Natural Sesquiterpene Lactones of the 4,15-iso-Atriplicolide Type are Inhibitors of Trypanothione Reductase
Abstract
:1. Introduction
2. Results and Discussion
2.1. Activity of Antitrypanosomal STLs against Trypanothione Reductase from Trypanosoma brucei (TbTR) and T. cruzi (TcTR)
2.2. Mode of Inhibition of TbTR by 4,15-iso-Atriplicolide Esters
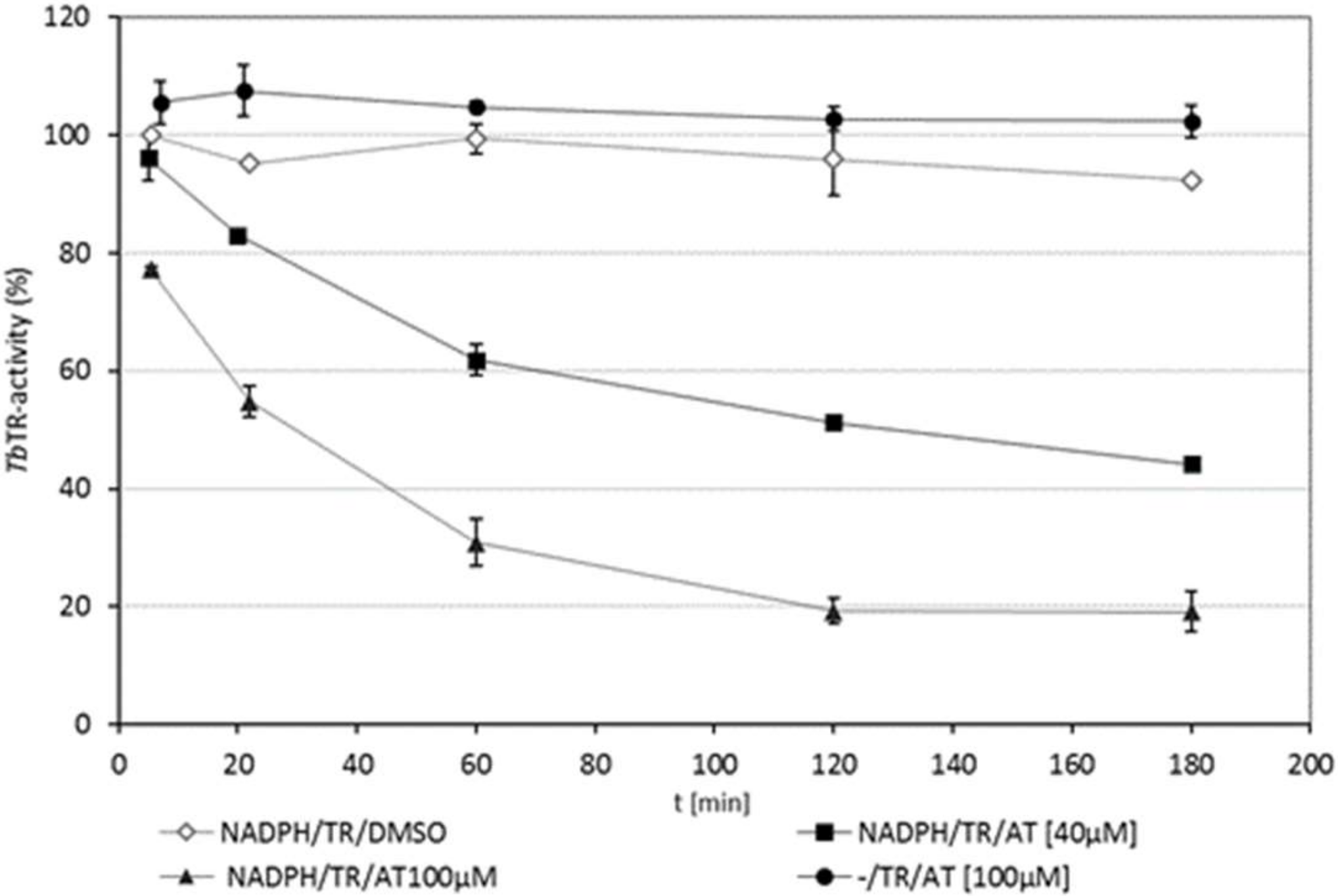
2.3. TbTR is Only Inhibited by 4,15-iso-Atriplicolide Esters in Its Reduced Dithiol State
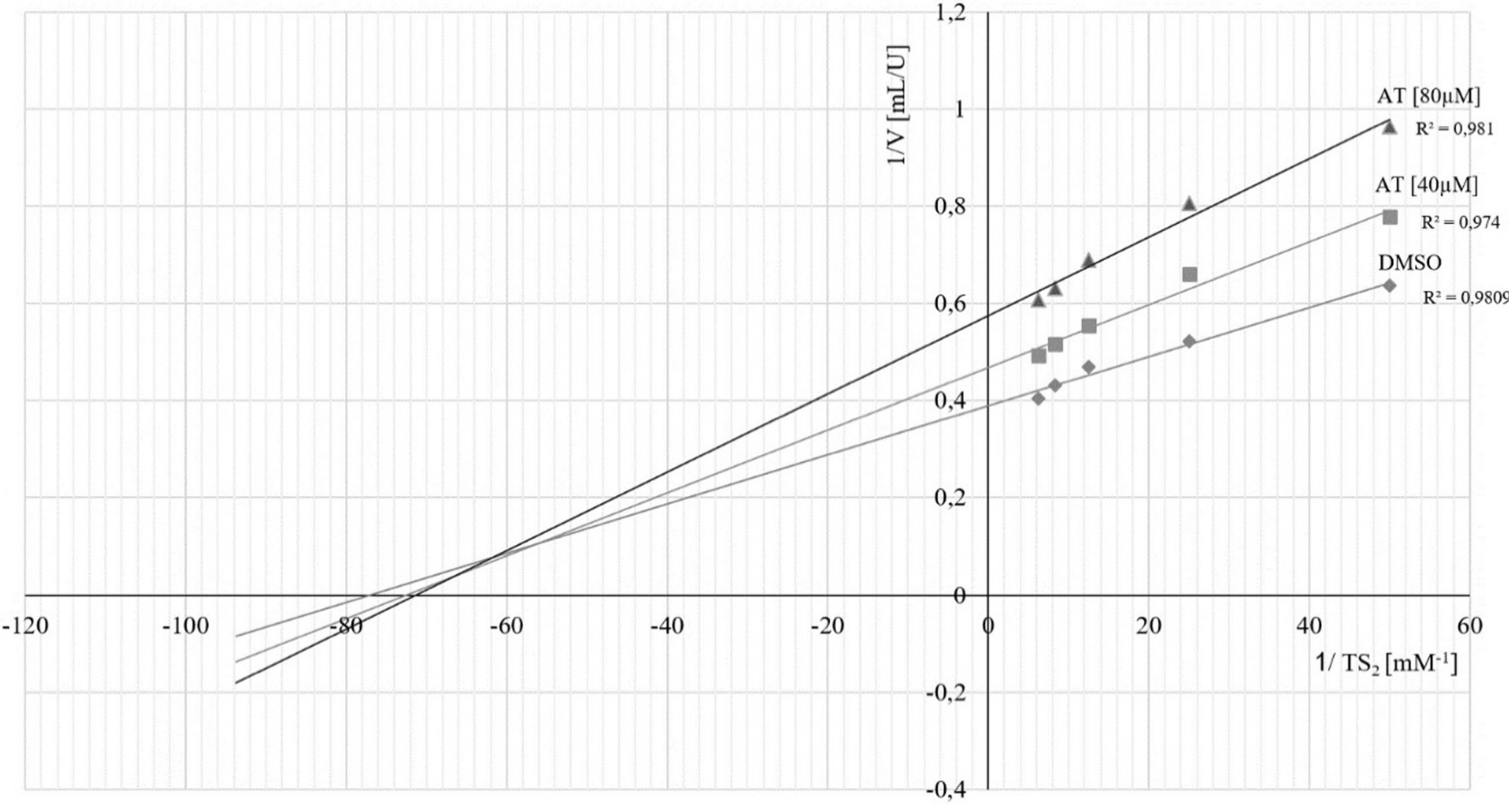
2.4. Kinetics of Inhibition of TbTR by 4,15-iso-Atriplicolide Esters
3. Materials and Methods
3.1. Compounds under Study
3.2. Enzmes
3.3. Enzyme Inhibition Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schmidt, T.J.; Nour, A.M.M.; Khalid, S.A.; Kaiser, M.; Brun, R. Quantitative structure—Antiprotozoal activity relationships of sesquiterpene lactones. Molecules 2009, 14, 2062–2076. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J.; Da Costa, F.B.F.; Lopes, N.P.N.; Kaiser, M.; Brun, R. In Silico prediction and experimental evaluation of furanoheliangolide sesquiterpene lactones as potent agents against Trypanosoma brucei rhodesiense. Antimicrob. Agents Chemother. 2014, 58, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Kimani, N.M.; Matasyoh, J.C.; Kaiser, M.; Nogueira, M.S.; Trossini, G.H.G.; Schmidt, T.J. Complementary quantitative structure-activity relationship models for the antitrypanosomal activity of sesquiterpene lactones. Int. J. Mol. Sci. 2018, 19, 3721. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J.; Brun, R.; Willuhn, G.; Khalid, S.A. Anti-trypanosomal activity of helenalin and some structurally related sesquiterpene lactones. Planta Med. 2002, 68, 750–751. [Google Scholar] [CrossRef] [PubMed]
- Galkina, A.; Krause, N.; Lenz, M.; Daniliuc, C.G.; Kaiser, M.; Schmidt, T.J. Antitrypanosomal activity of Sesquiterpene Lactones from Helianthus tuberosus L. including a new Furanoheliangolide with an unusual Structure. Molecules 2019, 24, 1068. [Google Scholar] [CrossRef] [PubMed]
- Comini, M.; Flohé, L. Trypanothione-Based Redox Metabolism of Trypanosomatids. In Trypanosomatid Diseases Molecular Routes to Drug Discovery; Jäger, T., Koch, O., Flohé, L., Eds.; Wiley-VCH: Weinheim, Germany, 2013; pp. 167–199. [Google Scholar]
- Leroux, A.E.; Krauth-Siegel, R.L. Thiol redox biology of trypanosomatids and potential targets for chemotherapy. Mol. Biochem. Parasitol. 2016, 206, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J. Structure–activity relationships of sesquiterpene lactones. In Studies in Natural Products Chemistry—Bioactive Natural Products (Part M); Ur Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 309–392. [Google Scholar]
- Patterson, S.; Alphey, M.S.; Jones, D.C.; Shanks, E.J.; Street, I.P.; Frearson, J.A.; Wyatt, P.G.; Gilbert, I.H.; Fairlamb, A.H. Dihydroquinazolines as a Novel Class of Trypanosoma brucei Trypanothione Reductase Inhibitors. Discovery, Synthesis, and Characterization of their Binding Mode by Protein Crystallography. J. Med. Chem. 2011, 54, 6514–6530. [Google Scholar] [CrossRef] [PubMed]
- Jockers-Scherübl, M.C.; Schirmer, R.H.; Krauth-Siegel, R.L. Trypanothione reductase from Trypanosoma cruzi. Catalytic properties of the enzyme and inhibition studies with trypanocidal compounds. Eur. J. Biochem. 1989, 180, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Persch, E.; Bryson, S.; Todoroff, N.K.; Eberle, C.; Thelemann, J.; Dirdjaja, N.; Kaiser, M.; Weber, M.; Derbani, H.; Brun, R.M.; et al. Binding to Large Enzyme Pockets. Small-Molecule Inhibitors of Trypanothione Reductase. ChemMedChem 2014, 9, 1880–1891. [Google Scholar] [CrossRef] [PubMed]
- Gallwitz, H.; Bonse, S.; Martinez-Cruz, A.; Schlichting, I.; Schumacher, K.; Krauth-Siegel, R.L. Ajoene is an Inhibitor and Subversive Substrate of Human Glutathione Reductase and Trypanosoma cruzi Trypanothione Reductase: Crystallographic, Kinetic, and Spectroscopic Studies. J. Med. Chem. 1999, 42, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Saravanamuthu, A.; Vickers, T.J.; Bond, C.S.; Peterson, M.R.; Hunter, W.N.; Fairlamb, A.H. Two Interacting Binding Sites for Quinacrine Derivatives in the Active Site of Trypanothione Reductase A template for drug design. J. Biol. Chem. 2004, 279, 29493–29500. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Bauer, H.; Melchers, J.; Ruppert, T.; Rattray, L.; Yardley, V.; Davioud-Charvet, E.; Krauth-Siegel, R.L. Irreversible Inactivation of Trypanothione Reductase by Unsaturated Mannich Bases: A Divinyl Ketone as Key Intermediate. J. Med. Chem. 2005, 48, 7400–7410. [Google Scholar] [CrossRef] [PubMed]
- Bisswanger, H. Enzyme kinetics. Principles and Methods, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Comini, M.A.; Dirdjaja, N.; Kaschel, M.; Krauth-Siegel, R.L. Preparative enzymatic synthesis of trypanothione and trypanothione analogues. Int. J. Parasitol. 1999, 39, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.C.; Ariza, A.; Chow, W.-H.A.; Oza, S.L.; Fairlamb, A.H. Comparative structural, kinetic and inhibitor studies of Trypanosoma brucei trypanothione reductase with T. cruzi. Mol. Biochem. Parasitol. 2010, 169, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, S.; Oufir, M.; Leroux, A.; Krauth-Siegel, R.L.; Becker, K.; Kaiser, M.; Brun, R.; Hamburger, M.; Adams, M. Cynaropicrin targets the trypanothione redox system in Trypanosoma brucei. Bioorg. Med. Chem. 2013, 21, 7202–7209. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
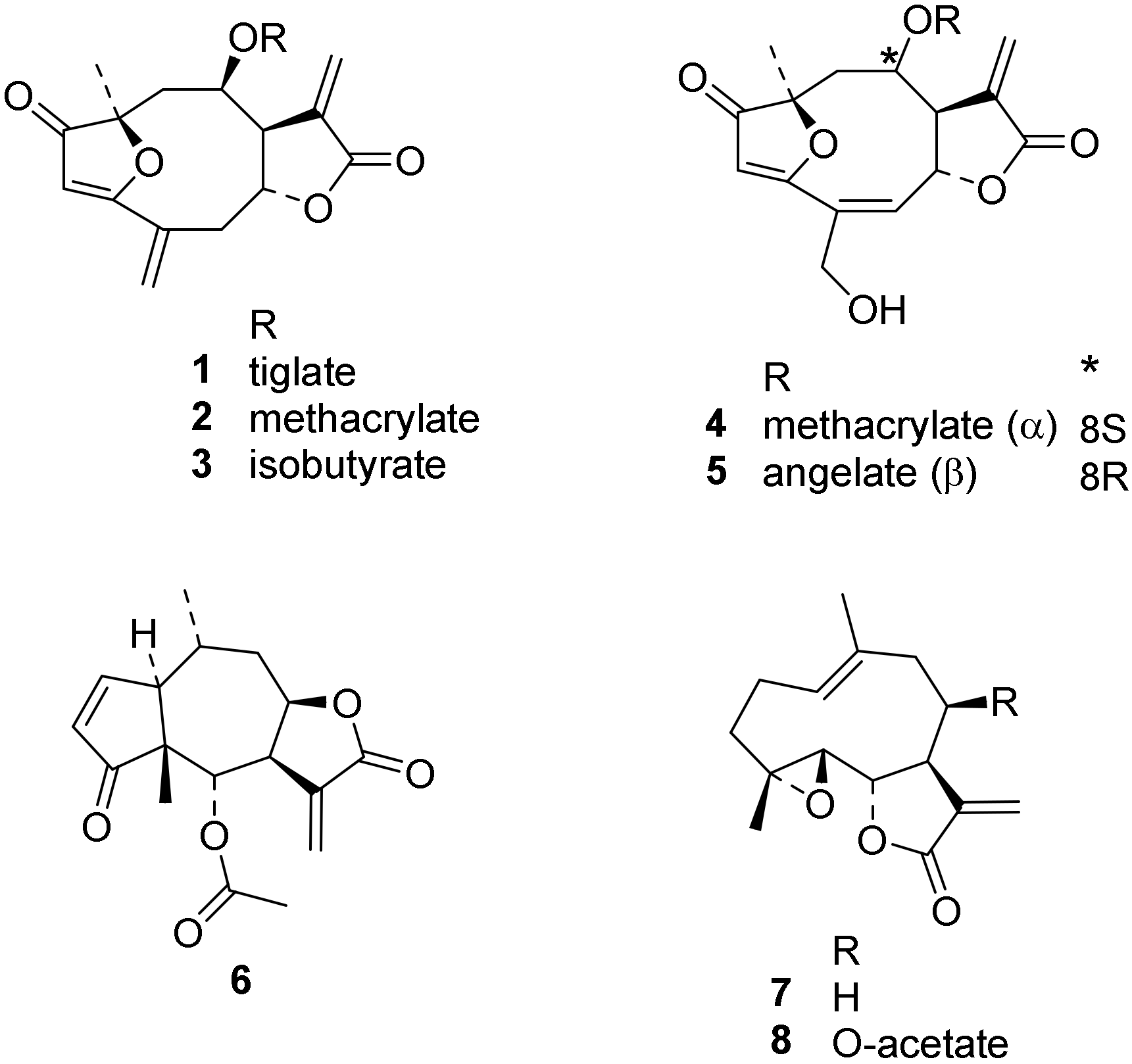

| Compound | Tbr | Tc | L6 | TcTR | TbTR | |||
|---|---|---|---|---|---|---|---|---|
| IC50 (µM) | % Inhibition at c = 100 µM Preincubation Time (min) | |||||||
| 0 | 15 | 0 | 15 | 30 | ||||
| 1 | 0.015 | 3.7 | 1.2 | 42 ± 0 | 89 ± 1 | 39 ± 3 | 87 ± 1 | 92 ± 0 |
| 2 | 0.077 | 1.6 | 0.52 | 0 ± 0 | 41 ± 1 | 6.0 ± 2.4 | 68 ± 1 | 84 ± 0 |
| 3 | 0.26 | 3.1 | 0.88 | 33 ± 2 | 80 ± 0 | 8.0 ± 2.8 | 62 ± 1 | 79 ± 0 |
| 4 | 0.073 | 1.1 | 0.49 | n.i. | n.t. | n.t. | n.t. | n.i. |
| 5 | 0.072 | 1.8 | 0.38 | 5 ± 8 | n.t. | n.t. | n.t. | 3 ± 3 |
| 6 | 0.063 | 0.54 | 0.81 | n.i. | n.t. | n.t. | n.t. | n.i * |
| 7 | 0.39 | 11 | 7.2 | n.i. | n.t. | n.t. | n.t. | 2 ± 8 |
| 8 | 0.23 | 11 | 4.7 | n.i. | n.t. | n.t. | n.t. | n.i. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenz, M.; Krauth-Siegel, R.L.; Schmidt, T.J. Natural Sesquiterpene Lactones of the 4,15-iso-Atriplicolide Type are Inhibitors of Trypanothione Reductase. Molecules 2019, 24, 3737. https://doi.org/10.3390/molecules24203737
Lenz M, Krauth-Siegel RL, Schmidt TJ. Natural Sesquiterpene Lactones of the 4,15-iso-Atriplicolide Type are Inhibitors of Trypanothione Reductase. Molecules. 2019; 24(20):3737. https://doi.org/10.3390/molecules24203737
Chicago/Turabian StyleLenz, Mairin, R. Luise Krauth-Siegel, and Thomas J. Schmidt. 2019. "Natural Sesquiterpene Lactones of the 4,15-iso-Atriplicolide Type are Inhibitors of Trypanothione Reductase" Molecules 24, no. 20: 3737. https://doi.org/10.3390/molecules24203737
APA StyleLenz, M., Krauth-Siegel, R. L., & Schmidt, T. J. (2019). Natural Sesquiterpene Lactones of the 4,15-iso-Atriplicolide Type are Inhibitors of Trypanothione Reductase. Molecules, 24(20), 3737. https://doi.org/10.3390/molecules24203737






