Synthesis of New Fused Heterocyclic 2-Quinolones and 3-Alkanonyl-4-Hydroxy-2-Quinolones
Abstract
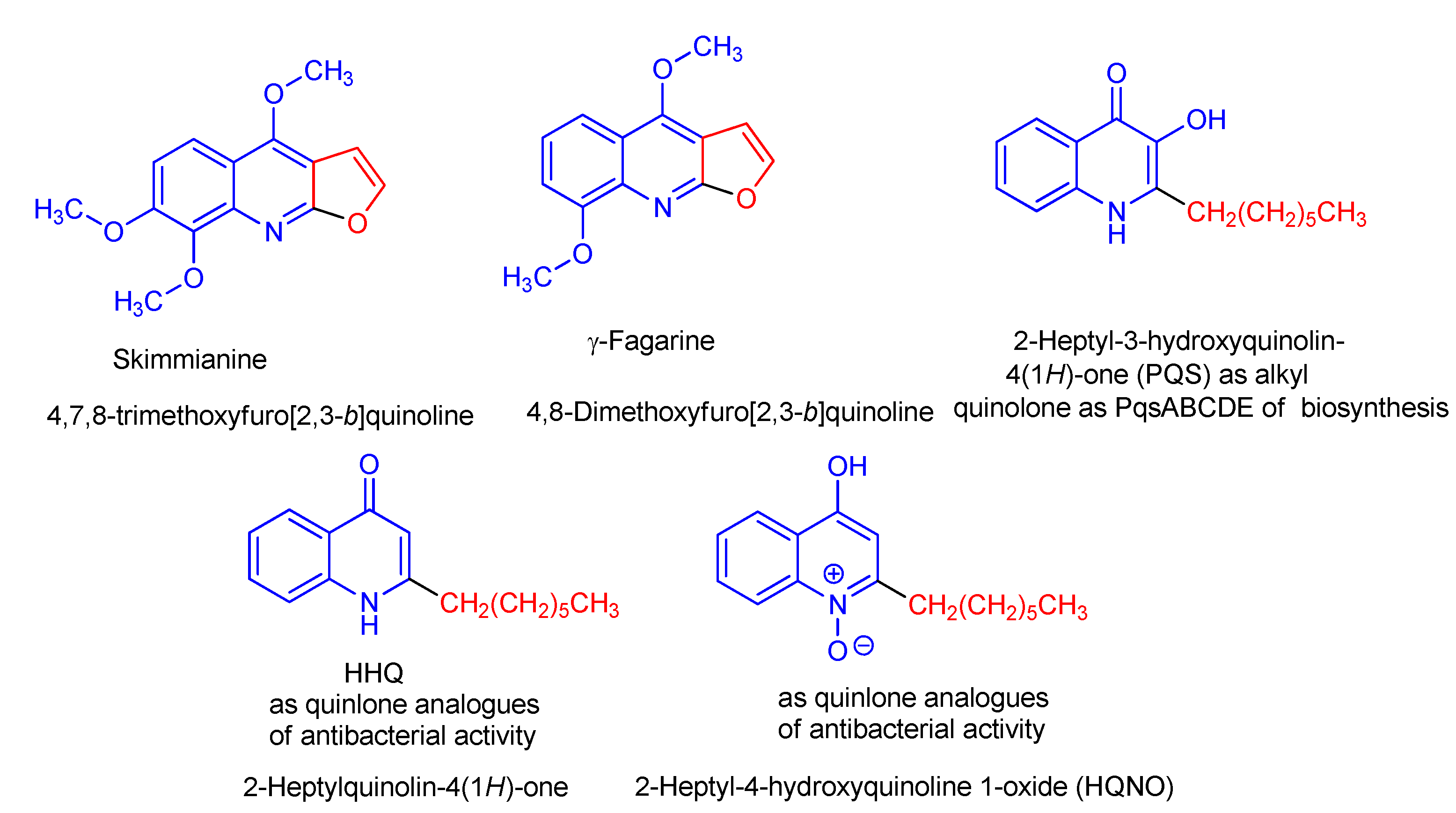
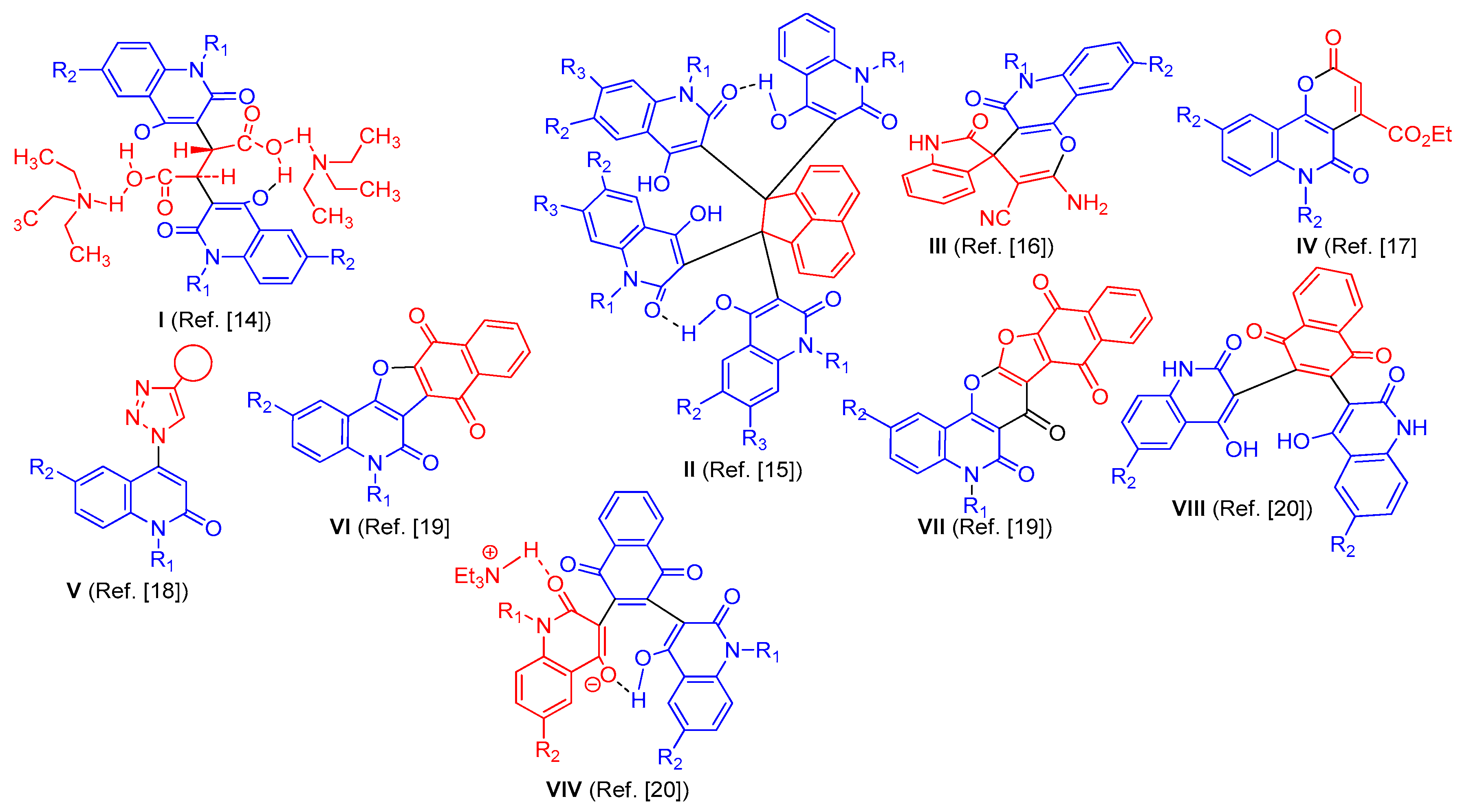
:1. Instruction
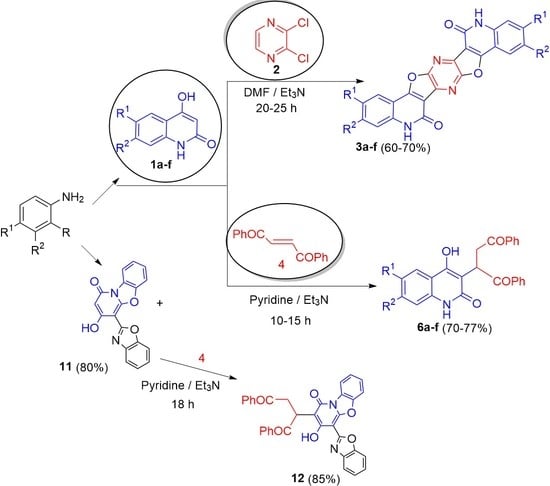
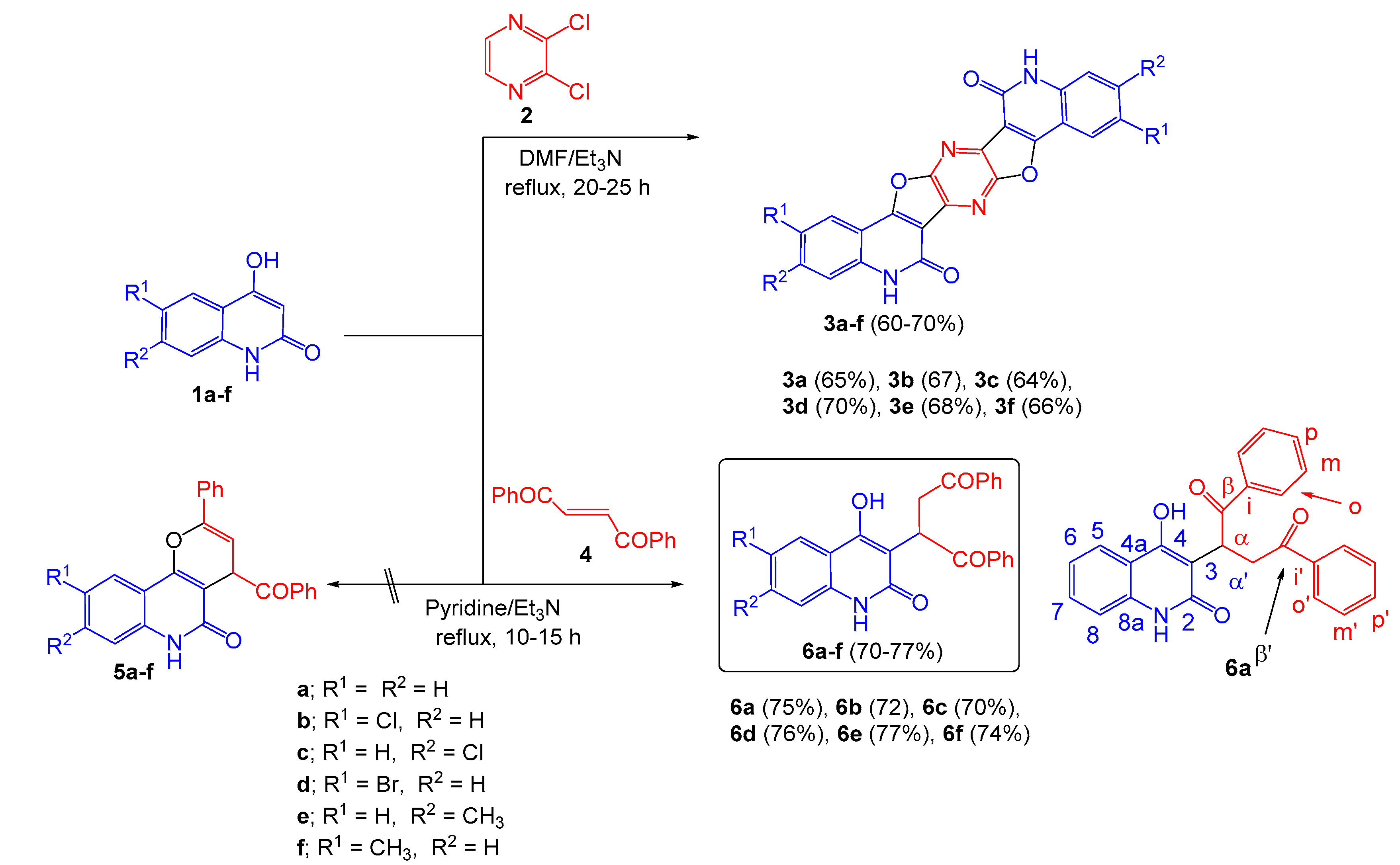
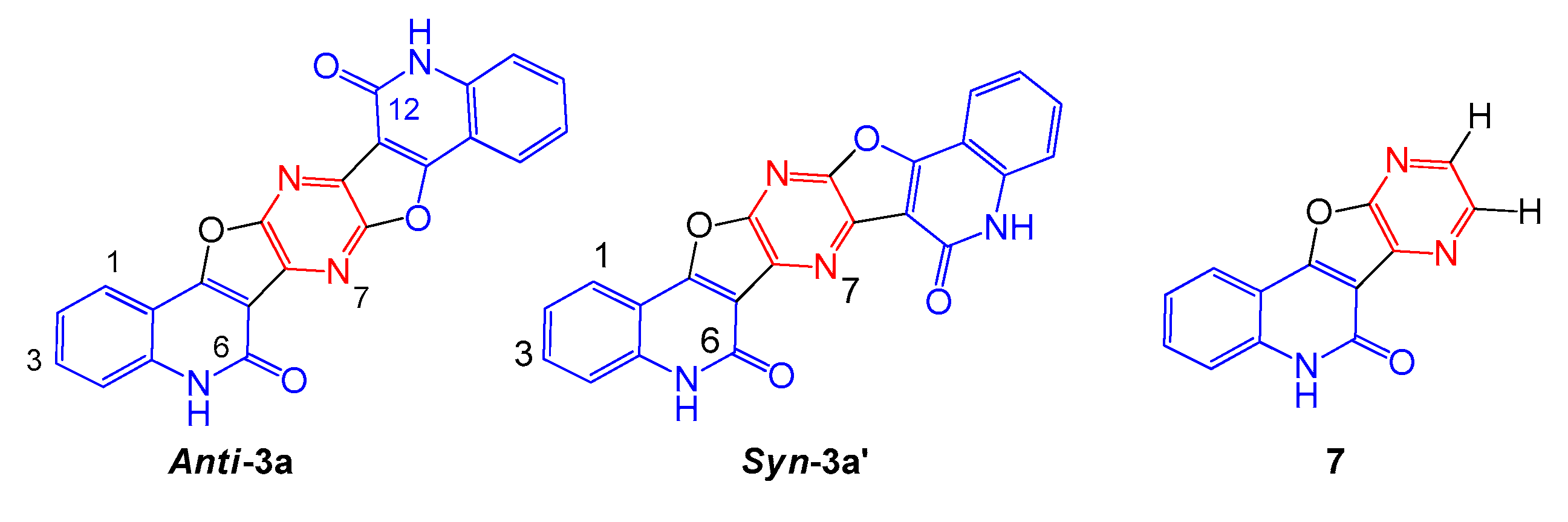
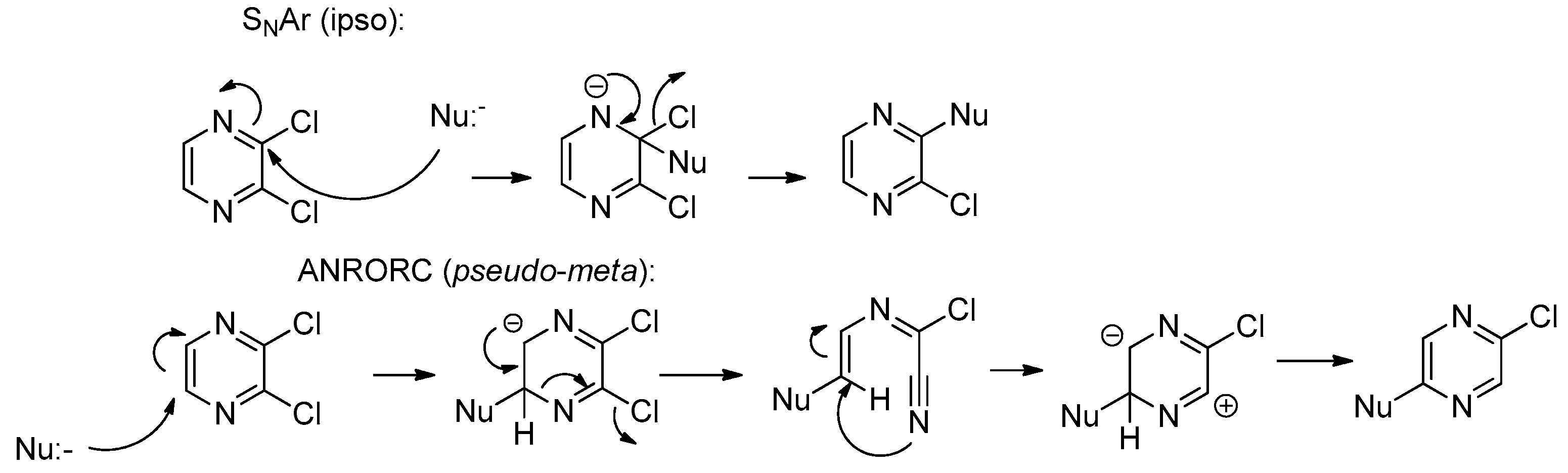
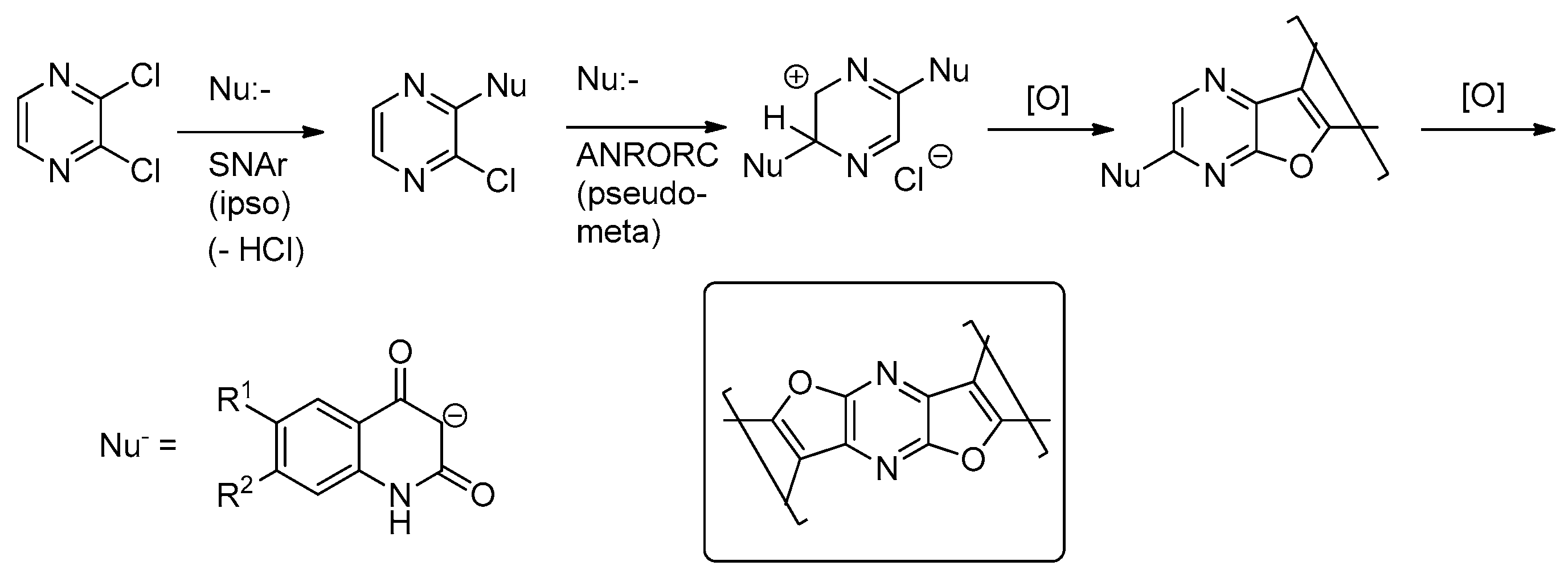
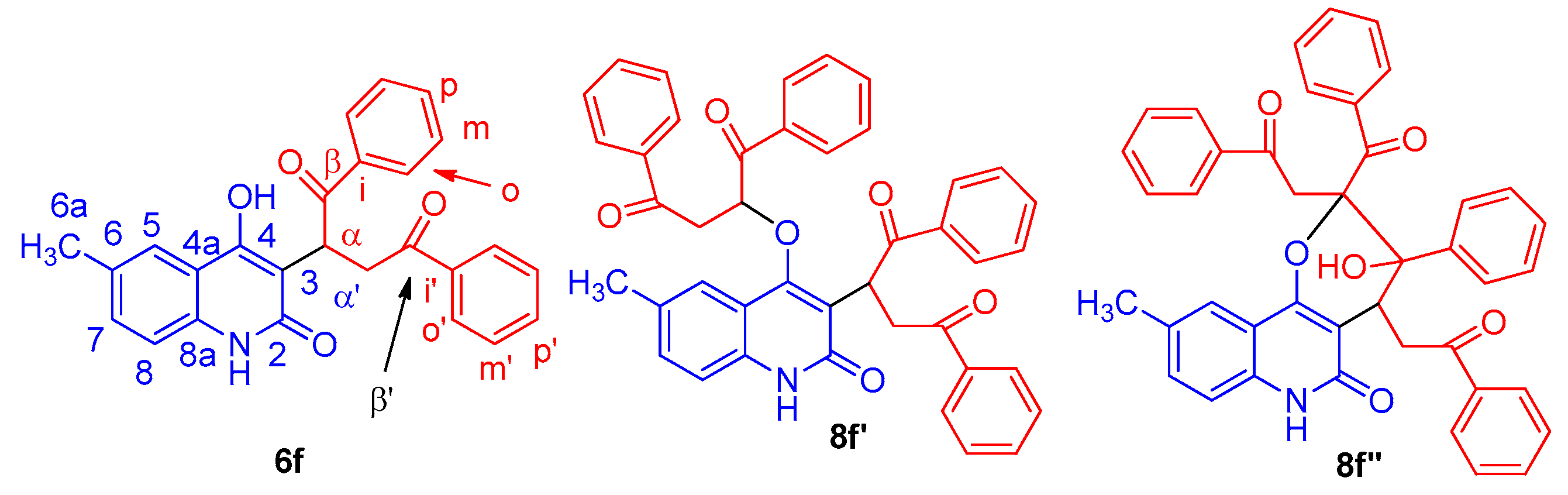
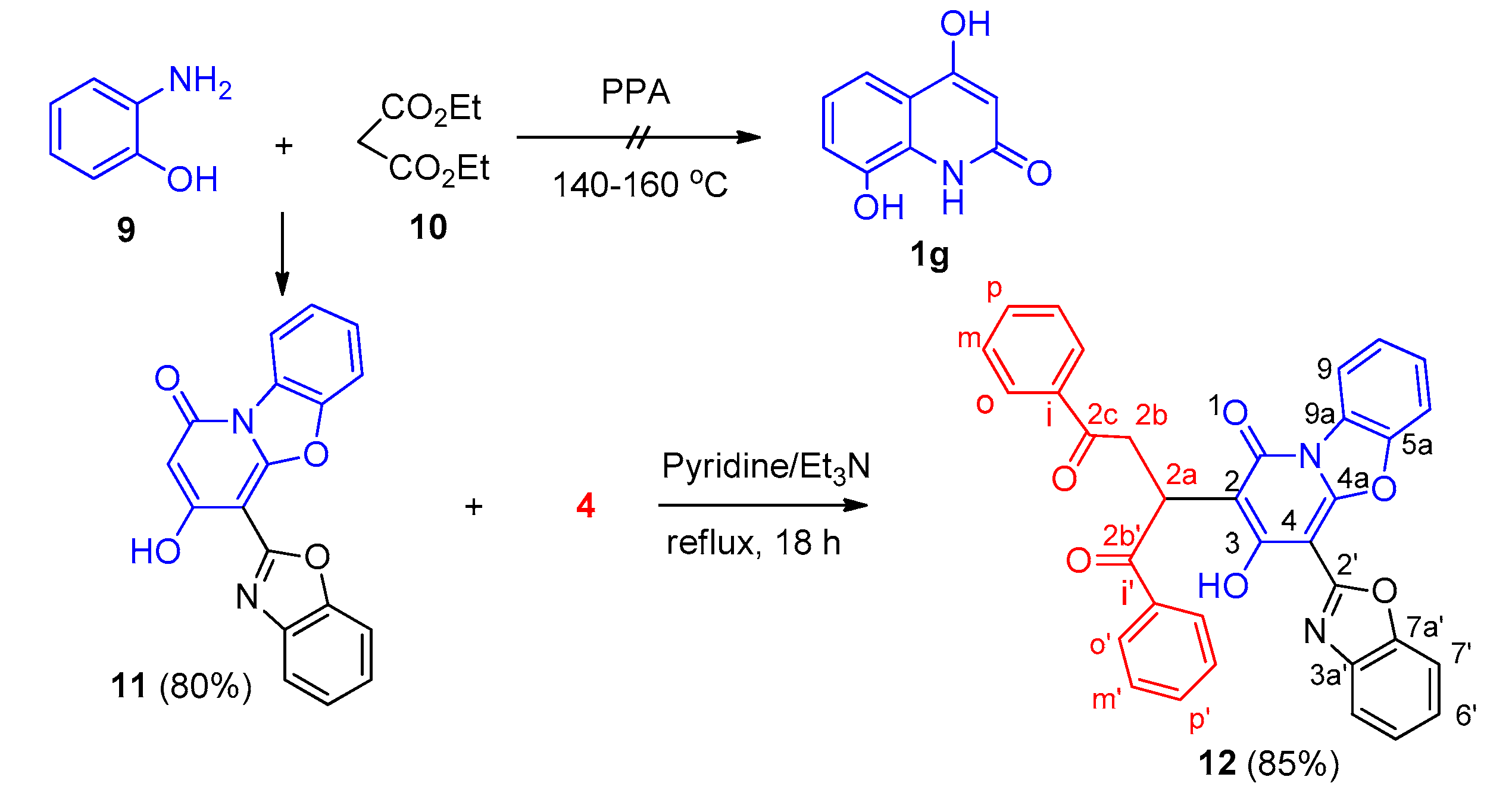
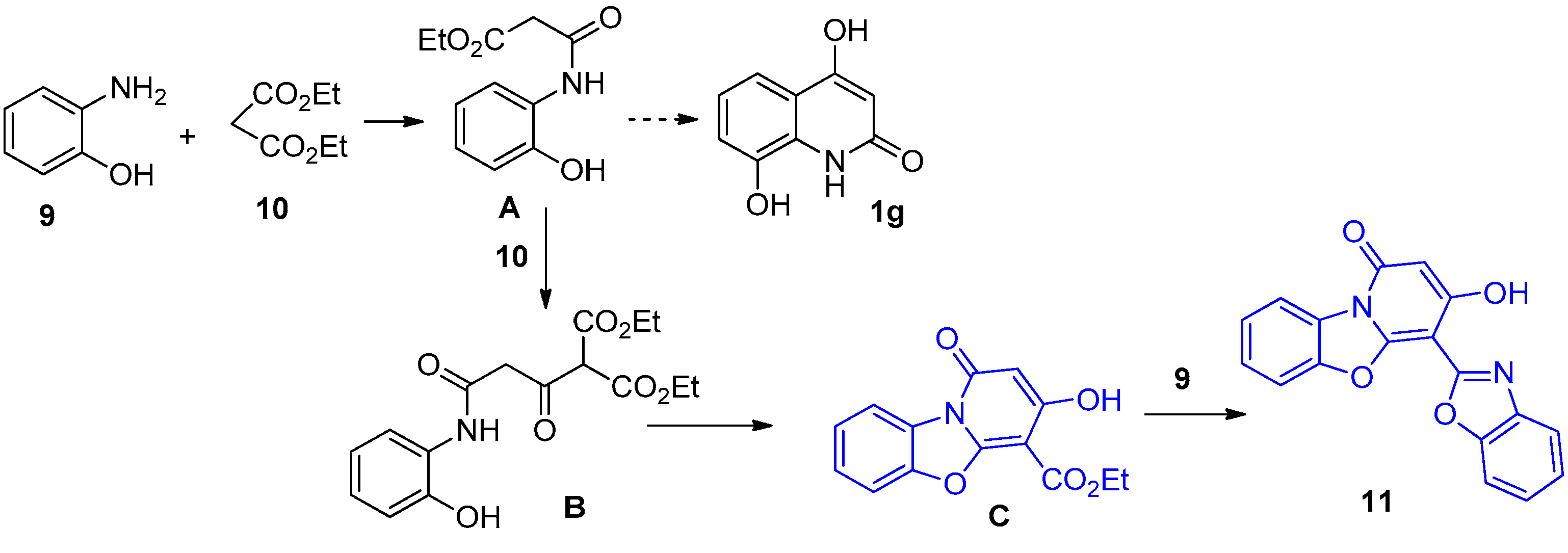
2. Results and Discussion
3. Experimental
3.1. Material and Methods
3.2. Starting Materials
3.2.1. Reaction of 1a–f with 2,3-Dichloropyrazine (2)
3.2.2. 5,12-Dihydropyrazino[2,3-c:5,6-c′]difuro[2,3-c:4,5-c′]diquinoline-6,14(5H,12H)-Dione (3a)
3.2.3. 2,10-Dichloro-5,12-Dihydropyrazino[2,3-c:5,6-c′]difuro[2,3-c:4,5-c′]-Diquinoline-6,14(5H,12H)-Dione (3b)
3.2.4. 3,11-Dichloro-5,12-Dihydropyrazino[2,3-c:5,6-c′]difuro[2,3-c:4,5-c′]diquinoline-6,14(5H,12H)-Dione (3c)
3.2.5. 2,10-Dibromo-5,12-Dihydropyrazino[2,3-c:5,6-c′]difuro[2,3-c:4,5-c′]diquinoline-6,14(5H,12H)-Dione (3d)
3.2.6. 5,12-Dihydro-3,11-Dimethylpyrazino[2,3-c:5,6-c′]difuro[2,3-c:4,5-c′]diquinoline-6,14(5H,12H)-Dione (3e)
3.2.7. 5,12-Dihydro-2,10-Dimethylpyrazino[2,3-c:5,6-c′]difuro[2,3-c:4,5-c′]diquinoline-6,14(5H,12H)-Dione (3f)
3.3. Reaction of 1a–f with (E)-1,2-Dibenzoylethene (4)
3.3.1. 2-(4-Hydroxy-2-oxo-1,2-Dihydroquinolin-3-yl)-1,4-Diphenylbutane-1,4-Dione (6a)
3.3.2. 2-(6-Chloro-4-Hydroxy-2-oxo-1,2-Dihydroquinolin-3-yl)-1,4-Diphenylbutane-1,4-Dione (6b)
3.3.3. 2-(7-Chloro-4-Hydroxy-2-oxo-1,2-Dihydroquinolin-3-yl)-1,4-Diphenylbutane-1,4-Dione (6c)
3.3.4. 2-(6-Bromo-4-Hydroxy-2-oxo-1,2-Dihydroquinolin-3-yl)-1,4-Diphenylbutane-1,4-Dione (6d)
3.3.5. 2-(4-Hydroxy-7-Methyl-2-oxo-1,2-Dihydroquinolin-3-yl)-1,4-Diphenylbutane-1,4-Dione (6e)
3.3.6. 2-(4-Hydroxy-6-Methyl-2-oxo-1,2-Dihydroquinolin-3-yl)-1,4-Diphenylbutane-1,4-Dione (6f)
3.3.7. 4-(Benzo[d]oxazol-2-yl)-3-Hydroxy-1H-Benzo[4,5]oxazolo[3,2-a]pyridine-1-one (11)
3.3.8. (4-(Benzo[d]oxazol-2-yl)-3-Hydroxy-1-oxo-1H-Benzo[4,5]oxazolo[3,2-a]pyridin-2-yl)-1,4-Diphenylbutane-1,4-Dione (12)
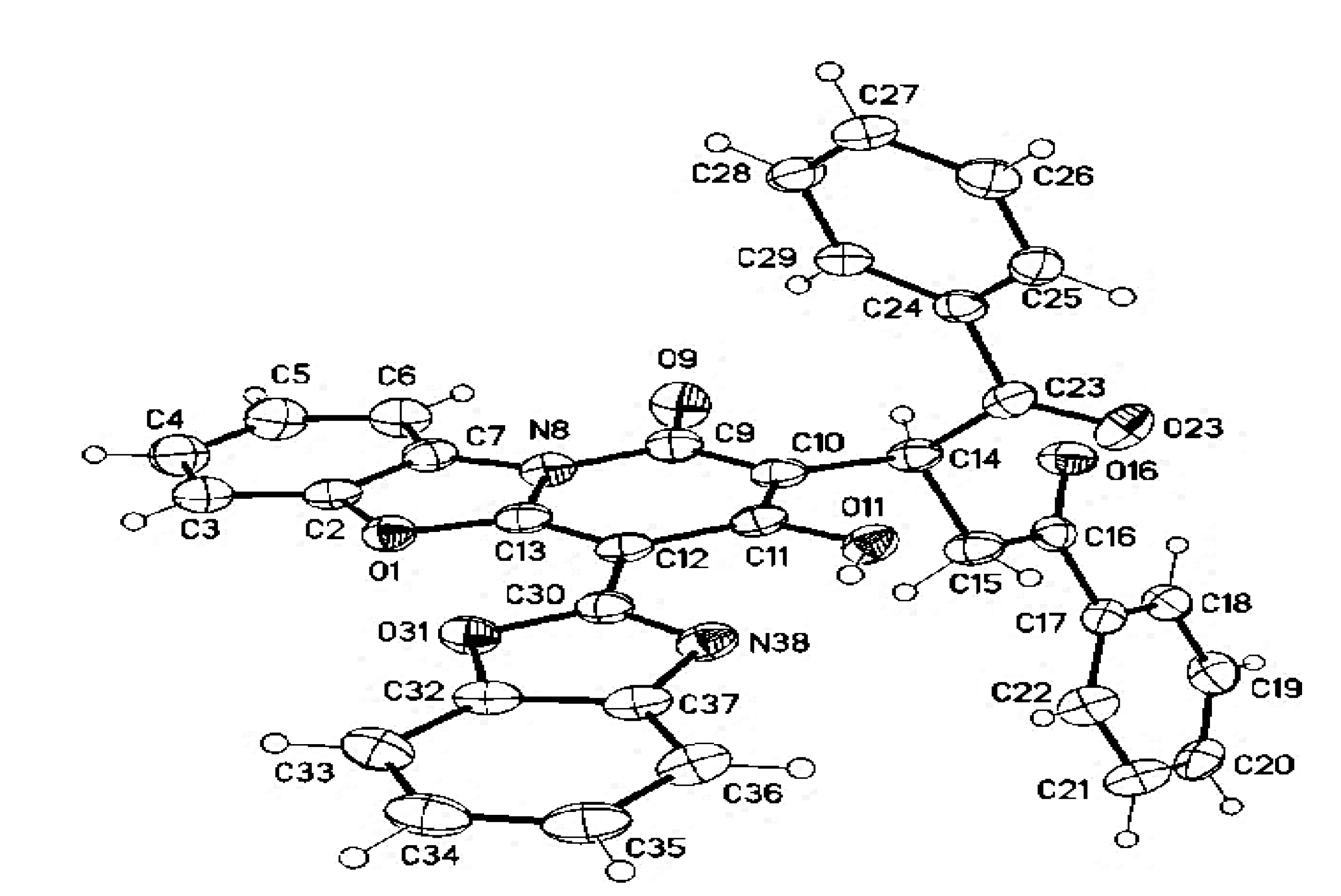
3.4. Crystal Structure Determinations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mizuta, M.; Kanamori, H. Mutagenic activities of dictamnine and g-fagarine from Dictamni radicis cortex (Rutaceae). Mutat. Res. Lett. 1985, 144, 221–225. [Google Scholar] [CrossRef]
- Zuo, Y.; Pu, J.; Chen, G.; Shen, W.; Wang, B. Study on the activity and mechanism of skimmianine against human non-small cell lung cancer. Nat. Prod. Res. 2019, 33, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Claerhout, S.; Sharma, S.; Skold, C.; Cavaluzzo, C.; Sandstrom, A.; Larhed, M.; Thirumal, M.; Parmar, V.S.; Van der Eycken, E.V. Synthesis of functionalized furopyrazines as restricted dipeptidomimetics. Tetrahedron 2012, 68, 3019–3029. [Google Scholar] [CrossRef]
- Pesci, E.C.; Milbank, J.B.J.; Pearson, J.P.; McKnight, S.; Kende, A.S.; Greenberg, E.P.; Iglewski, B.H. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 1999, 96, 11229–11234. [Google Scholar] [CrossRef]
- McGrath, S.; Wade, D.S.; Pesci, E.C. Dueling quorum sensing systems in Pseudomonas aeruginosa control the production of the Pseudomonas quinolone signal (PQS). FEMS Microbiol. Lett. 2004, 230, 27–34. [Google Scholar] [CrossRef]
- Diggle, S.P.; Gardner, A.; West, S.A.; Griffin, A.S. Evolutionary theory of bacterial quorum sensing: When is a signal not a signal? Phil. Trans. R. Soc. B 2007, 362, 1241–1249. [Google Scholar] [CrossRef]
- Dulcey, C.E.; Dekimpe, V.; Fauvelle, D.-A.; Milot, S.; Groleau, M.-C.; Doucet, N.; Rahme, L.G.; Lépine, F.; Déziel, E. The end of an old hypothesis: The Pseudomonas signalling molecules 4-hydroxy-2-alkylquinolines derive from fatty acids, not 3-ketofatty acids. Chem. Biol. 2013, 20, 1481–1491. [Google Scholar] [CrossRef]
- Wu, Y.; Seyedsayamdost, M.R. Synergy and target promiscuity drive structural divergence in bacterial alkylquinolone biosynthesis. Cell Chem. Biol. 2017, 24, 1437–1444. [Google Scholar] [CrossRef]
- Burden, D.A.; Osheroff, N. Mechanism of action of eukaryotic topoisomerase II and drugs targeted to the enzyme. Biochim. Biophys. Acta. 1998, 1400, 139–154. [Google Scholar] [CrossRef]
- Dang, Z.; Yang, Y.; Ji, R.; Zhang, S. Synthesis and antibacterial activity of novel fluoroquinolones containing substituted piperidines. Bioorg. Med. Chem. Lett. 2007, 17, 4523–4526. [Google Scholar] [CrossRef]
- Shindikar, A.V.; Viswanathan, C.L. Novel fluoroquinolones: Design, synthesis, and in vivo activity in mice against Mycobacterium tuberculosis H37Rv. Bioorg. Med. Chem. Lett. 2005, 15, 1803–1806. [Google Scholar] [CrossRef] [PubMed]
- Andriole, V.T. The quinolones: Past, present, and future. Clin. Infect. Dis. 41 Suppl 2005, 2, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-L.; Huang, Z.-S.; An, L.-K.; Bu, X.-Z.; Ma, L.; Li, Y.-M.; Chan, A.S.C.; Gu, L.-Q. Synthesis of Zwitter-ionic 4-hydroxycoumarin derivatives through a unique reaction of 4-hydroxycoumarins with p-benzoquinone and pyridine. Org. Lett. 2004, 6, 4853–4855. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.A.; El-Sheref, E.M.; Mourad, A.F.E.; Bakheet, M.E.M.; Bräse, S.; Nieger, M. One-pot synthesis of 2,3-bis-(4-hydroxy-2-oxo-1,2-dihydroquinolin-3-yl)succinates and arylmethylene-bis-3,3′-quinoline-2-ones. Chem. Pap. 2019, 73, 27–37. [Google Scholar] [CrossRef]
- Aly, A.A.; Ramadan, M.; El-Reedy, A.A.M. Reactions of 4-hydroxyquinolin-2(1H)-ones with acenaphthoquinone: Synthesis of new 1,2-dihydroacenaphthylene-spiro-tetrakis-(4-hydroxy-quinolin-2(1H)-ones). J. Heterocycl. Chem. 2019, 56, 642–645. [Google Scholar] [CrossRef]
- Aly, A.A.; El-Sheref, E.M.; Mourad, A.-F.E.; Brown, A.B.; Bräse, S.; Bakheet, M.E.M.; Nieger, M. Synthesis of spiro[indoline-3,4′-pyrano[3,2-c]quinolone]-3′-carbonitriles. Monatsh. Chem. 2018, 149, 635–644. [Google Scholar] [CrossRef]
- El-Sheref, E.M.; Aly, A.A.; Mourad, A.-F.E.; Brown, A.B.; Bräse, S.; Bakheet, M.E.M. Synthesis of pyrano[3,2-c]quinoline-4-carboxylates and 2-(4-oxo-1,4-dihydroquinolin-3-yl)fumarates. Chem. Pap. 2018, 72, 181–190. [Google Scholar] [CrossRef]
- El-Sheref, E.M.; Aly, A.A.; Ameen, M.A.; Brown, A.B. New 4-(1,2,3-triazolo)quinolin-2(1H)-ones via Cu-catalyzed [3+2] cycloaddition. Monatsh. Chem. 2019, 150, 747–756. [Google Scholar] [CrossRef]
- Aly, A.A.; El-Sheref, E.M.; Bakheet, M.E.M.; Mourad, M.A.E.; Bräse, S.; Ibrahim, M.A.A.; Nieger, M.; Garvalov, B.K.; Dalby, K.N.; Kaoud, T.S. Design, synthesis and biological evaluation of fused naphthofuro[3,2-c]quinoline-6,7,12-triones and pyrano[3,2-c]quinoline- 6,7,8,13-tetraones derivatives as ERK inhibitors with efficacy in BRAF-mutant melanoma. Bioorg. Chem. 2019, 82, 290–305. [Google Scholar] [CrossRef]
- Aly, A.A.; El-Sheref, E.M.; Bakheet, M.E.M.; Mourad, M.A.E.; Brown, A.B.; Bräse, S.; Nieger, M.; Ibrahim, M.A.A. Synthesis of novel 1,2-bis-quinolinyl-1,4-naphthoquinones: ERK2 inhibition, cytotoxicity and molecular docking studies. Bioorg. Chem. 2018, 81, 700–712. [Google Scholar] [CrossRef]
- Spartan ’18; Wavefunction, Inc.: Irvine, CA, USA. Available online: http://downloads.wavefun.com/Spartan18Brochure.pdf (accessed on 21 October 2019).
- Endres, A.H.; Schaffroth, M.; Paulus, F.; Reiss, H.; Wadepohl, H.; Rominger, F.; Krämer, R.; Bunz, U.H.F. Coronene-containing N-heteroarenes: 13 rings in a row. J. Am. Chem. Soc. 2016, 138, 1792–1795. [Google Scholar] [CrossRef] [PubMed]
- Bisballe, N.; Hedidi, M.; Demmer, C.S.; Chevallier, S.; Roisnel, T.; Dorcet, V.; Halauko, Y.S.; Ivashkevich, O.A.; Matulis, V.E.; Bentabed-Ababsa, G.; et al. Functionalization of oxazolo[4,5-b]pyrazines by deprotometallation. Eur. J. Org. Chem. 2018, 2018, 3904–3913. [Google Scholar] [CrossRef]
- Lont, P.J.; van der Plas, H.C.; Verbeek, A.J. Ring transformations in reactions of heterocyclic halogeno compounds with nucleophiles (XXV). Recl Trav. Chim. Pays-Bas 1978, 91, 949–957. [Google Scholar] [CrossRef]
- Van der Plas, H.C. The SN(ANRORC) mechanism: A new mechanism for nucleophilic substitution. Acc. Chem Res. 1978, 11, 462–468. [Google Scholar] [CrossRef]
- Van der Plas, H.C. Degenerate ring transformations of heterocyclic compounds. Adv. Heterocycl Chem. 1999, 74, 1–253. [Google Scholar]
- Rusinov, G.L.; Slepukhin, P.A.; Charusin, V.N.; Chupakhin, O.N. 2,3-Dichloro-1-alkyl- pyrazinium tetrafluoroborates: The synthesis and reactions with nucleophiles. Mendeleev Commun. 2001, 11, 78–80. [Google Scholar] [CrossRef]
- Rao, V.S.; Darbarwar, M. One pot synthesis of 7-[1,2-dihydro-4-hydroxy-1-methyl/phenyl- 2-oxo-3-quinolinyl]-5,7-dihydro-5-methyl/phenyl-6H-[1]-benzo-pyrano-[3,2-c]quinolin-6-ones. Synth. Commun. 1988, 18, 2267–2272. [Google Scholar] [CrossRef]
- Buckle, D.R.; Cantello, B.C.C.; Smith, H.; Spicer, B.A. 4-Hydroxy-3-nitro-2-quinolones and related compounds as inhibitors of allergic reactions. J. Med. Chem. 1975, 18, 726–732. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta. Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT: Integrated space-group and crystal-structure determination. Acta. Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aly, A.A.; Hassan, A.A.; Mohamed, N.K.; El-Haleem, L.E.A.; Bräse, S.; Polamo, M.; Nieger, M.; Brown, A.B. Synthesis of New Fused Heterocyclic 2-Quinolones and 3-Alkanonyl-4-Hydroxy-2-Quinolones. Molecules 2019, 24, 3782. https://doi.org/10.3390/molecules24203782
Aly AA, Hassan AA, Mohamed NK, El-Haleem LEA, Bräse S, Polamo M, Nieger M, Brown AB. Synthesis of New Fused Heterocyclic 2-Quinolones and 3-Alkanonyl-4-Hydroxy-2-Quinolones. Molecules. 2019; 24(20):3782. https://doi.org/10.3390/molecules24203782
Chicago/Turabian StyleAly, Ashraf A., Alaa A. Hassan, Nasr K. Mohamed, Lamiaa E. Abd El-Haleem, Stefan Bräse, Mika Polamo, Martin Nieger, and Alan B. Brown. 2019. "Synthesis of New Fused Heterocyclic 2-Quinolones and 3-Alkanonyl-4-Hydroxy-2-Quinolones" Molecules 24, no. 20: 3782. https://doi.org/10.3390/molecules24203782
APA StyleAly, A. A., Hassan, A. A., Mohamed, N. K., El-Haleem, L. E. A., Bräse, S., Polamo, M., Nieger, M., & Brown, A. B. (2019). Synthesis of New Fused Heterocyclic 2-Quinolones and 3-Alkanonyl-4-Hydroxy-2-Quinolones. Molecules, 24(20), 3782. https://doi.org/10.3390/molecules24203782