Anticancer Indole-Based Chalcones: A Structural and Theoretical Analysis
Abstract
1. Introduction
2. Results
Synthesis
3. Discussion
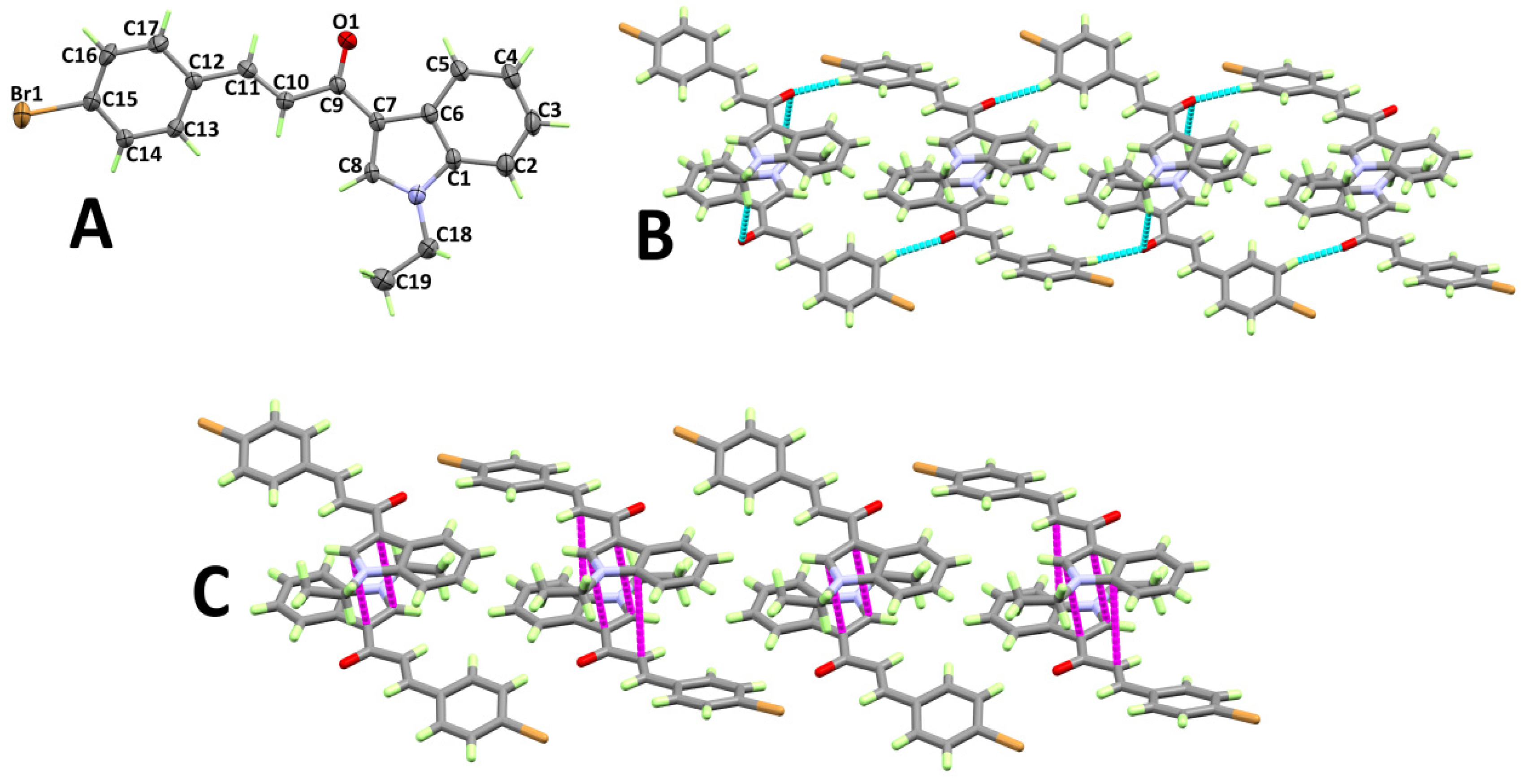
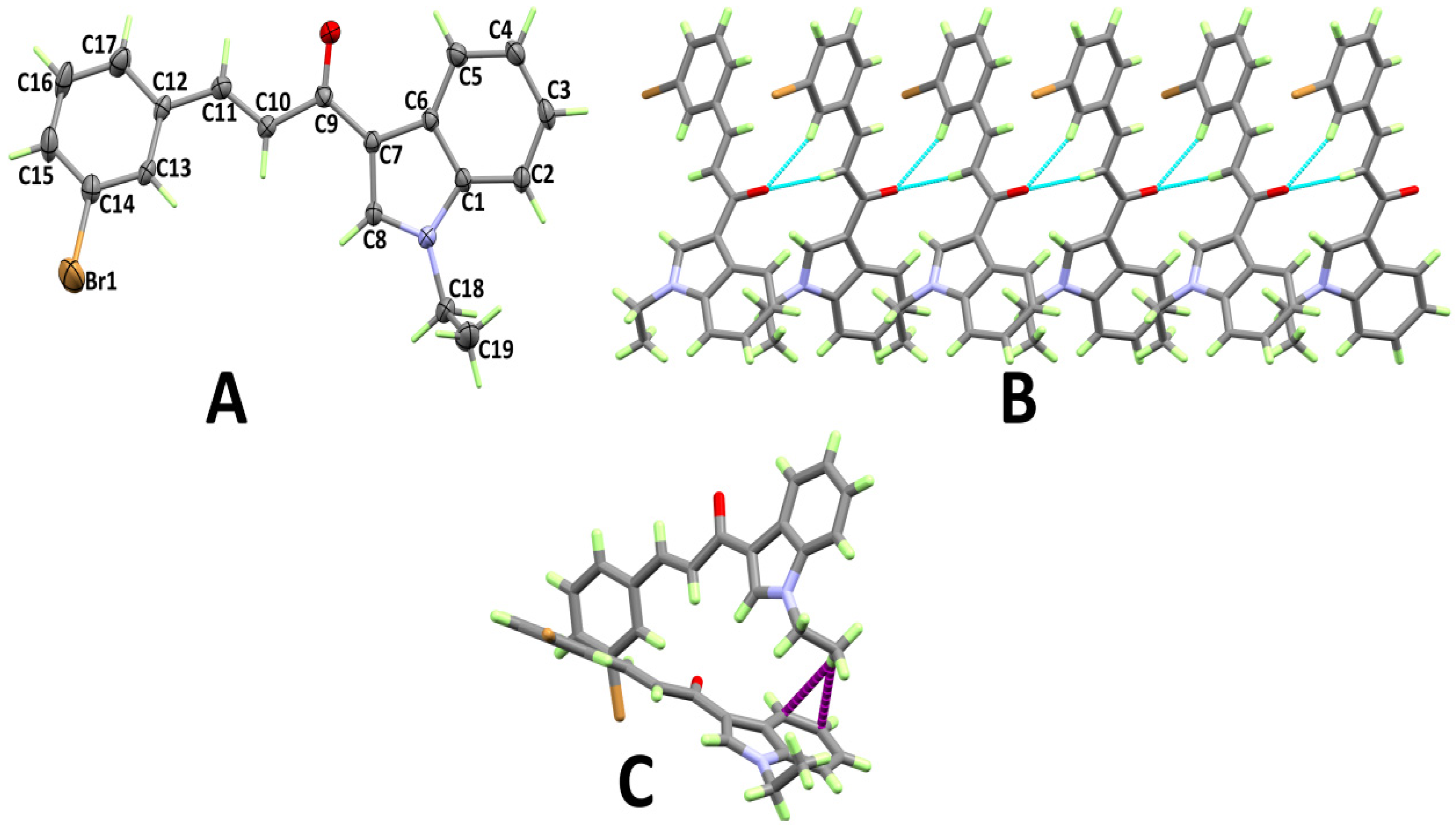
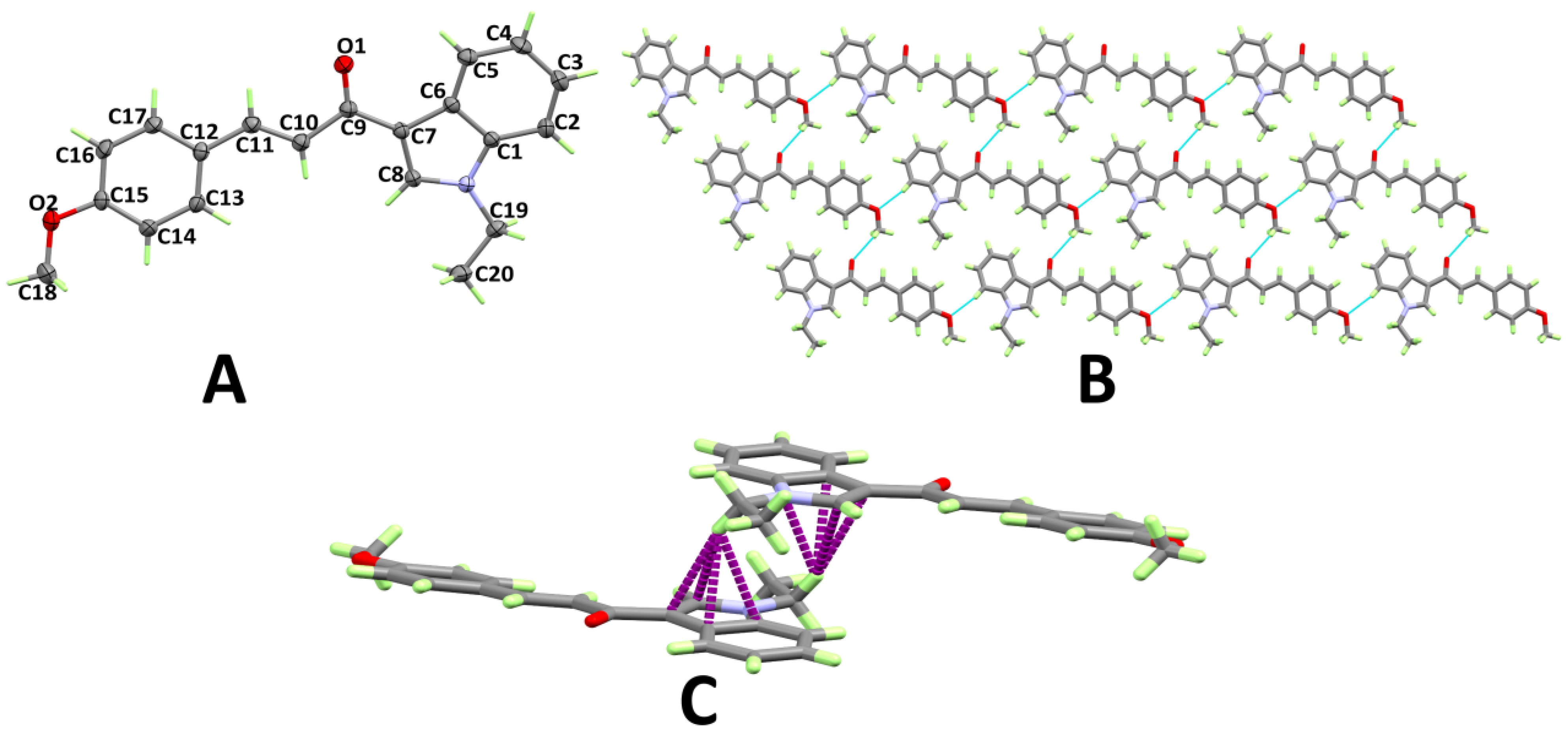
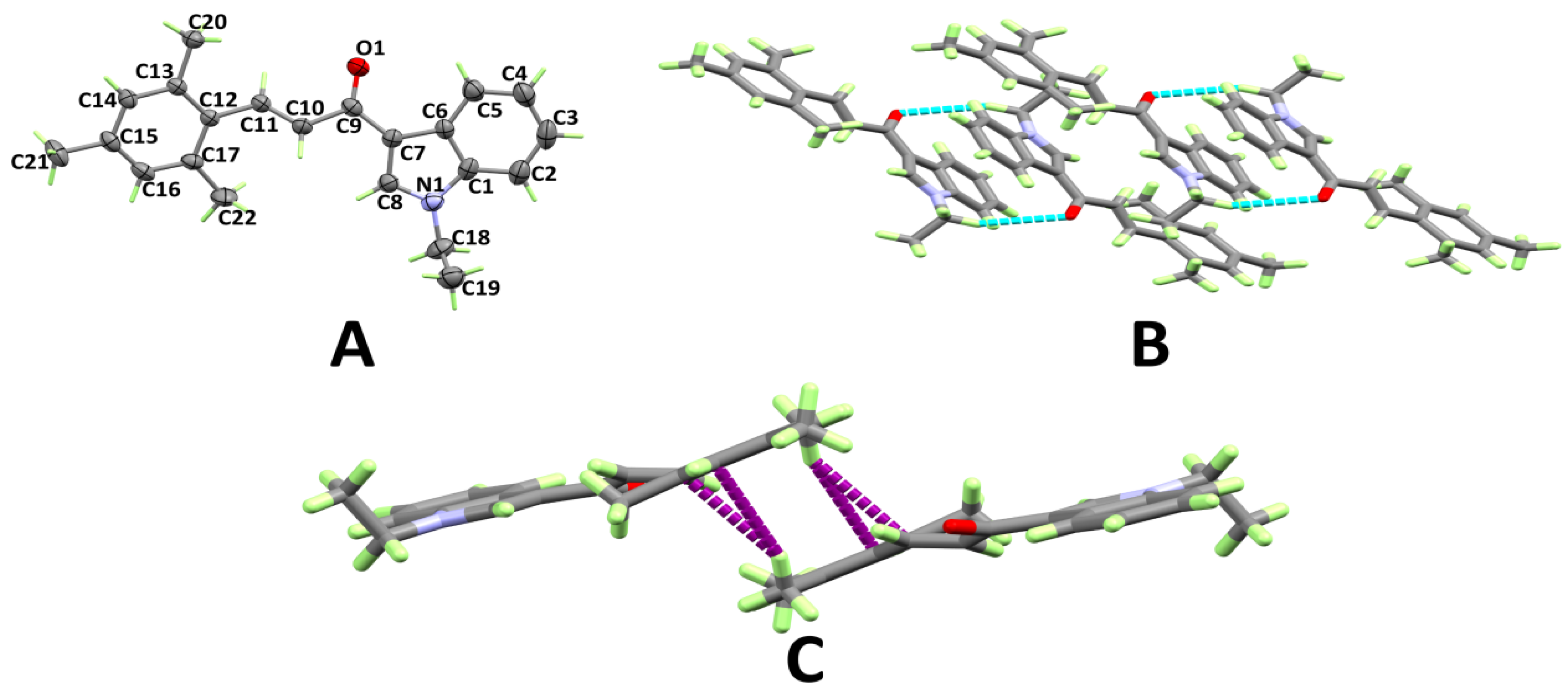
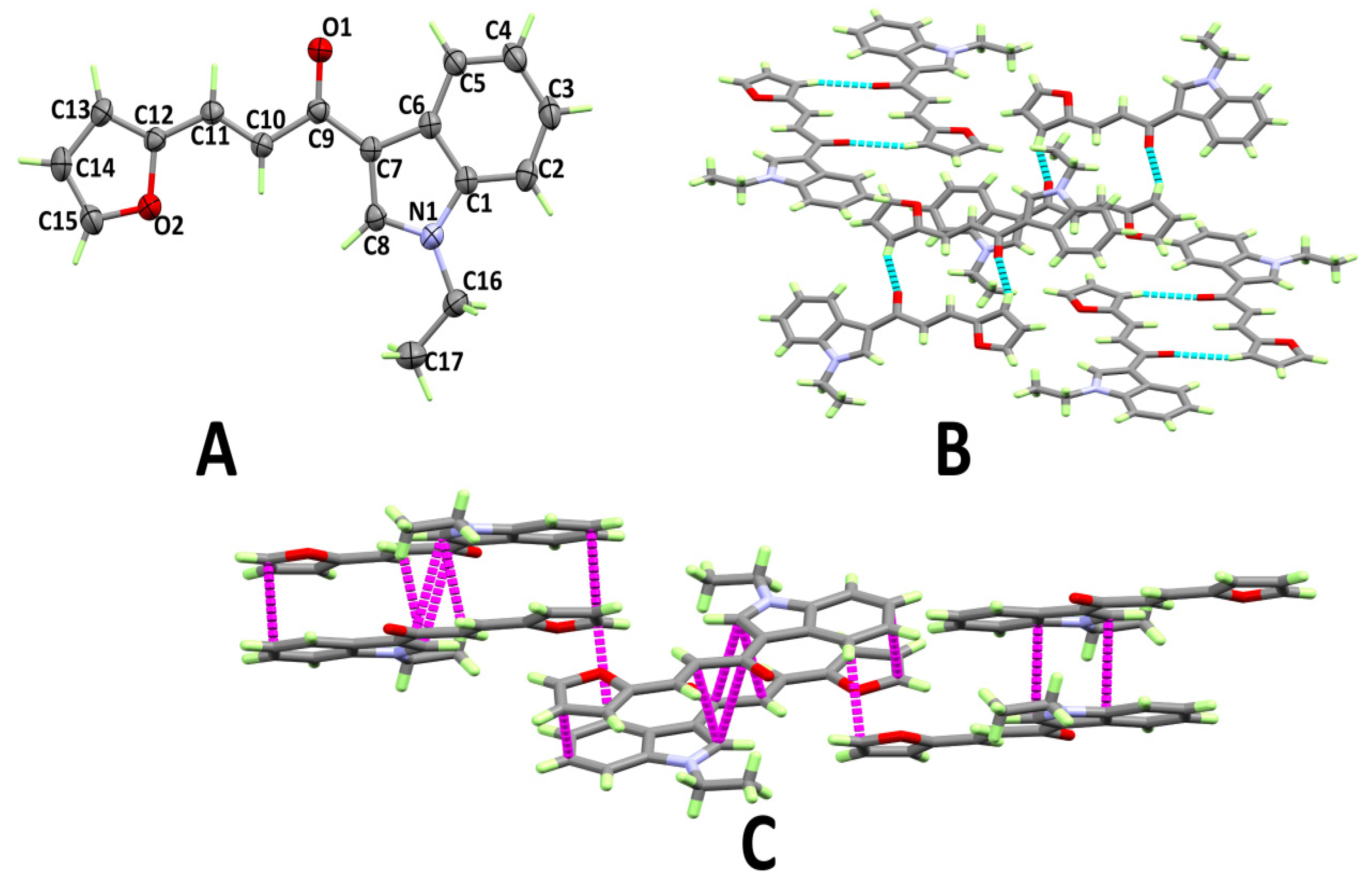
3.1. X-ray Structure
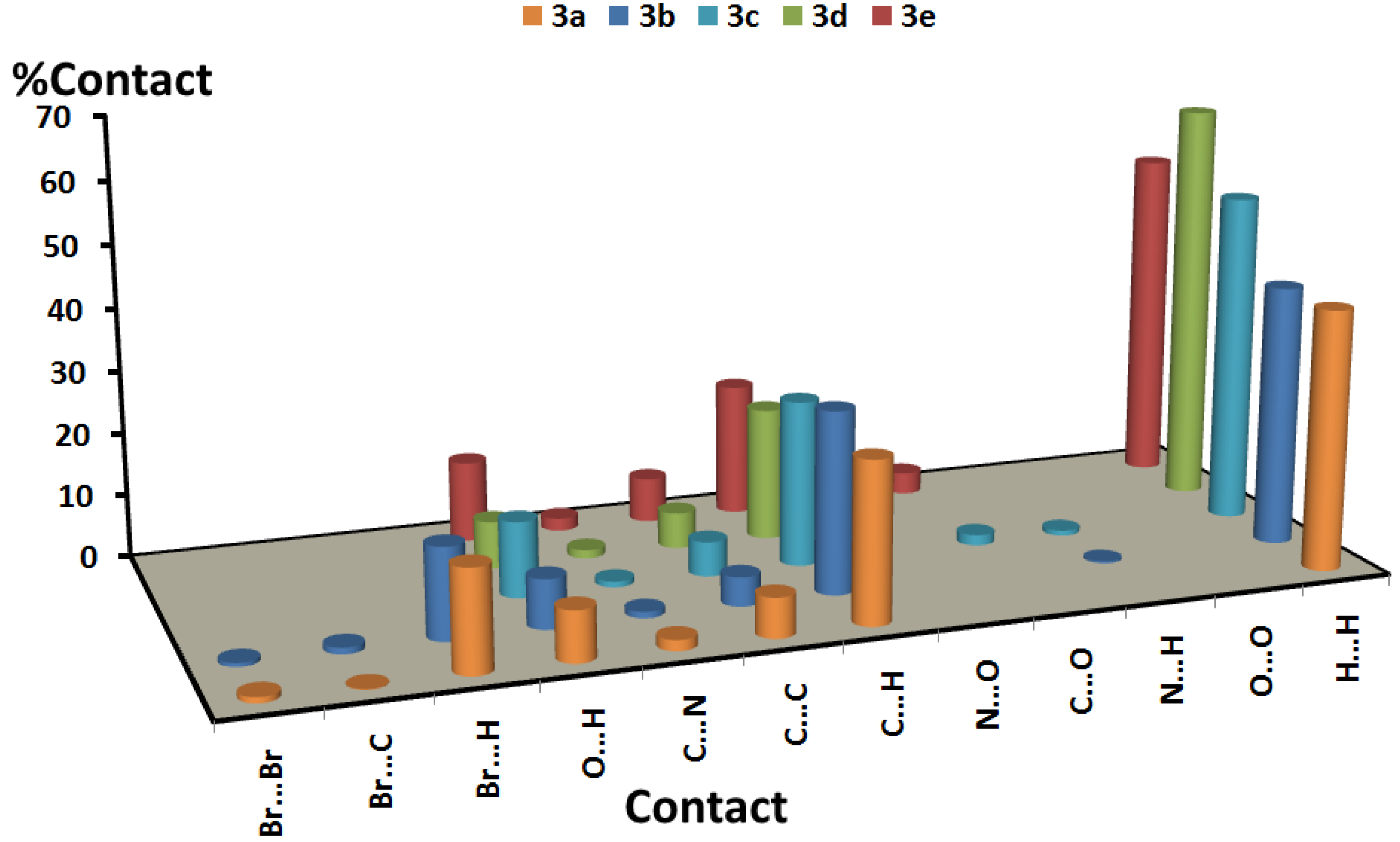
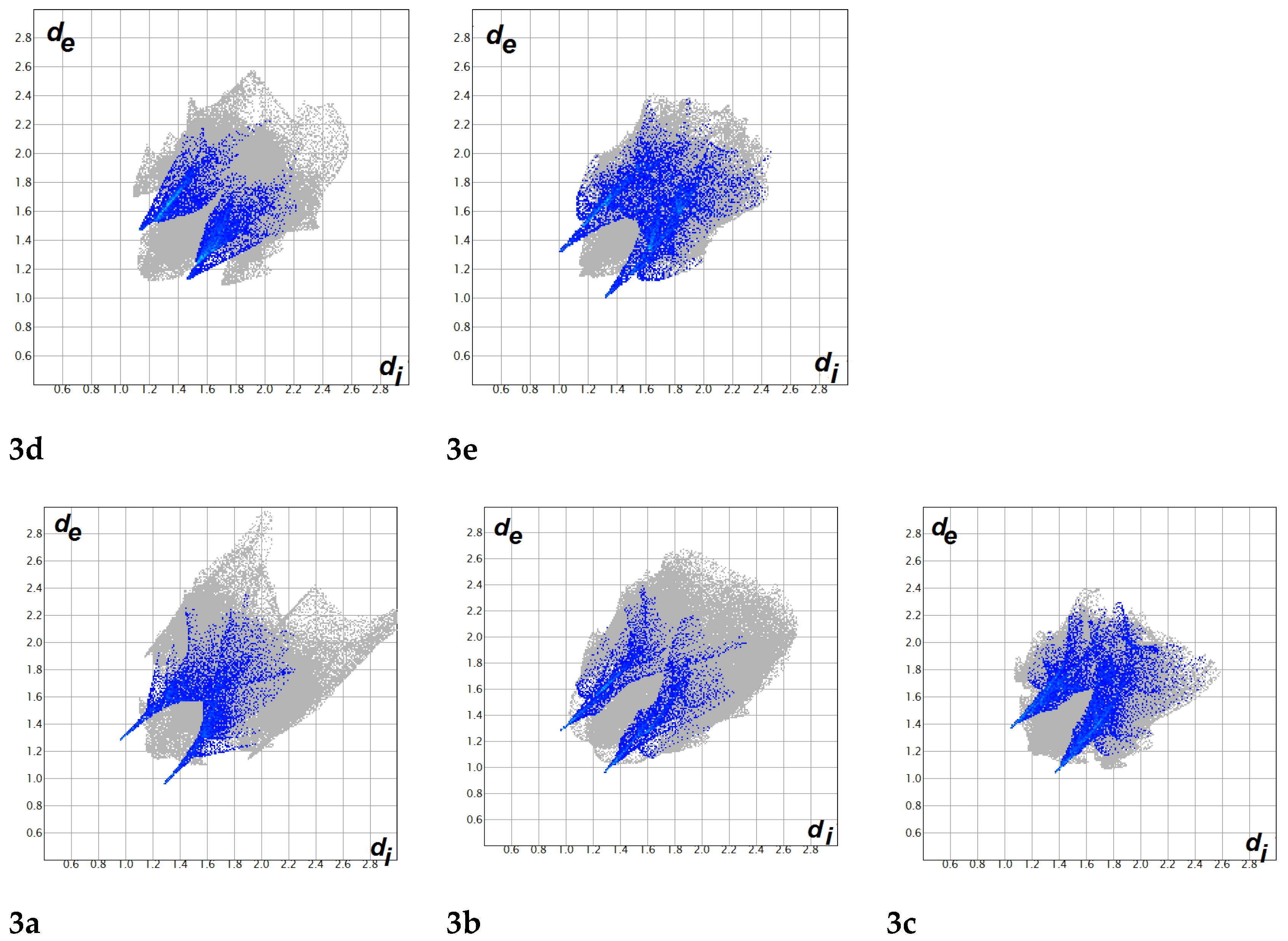
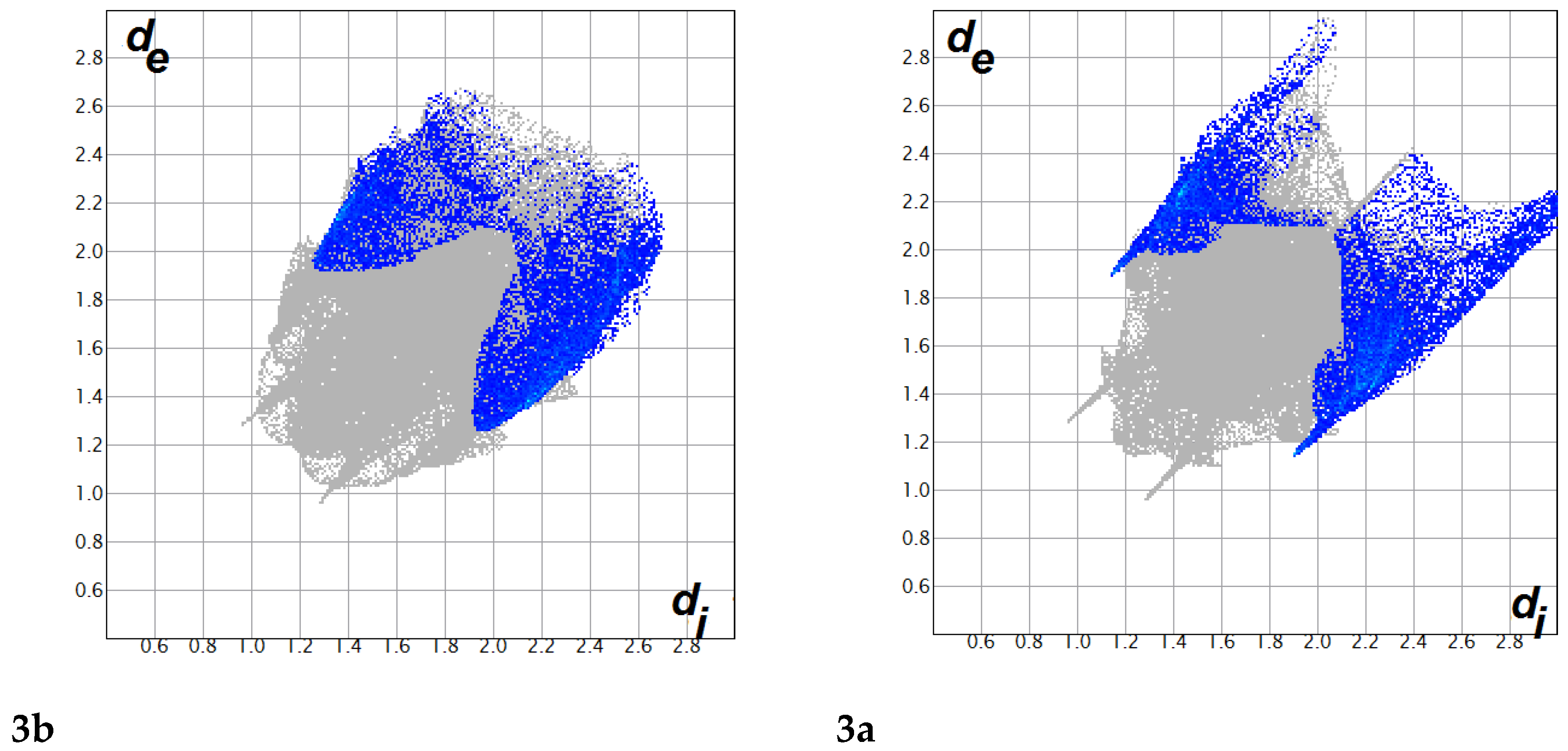
3.2. Analysis of Molecular Packing
3.3. Reactivity Studies
3.4. Biological Activity
4. Materials and Methods
4.1. General Procedure for the Synthesis of Chalcones 3a–e
4.2. X-ray Structure Measurements
4.3. DFT Calculations
4.4. Hirshfeld Surface Analysis
4.5. Anticancer Activity Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kawase, M.; Sakagami, H.; Motohashi, N. The chemistry of bioactive mesoionic heterocycles. Top. Heterocycl. Chem. 2009, 16, 135–152. [Google Scholar]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among US FDA approved pharmaceuticals: Miniperspective. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.K.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, C.-H.; Verma, A.K.; Choi, E.-H. Biomedical importance of indoles. Molecules 2013, 18, 6620–6662. [Google Scholar] [CrossRef] [PubMed]
- Reyes, F.; Fernández, R.; Rodríguez, A.; Francesch, A.; Taboada, S.; Ávila, C.; Cuevas, C. Aplicyanins A–F, new cytotoxic bromoindole derivatives from the marine tunicate Aplidium cyaneum. Tetrahedron 2008, 64, 5119–5123. [Google Scholar] [CrossRef]
- Liu, L.F.; Desai, S.D.; Li, T.-K.; Mao, Y.; Sun, M.; Sim, S.-P. Mechanism of action of camptothecin. Ann. N. Y. Acad. Sci. 2006, 922, 1–10. [Google Scholar] [CrossRef]
- Onyeibor, O.; Croft, S.L.; Dodson, H.I.; Feiz-Haddad, M.; Kendrick, H.; Millington, N.J.; Parapini, S.; Phillips, R.M.; Seville, S.; Shnyder, S.D.; et al. Synthesis of some cryptolepine analogues, assessment of their antimalarial and cytotoxic activities, and consideration of their antimalarial mode of action. J. Med. Chem. 2005, 48, 2701–2709. [Google Scholar] [CrossRef]
- Chien, C.M.; Yang, S.H.; Lin, K.L.; Chen, Y.L.; Chang, L.S.; Lin, S.R. Novel indoloquinoline derivative, IQDMA, suppresses STAT5 phosphorylation and induces apoptosis in HL-60 cells. Chem. Biol. Interact. 2008, 176, 40–47. [Google Scholar] [CrossRef]
- Franco, L.H.; de Kier Joffé, E.B.; Puricelli, L.; Tatian, M.; Seldes, A.M.; Palermo, J.A. Indole alkaloids from the tunicate aplidium m eridianum. J. Nat. Prod. 1998, 61, 1130–1132. [Google Scholar] [CrossRef]
- Pauletti, P.M.; Cintra, L.S.; Braguine, C.G.; Da Silva Filho, A.A.; Silva, M.L.A.; Cunha, W.R.; Januário, A.H. Halogenated indole alkaloids from marine invertebrates. Mar. Drugs 2010, 8, 1526–1549. [Google Scholar] [CrossRef]
- Wang, X.F.; Ohkoshi, E.; Wang, S.B.; Hamel, E.; Bastow, K.F.; Morris-Natschke, S.L.; Lee, K.H.; Xie, L. Synthesis and biological evaluation of N-alkyl-N-(4-methoxyphenyl) pyridin-2-amines as a new class of tubulin polymerization inhibitors. Bioorganic Med. Chem. 2013, 21, 632–642. [Google Scholar] [CrossRef][Green Version]
- Hu, M.J.; Zhang, B.; Yang, H.K.; Liu, Y.; Chen, Y.R.; Ma, T.Z.; Lu, L.; You, W.W.; Zhao, P.L. Design, synthesis and molecular docking studies of novel indole–pyrimidine hybrids as tubulin polymerization inhibitors. Chem. Biol. Drug Des. 2015, 86, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Geerlings, P.; De Proft, F.; Langenaeker, W. Conceptual Density Functional Theory. Chem. Rev. 2003, 103, 1793. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the Conceptual Density Functional Theory Indices to Organic Chemistry Reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef] [PubMed]
- Foresman, J.B.; Frisch, A. Exploring Chemistry with Electronic Structure Methods, 2nd ed.; Gaussian: Pittsburgh, PA, USA, 1996. [Google Scholar]
- Chang, R. Chemistry, 7th ed.; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Kosar, B.; Albayrak, C. Spectroscopic investigations and quantum chemical computational study of (E)-4-methoxy-2-[(p-tolylimino) methyl] phenol. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2011, 78, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, T.A. Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den einzelnen Elektronen eines Atoms. Physica 1934, 1, 104–113. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density Functional Theory of Atoms and Molecules; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Parr, R.G.; Szentpaly, L.V.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Chamorro, E.; Pérez, P. Understanding the Reactivity of Captodative Ethylenes in Polar Cycloaddition Reactions. A Theoretical Study. J. Org. Chem. 2008, 73, 4615. [Google Scholar]
- Gao, Y.H.; Yang, L.; Zhou, W.; Xu, L.W.; Xia, C.G. Highly efficient bimetallic iron-palladium catalyzed Michael-type Friedel–Crafts reactions of indoles with chalcones. Appl. Organomet. Chem. 2009, 23, 114–116. [Google Scholar] [CrossRef]
- Zinser, C.M.; Warren, K.G.; Nahra, F.; Al-Majid, A.; Barakat, A.; Islam, M.S.; Nolan, S.P.; Cazin, C.S. Palladate precatalysts for the formation of C–N and C–C bonds. Organometallics 2019, 38, 2812–2817. [Google Scholar] [CrossRef]
- Badria, F.A.; Atef, S.; Al-Majid, A.M.; Ali, M.; Elshaier, Y.A.M.M.; Ghabbour, H.A.; Islam, M.S.; Barakat, A. Synthesis and inhibitory effect of some indole-pyrimidine based hybrid heterocycles on α-glucosidase and α-amylase as potential hypoglycemic agents. Chem. Open 2019, 8, 1–11. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2007. [Google Scholar]
- Dennington, R., II; Keith, T.; Millam, J. GaussView, Version 4.1; Semichem Inc.: Shawnee Mission, KS, USA, 2007. [Google Scholar]
- Crystal Explorer 17. Available online: http://hirshfeldsurface.net (accessed on 6 September 2017).
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harbor Protocols 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Chiba, K.; Kawakami, K.; Tohyama, K. Simultaneous evaluation of cell viability by neutral red, MTT and crystal violet staining assays of the same cells. Toxicol. In Vitro 1998, 12, 251–258. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 3a–e are available from the authors. |

| D–H···A | D–H (Å) | H···A (Å) | D···A (Å) | D–H···A (°) | Symm. Codei |
|---|---|---|---|---|---|
| 3a | |||||
| C14–H14A···O1i | 0.930 | 2.390 | 3.307 (7) | 167.7 | x, 3/2 − y, −1/2 + z |
| C18–H18B···O1i | 0.971 | 2.733 | 3.420 (1) | 128.5 | 1 − x, 2 − y, 1 − z |
| 3b | |||||
| C10–H10A···O1i | 0.931 | 2.526 | 3.447 (5) | 170.8 | x, 3/2 − y, −1/2 + z |
| C13–H13A···O1i | 0.931 | 2.391 | 3.269 (5) | 157.1 | x, 3/2 − y, −1/2 + z |
| 3c | |||||
| C2–H2A···O2i | 0.930 | 2.562 | 3.474 (2) | 166.8 | −1 + x, −1 + y, 1 + z |
| C18–H18B···O1i | 0.960 | 2.617 | 3.441 (2) | 144.1 | x, −1 + y, z |
| 3d | |||||
| C18–H18B···O1i | 0.971 | 2.697 | 3.564 (5) | 149.0 | −x, 1 − y, 1 − z |
| 3e | |||||
| C13–H13A···O1i | 0.930 | 2.460 | 3.291 (3) | 148.8 | 1 − x, 2 − y, 1 − z |
| 3a | 3b | 3c | 3d | 3e | |
|---|---|---|---|---|---|
| Br…Br | 0.9 | 0.6 | |||
| Br…C | 0.1 | 1.0 | |||
| Br…H | 16.0 | 14.4 | |||
| O…H | 8.0 | 7.8 | 12.0 | 7.5 | 12.8 |
| C…N | 1.7 | 1.0 | 0.9 | 1.3 | 2.0 |
| C…C | 6.3 | 4.6 | 5.5 | 5.7 | 7.1 |
| C…H | 25.5 | 28.8 | 26.3 | 21.1 | 21.1 |
| N…O | |||||
| C…O | 1.7 | 3.5 | |||
| N…H | 0.3 | 0.8 | |||
| O…O | |||||
| H…H | 41.4 | 41.5 | 52.8 | 64.4 | 53.5 |
| 3a | 3b | 3c | 3d | 3e | |
|---|---|---|---|---|---|
| μ | 5.650 | 3.866 | 7.282 | 5.352 | 5.573 |
| EHOMO | −5.909 | −5.949 | −5.676 | −5.812 | −5.730 |
| ELUMO | −2.132 | −2.175 | −1.758 | −1.771 | −1.887 |
| ΔE | 3.777 | 3.773 | 3.918 | 4.041 | 3.843 |
| I | 5.91 | 5.95 | 5.68 | 5.81 | 5.73 |
| A | 2.13 | 2.18 | 1.76 | 1.77 | 1.89 |
| η | 3.78 | 3.77 | 3.92 | 4.04 | 3.84 |
| μ | -4.02 | -4.06 | -3.72 | -3.79 | -3.81 |
| x | 4.02 | 4.06 | 3.72 | 3.79 | 3.81 |
| S | 0.26 | 0.27 | 0.26 | 0.25 | 0.26 |
| ω | 2.14 | 2.19 | 1.76 | 1.78 | 1.89 |
| N | 3.46 | 3.42 | 3.69 | 3.56 | 3.64 |
| Type | Breast | Oral | Prostate | Colon | Liver | ||
|---|---|---|---|---|---|---|---|
| Cell line | MCF-7 | MDA-MB-231 | SAS | PC-3 | HCT-116 | HuH-7 | HepG2 |
| 3a | 30 ± 2 | 15 ± 1 | 30 ± 1.5 | 30 ± 2.2 | 20 ± 1.3 | 28.4 ± 1.67 | 20 ± 1.5 |
| 3b | >50 | 13 ± 0.5 | 29 ± 3 | >50 | 25 ± 2.1 | NA | 15 ± 0.57 |
| 3c | >50 | 19 ± 1.6 | NA | NA | NA | NA | 25 ± 0.97 |
| 3d | 50 ± 5.5 | 18.5 ± 1 | NA | NA | NA | NA | 25 ± 1.7 |
| 3e | 35 ± 3 | 18 ± 2.4 | 32 ± 2 | 42 ± 3 | 30 ± 2.5 | 29.2 ± 3.4 | 29 ± 1.4 |
| cisplatin | 9 ± 0.3 | 15 ± 1.6 | 4.5 ± 0.4 | 12 ± 1 | 8 ± 1.3 | 14.7 ± 2 | 10 ± 1.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badria, F.A.; Soliman, S.M.; Atef, S.; Islam, M.S.; Al-Majid, A.M.; Dege, N.; Ghabbour, H.A.; Ali, M.; El-Senduny, F.F.; Barakat, A. Anticancer Indole-Based Chalcones: A Structural and Theoretical Analysis. Molecules 2019, 24, 3728. https://doi.org/10.3390/molecules24203728
Badria FA, Soliman SM, Atef S, Islam MS, Al-Majid AM, Dege N, Ghabbour HA, Ali M, El-Senduny FF, Barakat A. Anticancer Indole-Based Chalcones: A Structural and Theoretical Analysis. Molecules. 2019; 24(20):3728. https://doi.org/10.3390/molecules24203728
Chicago/Turabian StyleBadria, Farid A., Saied M. Soliman, Saleh Atef, Mohammad Shahidul Islam, Abdullah Mohammed Al-Majid, Necmi Dege, Hazem A. Ghabbour, M. Ali, Fardous F. El-Senduny, and Assem Barakat. 2019. "Anticancer Indole-Based Chalcones: A Structural and Theoretical Analysis" Molecules 24, no. 20: 3728. https://doi.org/10.3390/molecules24203728
APA StyleBadria, F. A., Soliman, S. M., Atef, S., Islam, M. S., Al-Majid, A. M., Dege, N., Ghabbour, H. A., Ali, M., El-Senduny, F. F., & Barakat, A. (2019). Anticancer Indole-Based Chalcones: A Structural and Theoretical Analysis. Molecules, 24(20), 3728. https://doi.org/10.3390/molecules24203728