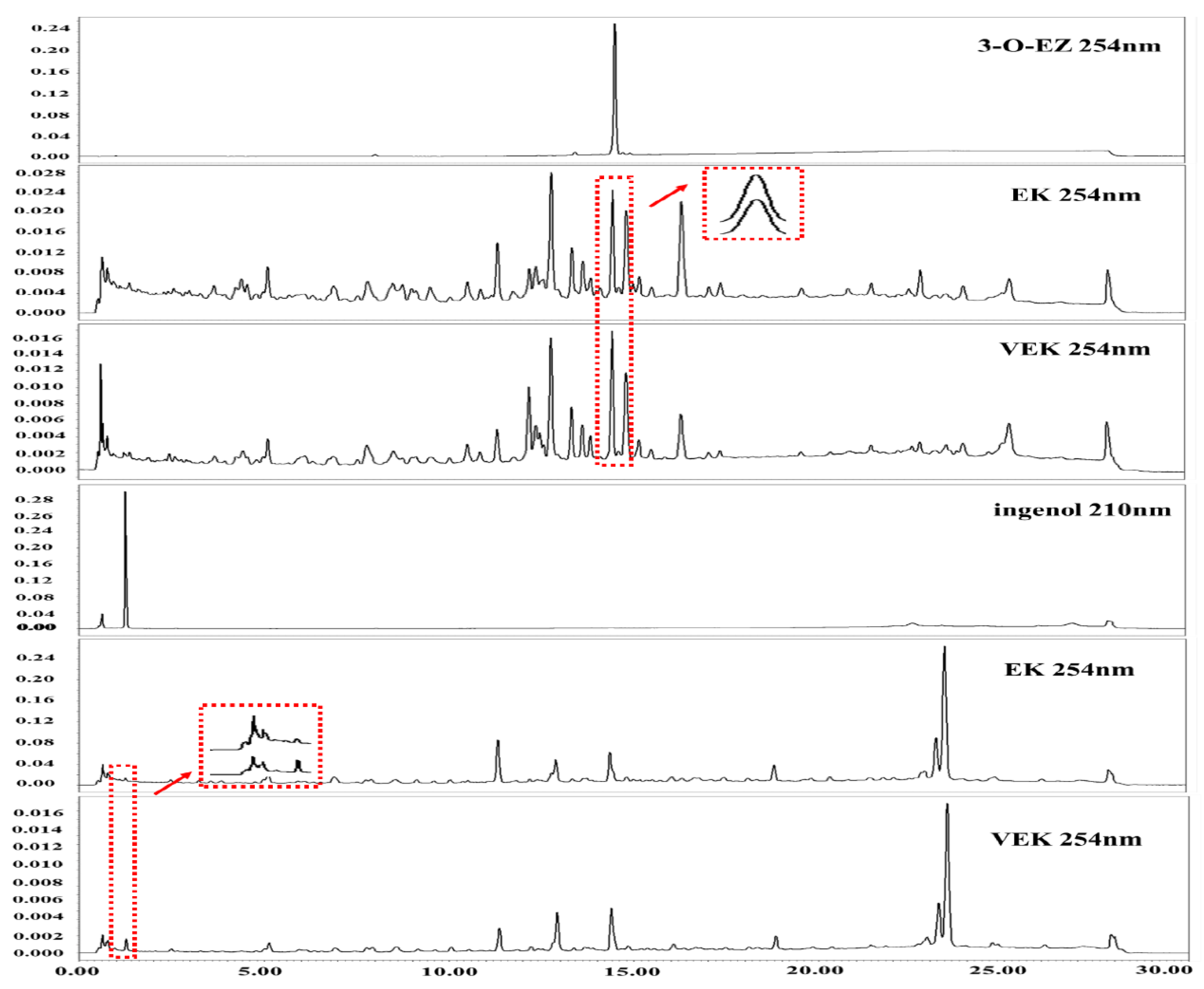
2.2. Peak Areas Comparison of 3-O-EZ and Ingenol in EK and VEK
2.3. Comparison of Toxicity of 3-O-EZ and Ingenol
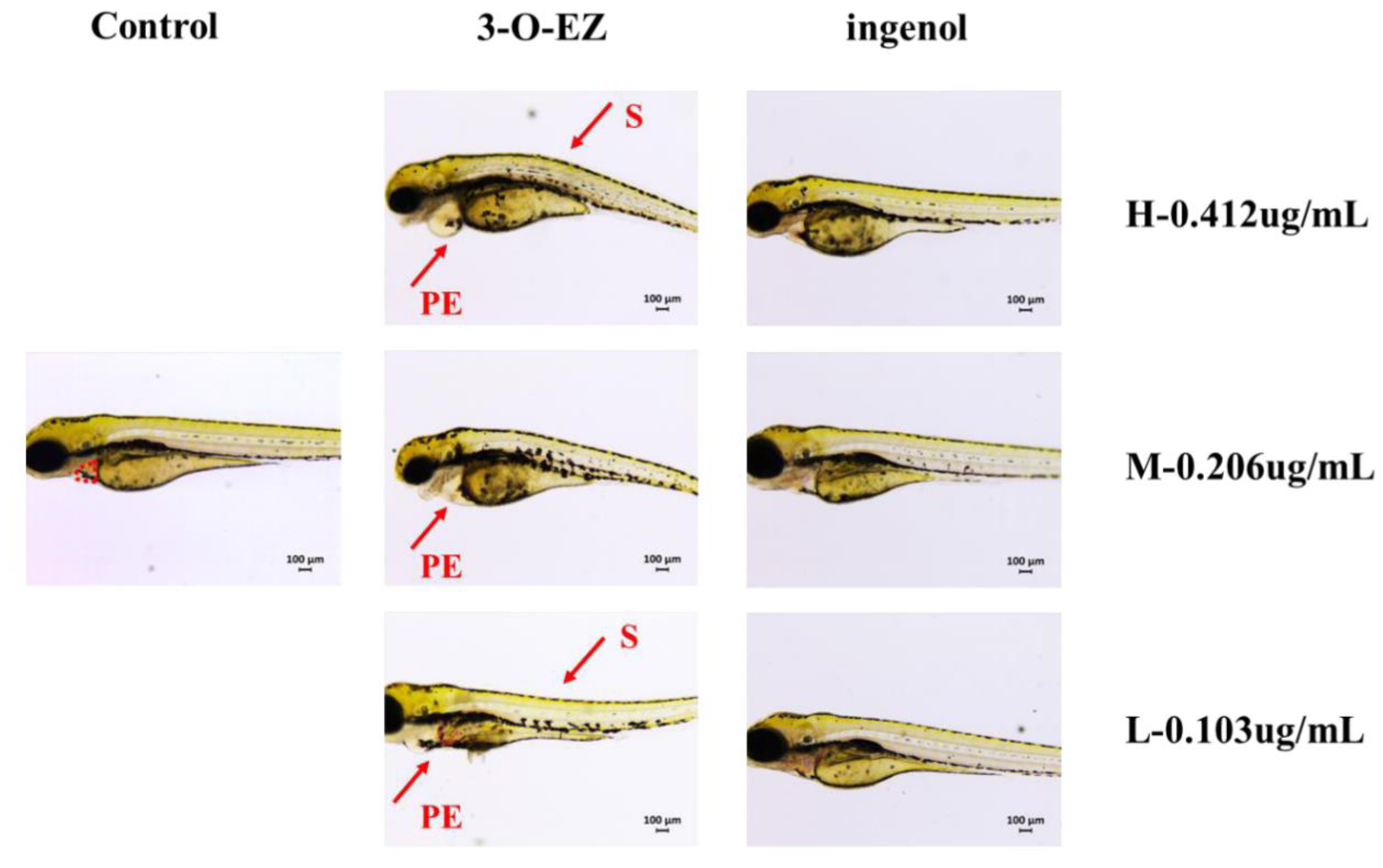
2.3.1. Acute Toxicity
2.3.2. Developmental Toxicity
2.3.3. Organic Toxicity
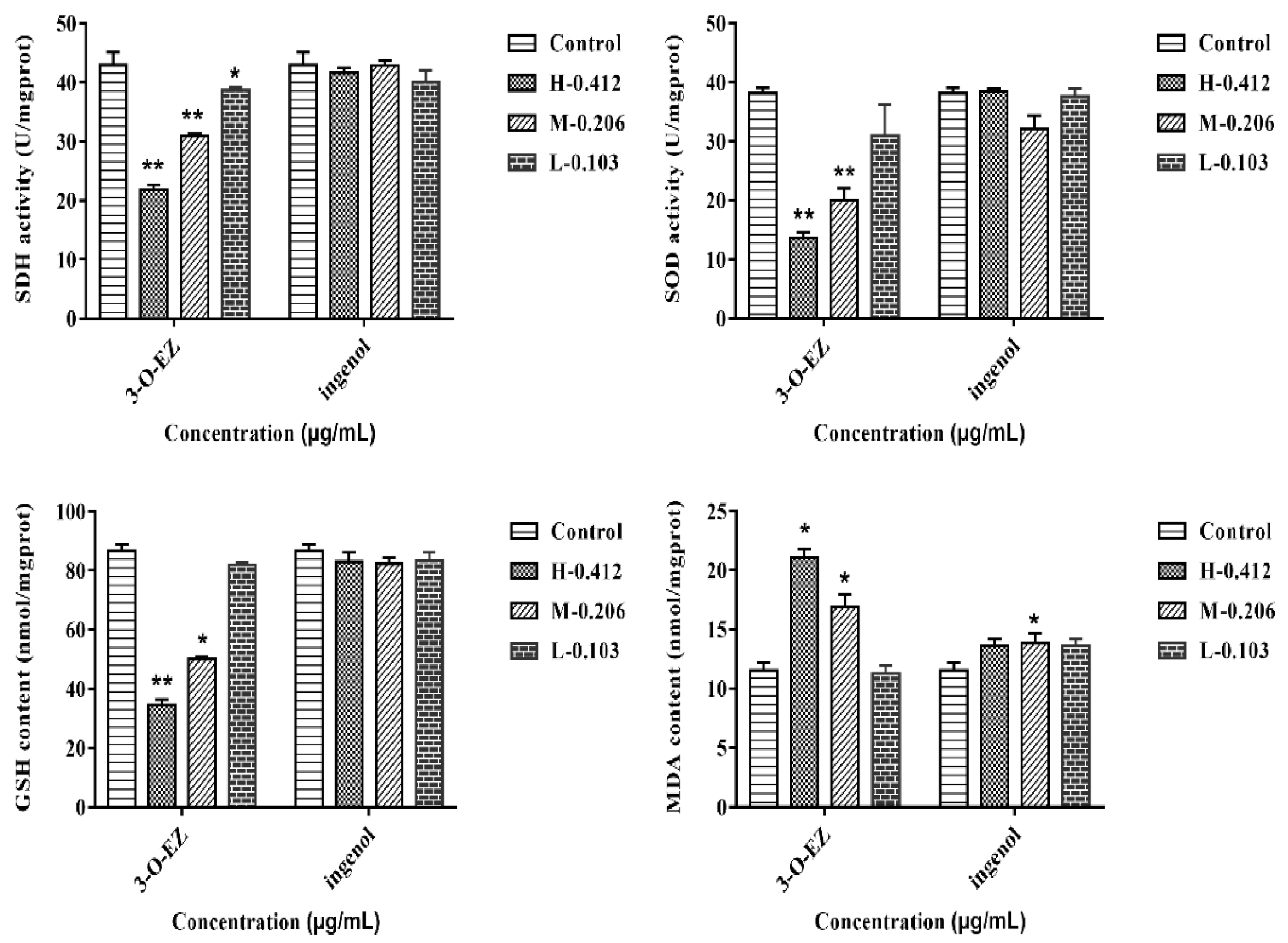
2.3.4. Measurement of SDH, SOD Activities, GSH and MDA Content
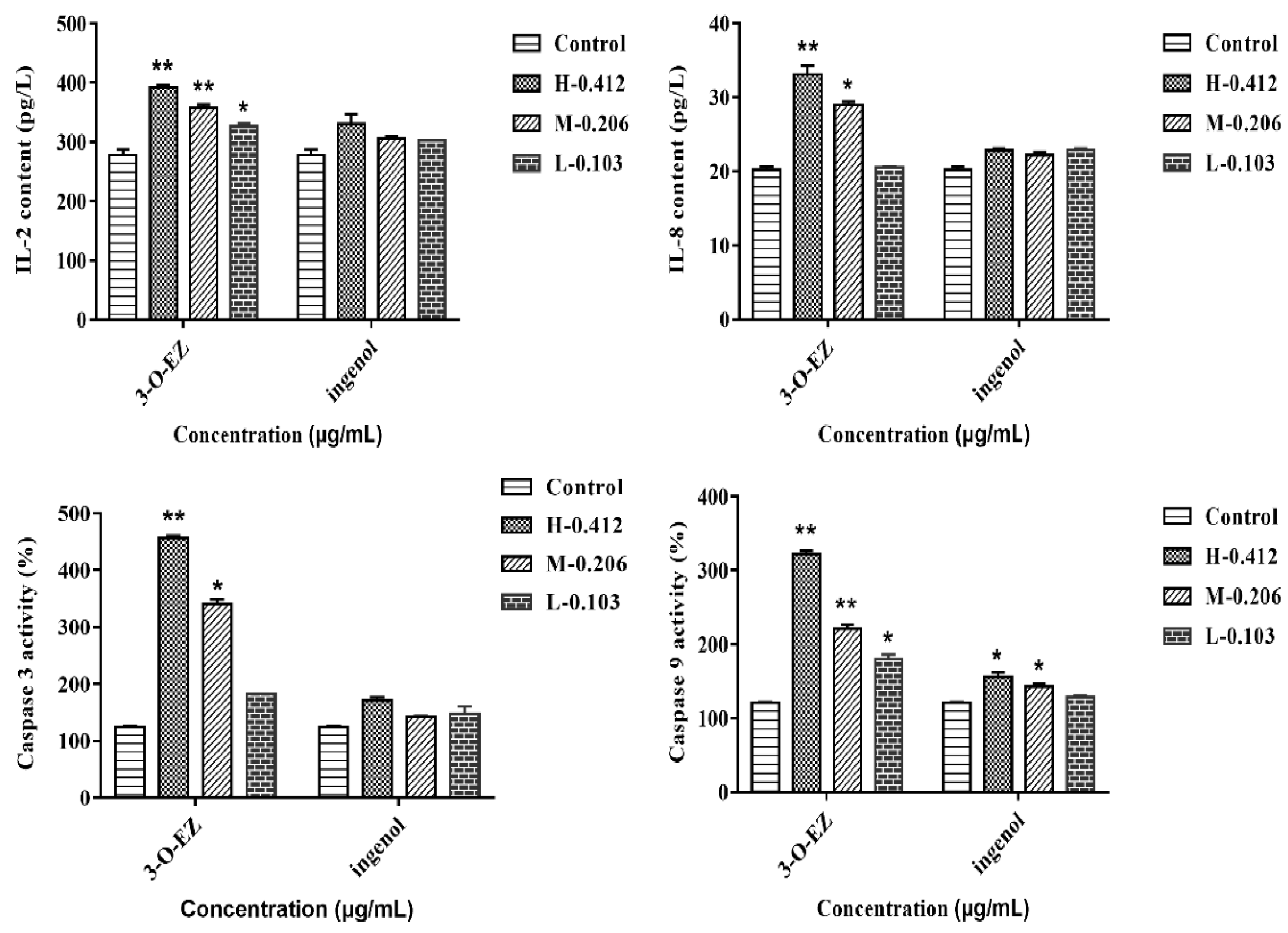
2.3.5. Measurement of Caspase Activity, IL-2 and IL-8 Content
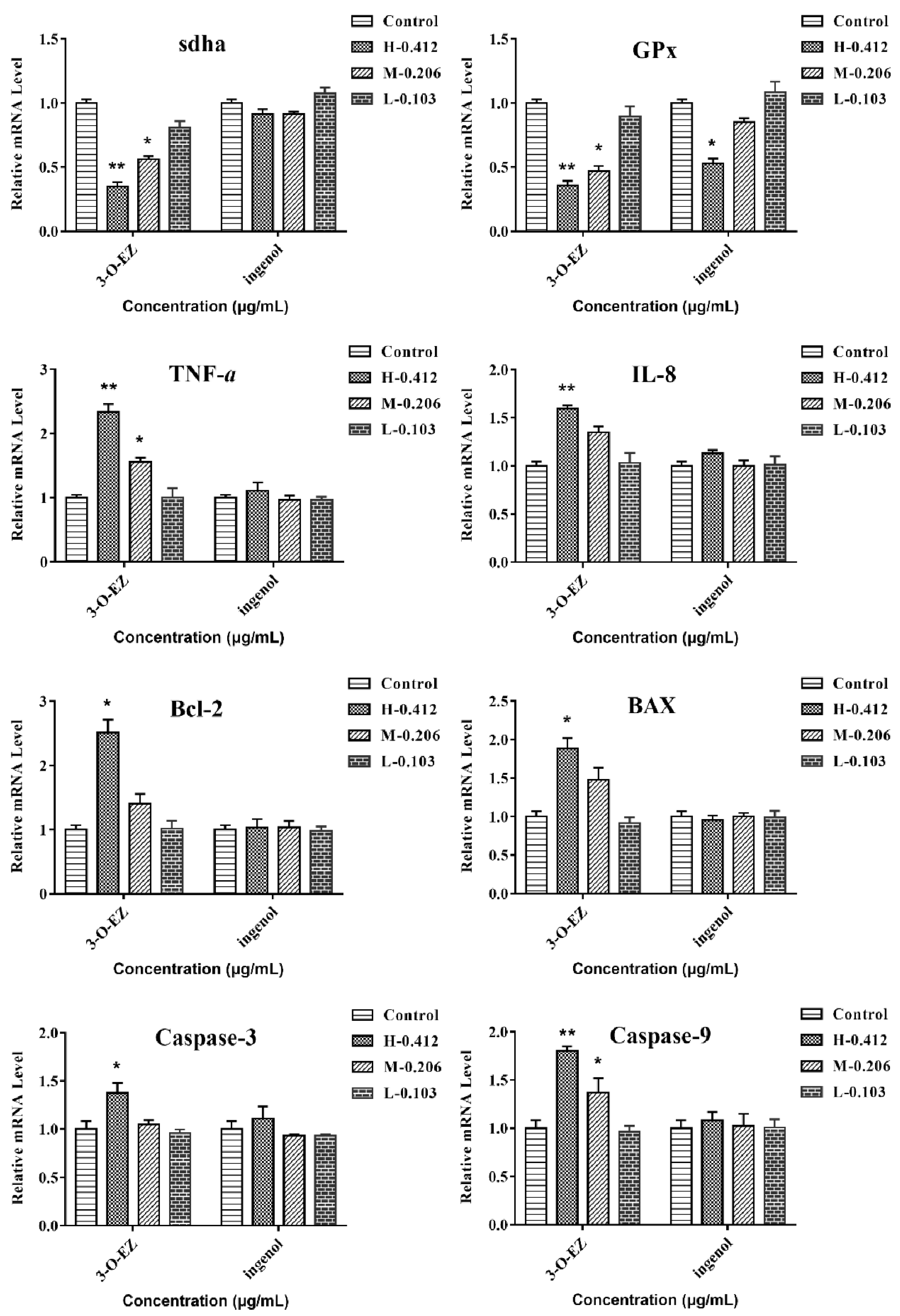
2.3.6. Gene Expression
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Zebrafish Embryos
4.3. Materials
4.4. Preparation and Analysis of Hydrolysate
4.4.1. Conversion Reaction
4.4.2. Purification and Analysis of Hydrolysate
4.5. Determination of the Peak Areas of 3-O-EZ and Hydrolysate in EK and VEK
4.6. Comparison of Toxicity of 3-O-EZ and Hydrolysate
4.6.1. Acute Toxicity
4.6.2. Developmental Toxicity
4.6.3. Cardiotoxicity
4.6.4. Gastrointestinal Toxicity
4.6.5. SDH and SOD Activity, GSH and MDA Content Analysis
4.6.6. Caspase Activity as well as IL-2 and IL-8 Content Analysis
4.6.7. Gene Expression Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EK | Euphorbia kansui |
| VEK | EK stir-fried with vinegar |
| SV | Sinus venosus |
| BA | Bulbus arteriosus |
| GI | gastrointestinal |
| SDH | Succinate Dehydrogenase |
| SOD | Superoxide Dismutase |
| GSH | Glutathione |
| MDA | Malondialdehyde |
| IL-2 | Interleukin-2 |
| IL-8 | Interleukin-8 |
| EBO | electro-thermostatic blast oven |
| DAD | Diode Array Detector |
| PBS | Phosphate Buffered Saline |
| qRT-PCR | quantitative real-time polymerase chain reaction |
| ANOVA | One-way analysis of variance |
References
- Liu, M.; Cao, Y.; Lv, D.; Zhang, W.; Zhu, Z.; Zhang, H.; Chai, Y. Effect of processing on the alkaloids in Aconitum tubers by HPLC-TOF/MS. J. Pharm. Anal. 2017, 7, 170–175. [Google Scholar] [CrossRef]
- Wu, X.J.; Ma, F.S.; Zhen, G.L.; Liang, Y.; Chen, H.B.; Liu, D. Toxicity research progress on indole alkaloids of strychni semen. Pharm. Clin. Chin. Mate 2016, 32, 231–235. [Google Scholar]
- Guo, J.; Meng, H.; Li, H.H.; Wang, Q.F. Determination of strychnine, brucine, strychnine N-oxide, and brucine N-oxide in plasma samples after the oral administration of processed semen strychni extract by high-performance liquidchromatography with ultrasound-assisted mixed cloud point extraction. J. Sep. Sci. 2016, 39, 2553–2561. [Google Scholar] [CrossRef]
- Cai, B.C.; Hattori, M.; Namba, T. Processing of nux vomica. II. Changes in alkaloid composition of the seeds of Strychnos nux-vomica on traditional drug-processing. Chem. Pharm. Bull. (Tokyo) 1990, 38, 1295. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2015; Volume 1, p. 88. [Google Scholar]
- Liu, Q.; Li, W.; Huang, L.; Asada, Y.; Morris-Natschke, S.L.; Chen, C.H.; Lee, K.H.; Koike, K. Identification, structural modification, and dichotomous effects on human immunodeficiency virus type 1 (HIV-1) replication of ingenane esters from Euphorbia Kansui. Eur. J. Med. Chem. 2018, 156, 618–627. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, K.C.; Lou, J.W.; Guo, S.C.; Zhang, Y.; Yao, W.F.; Tang, Y.P.; Wu, J.H.; Zhang, L. Simultaneous quantification of twelve compounds in ethyl acetate extracts of Euphorbia kansui before and after fry-baked with vinegar by UPLC-MS/MS and its toxic effect on zebrafish. J. Pharm. Biomed. Anal. 2018, 155, 169–176. [Google Scholar] [CrossRef]
- Lou, J.W.; Cao, L.L.; Zhang, Q.; Jiang, D.J.; Yao, W.F.; Bao, B.H.; Cao, Y.D.; Tang, Y.P.; Zhang, L.; Wang, K.; et al. The toxicity and efficacy evaluation of different fractions of Kansui-stir fried with vinegar on Walker-256 tumor-bearing malignant ascites effusion rats and normal rats. J. Ethnopharmacol. 2018, 219, 257–268. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Q.R.; Lou, J.W.; Chen, P.D.; Yao, W.F.; Tao, W.W.; Tang, Y.P.; Dai, G.C.; Wang, K.; Zhang, L. Chemical Constituents from Euphorbia kansui. Molecules 2017, 22, 2176. [Google Scholar] [CrossRef]
- Hou, J.J.; Shen, Y.; Yang, Z.; Fang, L.; Cai, L.Y.; Yao, S.; Long, H.L.; Wu, W.Y.; Guo, D.A. Anti-proliferation activity of terpenoids isolated from Euphorbia kansui inhuman cancer cells and their structure-activity relationship. Chin. J. Nat. Med. 2017, 15, 766–774. [Google Scholar]
- Zhang, L.; Gao, L.; Li, Z.J.; Yan, X.J.; Yang, Y.J.; Tang, Y.P.; Cao, Y.D.; Ding, A.W. Bio-guided isolation of the cytotoxic terpenoids from the roots of Euphorbia kansui against human normal cell lines L-O2 and GES-1. Int. J. Mol. Sci. 2012, 13, 11247–11259. [Google Scholar] [CrossRef]
- Gao, J.; Gao, L.; Zhang, L.; Yao, W.F.; Cao, Y.D.; Bao, B.H.; Ding, A.W. 3-O-(2′E,4′Z-decadienoyl)-20-O-acetylingenol induces apoptosis in intestinal epithelial cells of rats via mitochondrial pathway. J. Ethnopharmacol. 2015, 174, 331–338. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.J.; Shu, X.Y.; Tang, Y.P.; Ding, A.W.; Duan, J.A. Determination of three diterpenoids in Euphorbia Kansui and its vinegar products by HPLC. Chin. Tradit. Herb. Drugs 2010, 41, 1987–1990. [Google Scholar]
- Shu, X.; Jiang, X.W.; Cheng, B.C.Y.; Ma, S.C.; Chen, G.Y.; Yu, Z.L. Ultra-performance liquid chromatography-quadrupole/time-of-flight mass spectrometry analysis of the impact of processing on toxic components of Kansui Radix. BMC Complement. Altern. Med. 2016, 16, 73. [Google Scholar] [CrossRef]
- Le Fol, V.; Brion, F.; Hillenweck, A.; Perdu, E.; Bruel, S.; Aït-Aïssa, S.; Cravedi, J.-P.; Zalko, D. Comparison of the In Vivo Biotransformation of Two Emerging Estrogenic Contaminants, BP2 and BPS, in Zebrafish Embryos and Adults. Int. J. Mol. Sci. 2017, 18, 704. [Google Scholar] [CrossRef]
- Zhao, C.J.; Jia, Z.; Li, E.W.; Zhao, X.; Han, T.; Tian, J.H.; Li, F.R.; Zou, D.X.; Lin, R.C. Hepatotoxicity evaluation of Euphorbia kansui on zebrafish larvae in vivo. Phytomedicine 2019, 62, 152959. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, H.; Yan, Y.; Lv, P.; Wu, W. Toxicity Evaluation and Biomarker Selection with Validated Reference Gene in Embryonic Zebrafish Exposed to Mitoxantrone. Int. J. Mol. Sci. 2018, 19, 3516. [Google Scholar] [CrossRef]
- Jia, Z.J.; Ding, Y.L. New diterpenoids from Euphorbia sieboldiana. Planta. Med. 1991, 57, 569–571. [Google Scholar] [CrossRef]
- Jiao, W.; Lu, L.; Deng, M.C.; Shao, H.W.; LU, R.H. Studies on Chemical constituents in seeds of Euphorbia lathyris. Chin. Tradit. Herb. Drugs 2010, 41, 181–187. [Google Scholar]
- Lu, J.L.; Zhu, X.U.; Liu, L.; Zhang, X.Q.; Xu, L.; Chen, Z.P.; Li, W.D. Study on Fingerprints of Traditional Chinese Medicine Processing Accessories of Rice Vinegar. J. Nanjing Univ. Chin. Med. 2017, 33, 463–469. [Google Scholar]
- Yan, Z.Y.; Huang, X.Y.; Xie, Y.Z.Y.; Song, M.R.; Zhu, K.; Ding, S.Y. Macrolides induce severe cardiotoxicity and developmental toxicity in zebrafish embryos. Sci. Total Environ. 2019, 649, 1414–1421. [Google Scholar] [CrossRef]
- Liu, H.C.; Wu, Q.; Chu, T.Y.; Mo, Y.Y.; Cai, S.Y.; Chen, M.L.; Zhu, G.N. High-dose acute exposure of paraquat induces injuries of swim bladder, gastrointestinal tract and liver via neutrophil-mediated ROS in zebrafish and their relevance for human health risk assessment. Chemosphere 2018, 205, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, W.X.; Mu, X.Y.; Qi, S.Z.; Fu, B.; Wang, C.J. Biological response of zebrafish embryos after short-term exposure to thifluzamide. Sci. Rep. 2016, 6, 38485. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.Z.; Chen, H.X.; Cui, W.; Guo, X.G.; Fang, J.S.; Liu, A.L.; Chen, Y.L.; Lee, S.M.Y. FGF2 Prevents Sunitinib-Induced Cardiotoxicity in Zebrafish and Cardiomyoblast H9c2 Cells. Cardiovasc. Toxicol. 2016, 16, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, L.; Wang, Y.; He, B.N.; Kong, B.D.; Zhu, J.B.; Jin, Y.X.; Fu, Z.W. Evaluation of development, locomotor behavior, oxidative stress, immune responses and apoptosis in developing zebrafish (Danio rerio) exposed to TBECH (tetrabromoethylcyclohexane). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 217, 106–113. [Google Scholar] [CrossRef]
- Yao, H.Z.; Xu, X.; Zhou, Y.; Xu, C. Impacts of isopyrazam exposure on the development of early-life zebrafish (Danio rerio). Environ. Sci. Pollut. Res. Int. 2018, 25, 23799–23808. [Google Scholar] [CrossRef]
- Metryka, E.; Chibowska, K.; Gutowska, I.; Falkowska, A.; Kupnicka, P.; Barczak, K.; Chlubek, D.; Baranowska-Bosiacka, I. Lead (Pb) Exposure Enhances Expression of Factors Associated with Inflammation. Int. J. Mol. Sci. 2018, 19, 1813. [Google Scholar] [CrossRef]
- Nantachit, N.; Sunintaboon, P.; Ubol, S. Responses of primary human nasal epithelial cells to EDIII-DENV stimulation: The first step to intranasal dengue vaccination. Virol. J. 2016, 13, 142. [Google Scholar] [CrossRef]
- Chen, J.-R.; Lai, Y.-H.; Tsai, J.-J.; Hsiao, C.-D. Live Fluorescent Staining Platform for Drug-Screening and Mechanism-Analysis in Zebrafish for Bone Mineralization. Molecules 2017, 22, 2068. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, K.; Hassan, H.M.; Guo, H.L.; Ding, P.P.; Han, L.W.; He, Q.X.; Chen, W.Y.; Hsiao, C.D.; Zhang, L.Y.; et al. Liver Fatty Acid Binding Protein Deficiency Provokes Oxidative Stress, Inflammation, and Apoptosis-Mediated Hepatotoxicity Induced by Pyrazinamide in Zebrafish Larvae. Antimicrob. Agents Chemother. 2016, 60, 7347–7356. [Google Scholar] [Green Version]
Sample Availability: Samples of the compounds.3-O-(2′E,4′Z-decadienoyl)-20-O-acetylingenol and ingenol are available from the authors. |
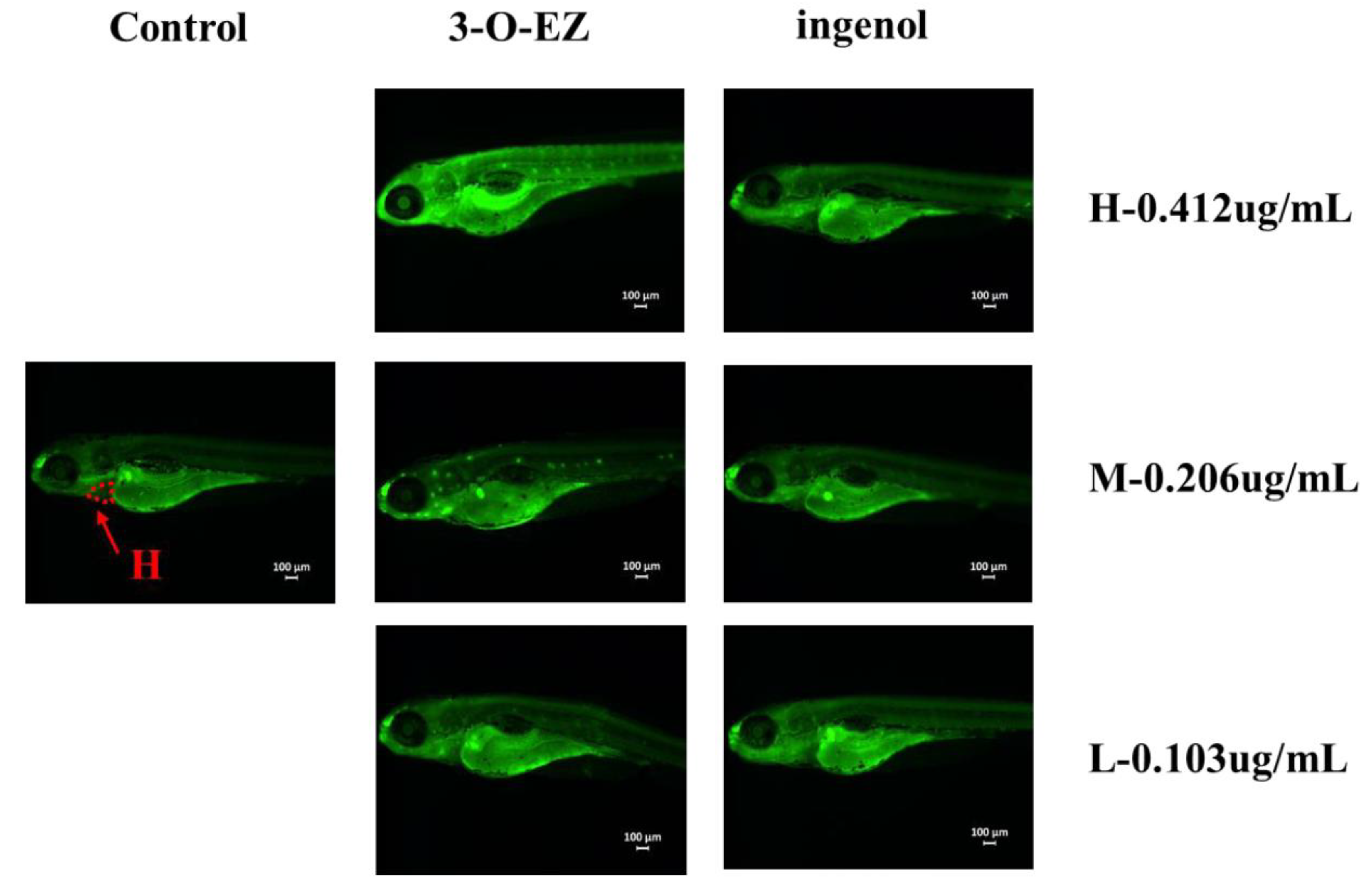
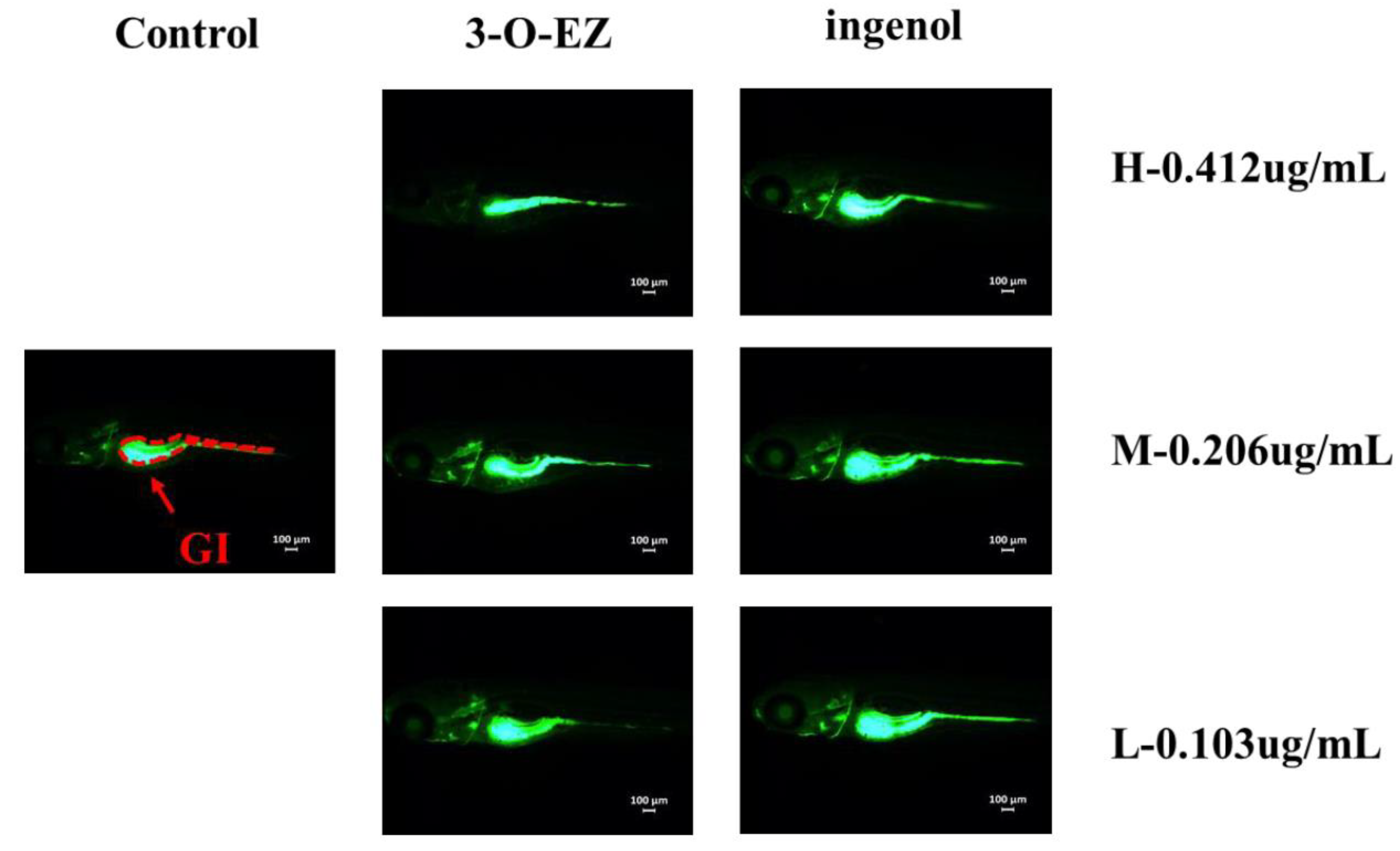
| Group | Concentration (μg/mL) | Heart Rate | SV-BA |
|---|---|---|---|
| Control | 0.1%(DMSO) | 52.90 ± 1.52 | 171.09 ± 4.08 |
| 3-O-EZ | 0.412 | 26.20 ± 1.99 ** | 286.32 ± 3.04 ** |
| 0.206 | 38.80 ± 1.93 ** | 263.79 ± 2.23 ** | |
| 0.103 | 50.60 ± 1.83 | 193.24 ± 2.68 * | |
| ingenol | 0.412 | 51.60 ± 1.35 | 194.48 ± 1.48 |
| 0.206 | 51.80 ± 1.99 | 171.62 ± 3.44 | |
| 0.103 | 52.50 ± 2.27 | 174.34 ± 2.38 |
| Group | Concentration (μg/mL) | Gastrointestinal Motility Rate | Gastrointestinal Area |
|---|---|---|---|
| Control | 0.1%(DMSO) | 10.50 ± 0.97 | 97,771 ± 1073 |
| 3-O-EZ | 0.412 | 3.90 ± 0.99 ** | 61,222 ± 2012 ** |
| 0.206 | 7.20 ± 1.03 ** | 86,111 ± 1962 ** | |
| 0.103 | 9.20 ± 1.40 | 93,591 ± 1411 ** | |
| ingenol | 0.412 | 10.10 ± 1.10 | 101,825 ± 1835 |
| 0.206 | 9.80 ± 1.14 | 102,635 ± 2335 | |
| 0.103 | 10.60 ± 1.35 | 101,366 ± 1618 |
| Gene | Primer | References | |
|---|---|---|---|
| β-actin | Forward | 5′-AGAGCTATGAGCTGCCTGACG-3′ | [30] |
| Reverse | 5′-CCGCAAGATTCCATACCCA-3′ | ||
| sdha | Forward | 5′-TGGTATGCCGTTCAGCCGTA-3′ | [23] |
| Reverse | 5′-GGCCAAGTCTTTGGCATTGG-3′ | ||
| GPx | Forward | 5′-AGATGTCATTCCTGCACACG-3′ | [23] |
| Reverse | 5′-AAGGAGAAGCTTCCTCAGCC-3′ | ||
| TNF-α | Forward | 5′-GCTGGATCTTCAAAGTCGGGTGTA-3 | [30] |
| Reverse | 5′-TGTGAGTCTCAGCACACTTCCATC-3′ | ||
| IL-8 | Forward | 5′-GTCGCTGCATTGAAACAGAA-3′ | [23] |
| Reverse | 5′-CTTAACCCATGGAGCAGAGG-3′ | ||
| Bcl-2 | Forward | 5′-TCACTCGTTCAGACCCTCAT-3′ | [30] |
| Reverse | 5′-ACGCTTTCCACGCACAT-3′ | ||
| Bax | Forward | 5′-GGCTATTTCAACCAGGGTTCC-3′ | [30] |
| Reverse | 5′-TGCGAATCACCAATGCTGT-3′ | ||
| Caspase-3 | Forward | 5′-CCGCTGCCCATCACTA-3′ | [23] |
| Reverse | 5′-ATCCTTTCACGACCATCT-3′ | ||
| Caspase-9 | Forward | 5′-CTGAGGCAAGCCATAATCG-3′ | [30] |
| Reverse | 5′-AGAGGACATGGGAATAGCGT-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Zhang, Y.; Zhou, S.-K.; Wang, K.; Zhang, M.; Chen, P.-D.; Yao, W.-F.; Tang, Y.-P.; Wu, J.-H.; Zhang, L. Toxicity Reduction of Euphorbia kansui Stir-Fried with Vinegar Based on Conversion of 3-O-(2′E,4′Z-Decadi-enoyl)-20-O-acetylingenol. Molecules 2019, 24, 3806. https://doi.org/10.3390/molecules24203806
Zhang Q, Zhang Y, Zhou S-K, Wang K, Zhang M, Chen P-D, Yao W-F, Tang Y-P, Wu J-H, Zhang L. Toxicity Reduction of Euphorbia kansui Stir-Fried with Vinegar Based on Conversion of 3-O-(2′E,4′Z-Decadi-enoyl)-20-O-acetylingenol. Molecules. 2019; 24(20):3806. https://doi.org/10.3390/molecules24203806
Chicago/Turabian StyleZhang, Qiao, Yi Zhang, Shi-Kang Zhou, Kan Wang, Min Zhang, Pei-Dong Chen, Wei-Feng Yao, Yu-Ping Tang, Jian-Hua Wu, and Li Zhang. 2019. "Toxicity Reduction of Euphorbia kansui Stir-Fried with Vinegar Based on Conversion of 3-O-(2′E,4′Z-Decadi-enoyl)-20-O-acetylingenol" Molecules 24, no. 20: 3806. https://doi.org/10.3390/molecules24203806
APA StyleZhang, Q., Zhang, Y., Zhou, S.-K., Wang, K., Zhang, M., Chen, P.-D., Yao, W.-F., Tang, Y.-P., Wu, J.-H., & Zhang, L. (2019). Toxicity Reduction of Euphorbia kansui Stir-Fried with Vinegar Based on Conversion of 3-O-(2′E,4′Z-Decadi-enoyl)-20-O-acetylingenol. Molecules, 24(20), 3806. https://doi.org/10.3390/molecules24203806