Aristolochic Acid Affects Upper Tract Urothelial Cancer Behavior through the MAPK Pathway
Abstract
1. Introduction
2. Results
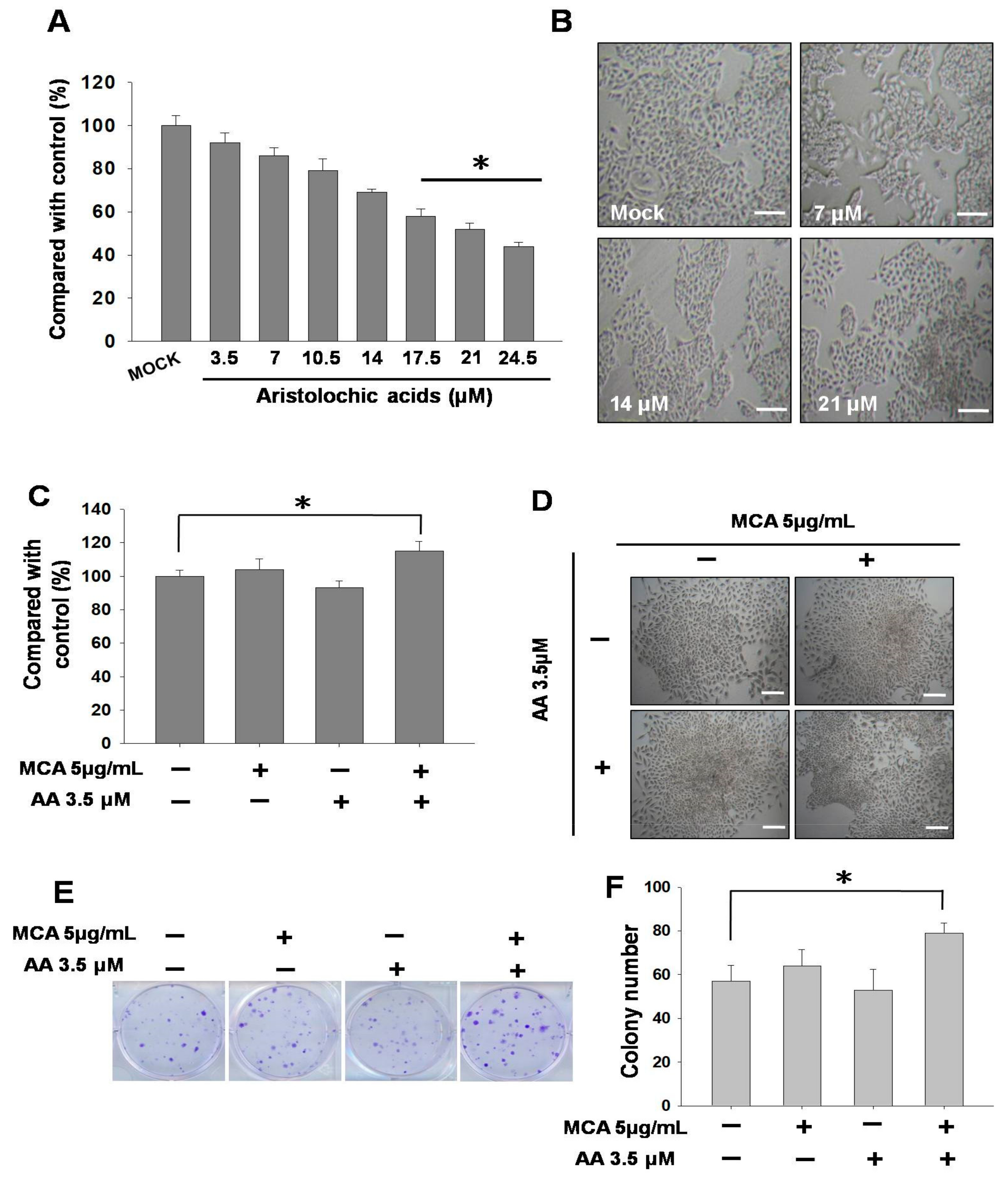
2.1. Cytotoxicity of Aristolochic Acid (AA) in SV-HUC-1 Cells and the Effect of a Low Concentration of AA on MCA-Induced Tumorigenic Transformation
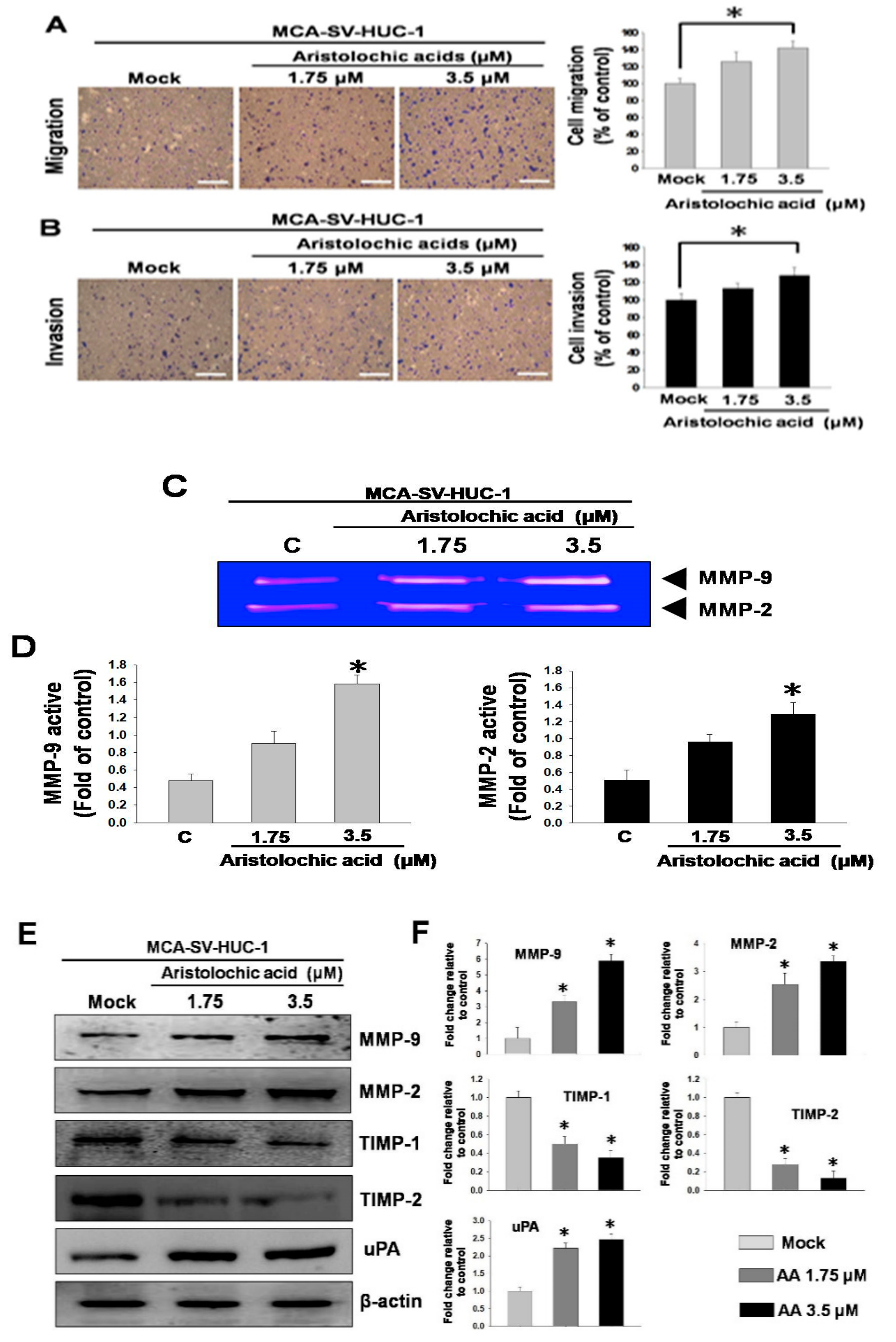
2.2. Changes in Cell Behavior and Matrix Metalloproteinase after Exposure to Aristolochic Acid
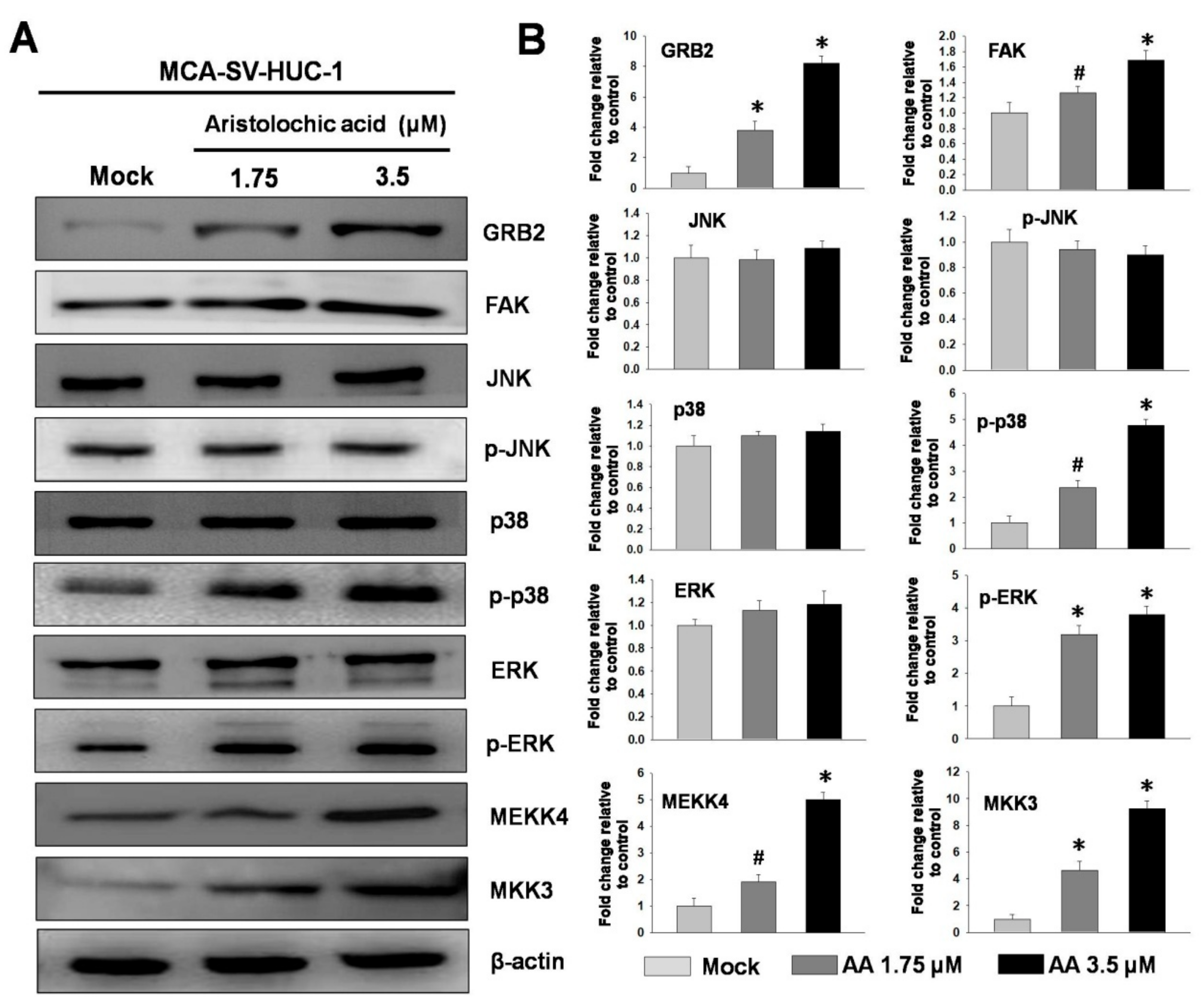
2.3. Aristolochic Acid Induces Cell Migration via Signal Transduction of ERK and p38 MAPK
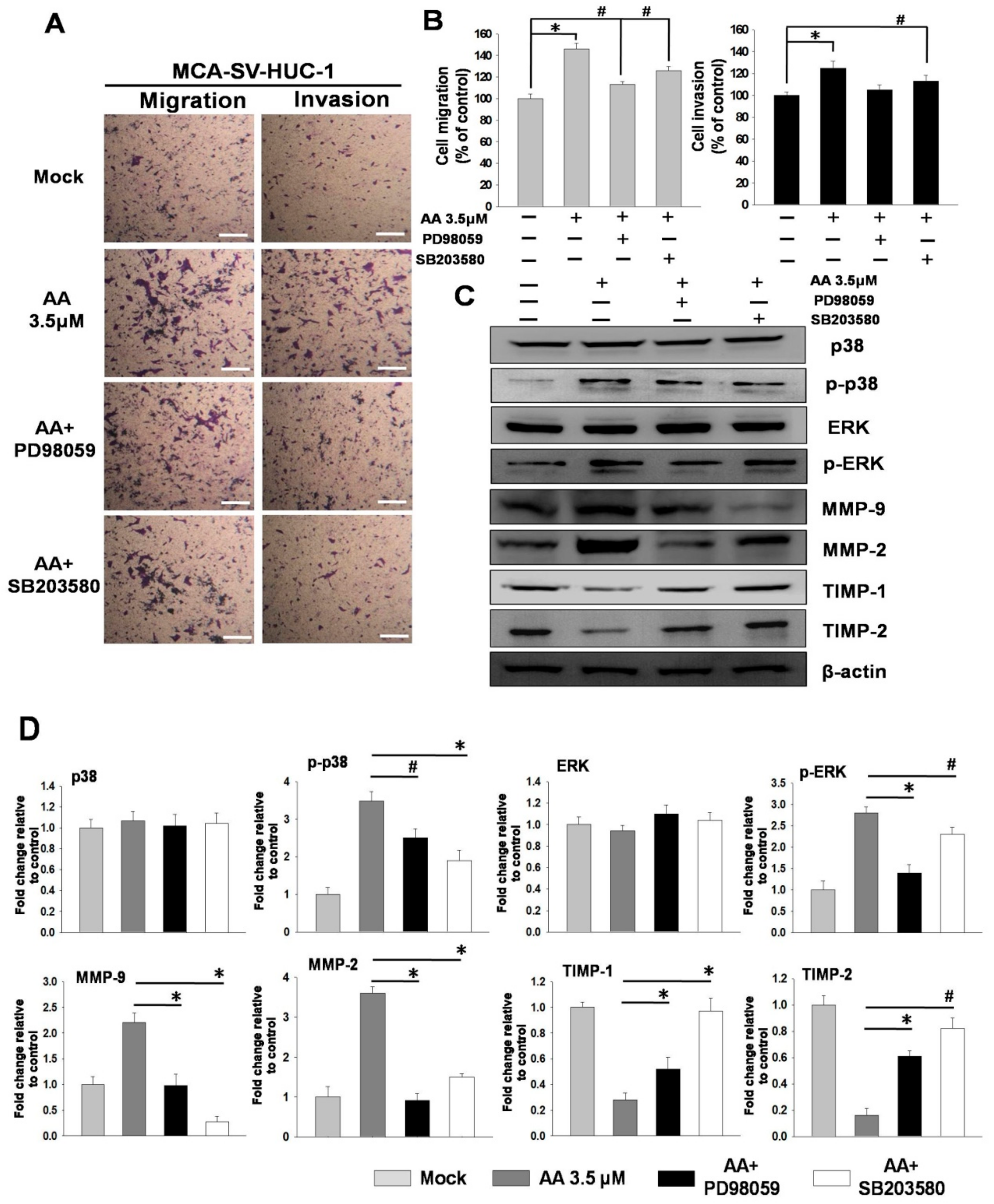
2.4. MAPK Inhibitors Block Aristolochic Acid-Induced Cell Migration through the ERK and p38 MAPK Pathways
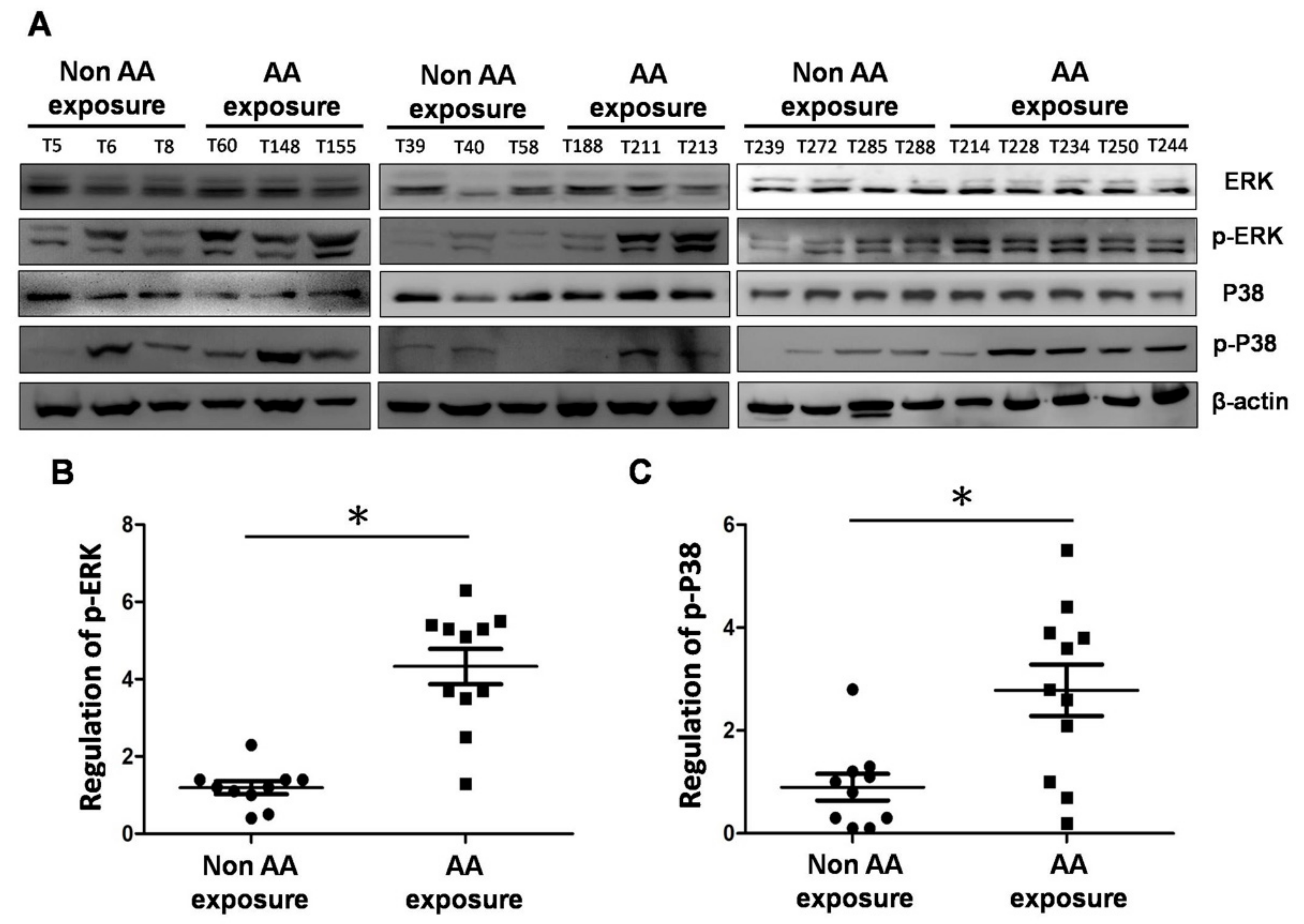
2.5. Expression of MAPK Proteins in UTUC tissues of Patients Exposed to Aristolochic Acid
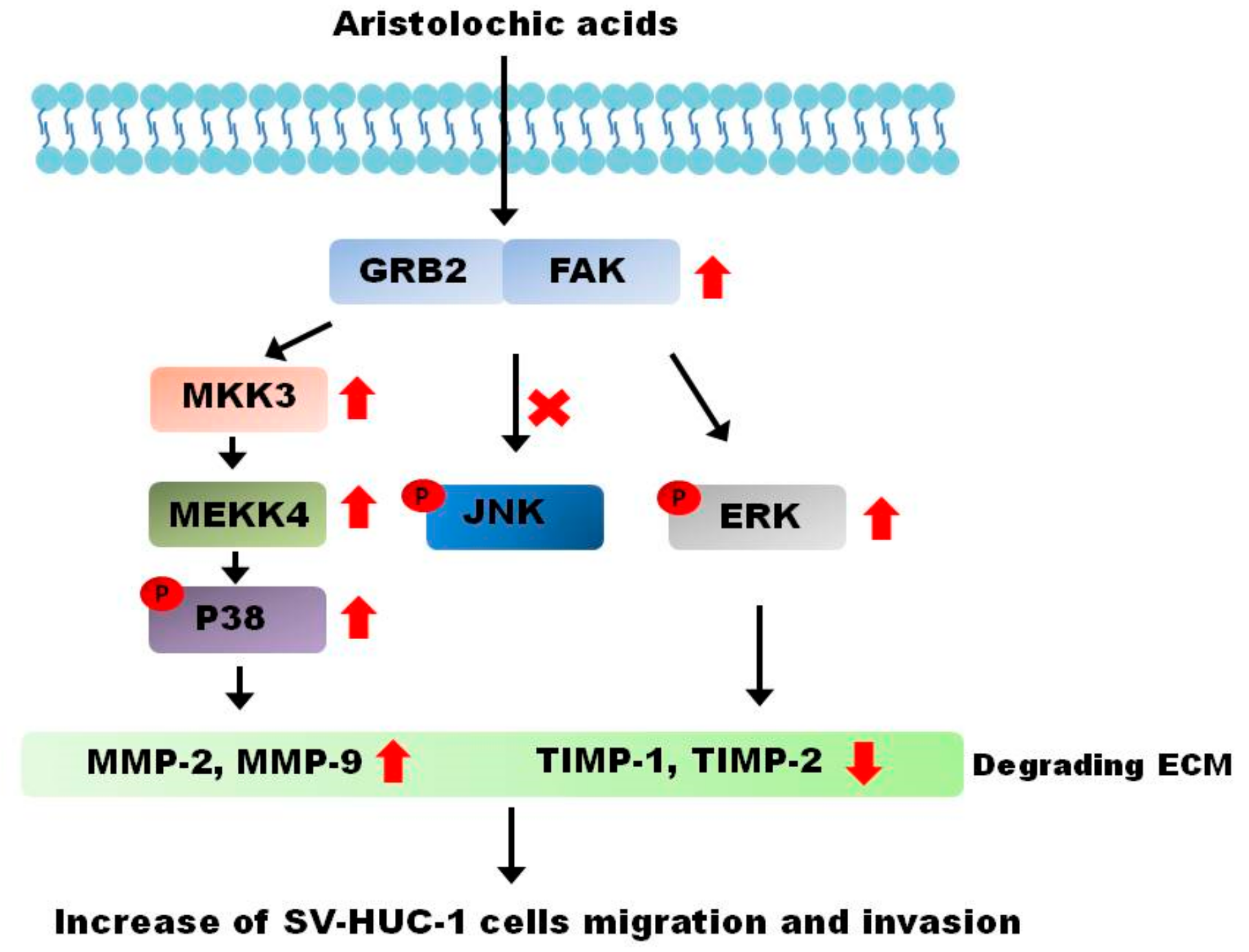
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Cell Culture and Treatment with Aristolochic Acid Induced Tumorigenic Transformation
4.2.2. Cytotoxicity Assay
4.2.3. MCA-SV-HUC-1 Treats MAPKs Inhibitor and Culture
4.2.4. Cell Migration and Invasion Assay
4.2.5. Clonogenic Assay
4.2.6. Determination of MMP-2/MMP-9 Activities by Gelatin Zymography
4.2.7. Protein Extraction and Immunostaining
4.2.8. The Human Samples
4.2.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hung, C.F.; Yang, C.K.; Ou, Y.C. Urologic cancer in Taiwan. Jpn. J. Clin. Oncol. 2016, 46, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Chen, Y.T.; Chung, H.J.; Liu, J.S.; Hsu, C.C.; Tarng, D.C. Kidney disease progression in patients of upper tract urothelial carcinoma following unilateral radical nephroureterectomy. Ren. Fail. 2016, 38, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.B.; Mitchell, J.P. Carcinoma of the ureter—A review of 54 cases. Brit. J. Urol. 1973, 45, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Cutress, M.L.; Stewart, G.D.; Zakikhani, P.; Phipps, S.; Thomas, B.G.; Tolley, D.A. Ureteroscopic and percutaneous management of upper tract urothelial carcinoma (UTUC): Systematic review. BJU Int. 2012, 110, 614–628. [Google Scholar] [CrossRef]
- Ouzzane, A.; Colin, P. Bladder cancer: Tumour recurrence after radical nephroureterectomy for UTUC. Nat. Rev. 2014, 11, 15–16. [Google Scholar] [CrossRef]
- Yang, M.H.; Chen, K.K.; Yen, C.C.; Wang, W.S.; Chang, Y.H.; Huang, W.J.; Fan, F.S.; Chiou, T.J.; Liu, J.H.; Chen, P.M. Unusually high incidence of upper urinary tract urothelial carcinoma in Taiwan. Urology 2002, 59, 681–687. [Google Scholar] [CrossRef]
- Chen, C.Y.; Liao, Y.M.; Tsai, W.M.; Kuo, H.C. Upper urinary tract urothelial carcinoma in eastern Taiwan: High proportion among all urothelial carcinomas and correlation with chronic kidney disease. J. Formos. Med. Assoc. 2007, 106, 992–998. [Google Scholar] [CrossRef]
- Lee, Y.C.; Wu, W.J.; Lin, H.H.; Li, W.M.; Huang, C.N.; Hsu, W.C.; Chang, L.L.; Li, C.C.; Yeh, H.C.; Li, C.F.; et al. Prognostic Value of Leptin Receptor Overexpression in Upper Tract Urothelial Carcinomas in Taiwan. Clin. Genitourin. Canc. 2017, 15, e653–e659. [Google Scholar] [CrossRef]
- Shen, C.H.; Chiou, H.Y.; Tung, M.C.; Wu, C.C.; Kao, W.T.; Wang, Y.H.; Juang, G.D. Clinical and demographic characteristics among patients with urothelial carcinomas of the upper urinary tract and bladder in Taiwan. J. Chin. Med. Assoc. 2017, 80, 563–568. [Google Scholar] [CrossRef]
- Nortier, J.L.; Schmeiser, H.H.; Muniz Martinez, M.C.; Arlt, V.M.; Vervaet, C.; Garbar, C.H.; Daelemans, P.; Vanherweghem, J.L. Invasive urothelial carcinoma after exposure to Chinese herbal medicine containing aristolochic acid may occur without severe renal failure. Nephrol. Dial. Transplant. 2003, 18, 426–428. [Google Scholar] [CrossRef]
- Mei, N.; Arlt, V.M.; Phillips, D.H.; Heflich, R.H.; Chen, T. DNA adduct formation and mutation induction by aristolochic acid in rat kidney and liver. Mutat. Res. 2006, 602, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Vanherweghem, J.L.; Depierreux, M.; Tielemans, C.; Abramowicz, D.; Dratwa, M.; Jadoul, M.; Richard, C.; Vandervelde, D.; Verbeelen, D.; Vanhaelen-Fastre, R.; et al. Rapidly progressive interstitial renal fibrosis in young women: Association with slimming regimen including Chinese herbs. Lancet 1993, 341, 387–391. [Google Scholar] [CrossRef]
- Chen, C.H.; Dickman, K.G.; Huang, C.Y.; Moriya, M.; Shun, C.T.; Tai, H.C.; Huang, K.H.; Wang, S.M.; Lee, Y.J.; Grollman, A.P.; et al. Aristolochic acid-induced upper tract urothelial carcinoma in Taiwan: Clinical characteristics and outcomes. Int. J. Canc. 2013, 133, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Chung, J.G.; Wu, H.C.; Bau, D.T.; Wu, K.Y.; Kao, S.T.; Hsiang, C.Y.; Ho, T.Y.; Chiang, S.Y. Aristolochic acid suppresses DNA repair and triggers oxidative DNA damage in human kidney proximal tubular cells. Oncol. Rep. 2010, 24, 141–153. [Google Scholar] [CrossRef]
- Xiong, G.; Yao, L.; Hong, P.; Yang, L.; Ci, W.; Liu, L.; He, Q.; Gong, K.; Li, X.; Zhou, L. Aristolochic acid containing herbs induce gender-related oncological differences in upper tract urothelial carcinoma patients. Canc. Manag. Res. 2018, 10, 6627–6639. [Google Scholar] [CrossRef]
- Wang, S.M.; Lai, M.N.; Chen, P.C.; Pu, Y.S.; Lai, M.K.; Hwang, J.S.; Wang, J.D. Increased upper and lower tract urothelial carcinoma in patients with end-stage renal disease: A nationwide cohort study in Taiwan during 1997-2008. BioMed Res. Int. 2014, 2014, 149750. [Google Scholar] [CrossRef]
- Aydin, S.; Ambroise, J.; Cosyns, J.P.; Gala, J.L. TP53 mutations in p53-negative dysplastic urothelial cells from Belgian AAN patients: New evidence for aristolochic acid-induced molecular pathogenesis and carcinogenesis. Mutat. Res. 2017, 818, 17–26. [Google Scholar] [CrossRef]
- Karanovic, S.; Lela, I.V.; Jelakovic, B.; Dickman, K.G.; Peic, A.K.; Dittrich, D.; Knezevic, M.; Matijevic, V.; Fernandes, A.S.; Miller, F. Variation in presentation and presence of DNA adducts and p53 mutations in patients with endemic nephropathy--an environmental form of the aristolochic acid nephropathy. Kidney Blood Pressure Res. 2013, 37, 1–8. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, E.B.; Lee, J.; Jung, O.; Ryu, B.J.; Kim, S.H.; Cho, J.Y.; Ryou, C.; Lee, S.Y. Emetine inhibits migration and invasion of human non-small-cell lung cancer cells via regulation of ERK and p38 signaling pathways. Chem.-Biol. Interact. 2015, 242, 25–33. [Google Scholar] [CrossRef]
- Wu, Y.J.; Neoh, C.A.; Tsao, C.Y.; Su, J.H.; Li, H.H. Sinulariolide Suppresses Human Hepatocellular Carcinoma Cell Migration and Invasion by Inhibiting Matrix Metalloproteinase-2/-9 through MAPKs and PI3K/Akt Signaling Pathways. Int. J. Mol. Sci. 2015, 16, 16469–16482. [Google Scholar] [CrossRef]
- Lai, M.N.; Lai, J.N.; Chen, P.C.; Tseng, W.L.; Chen, Y.Y.; Hwang, J.S.; Wang, J.D. Increased risks of chronic kidney disease associated with prescribed Chinese herbal products suspected to contain aristolochic acid. Nephrology 2009, 14, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Dickman, K.G.; Moriya, M.; Zavadil, J.; Sidorenko, V.S.; Edwards, K.L.; Gnatenko, D.V.; Wu, L.; Turesky, R.J.; Wu, X.R.; et al. Aristolochic acid-associated urothelial cancer in Taiwan. Proc. Nat. Acad. Sci. USA 2012, 109, 8241–8246. [Google Scholar] [CrossRef] [PubMed]
- Luciano, R.L.; Perazella, M.A. Aristolochic acid nephropathy: Epidemiology, clinical presentation, and treatment. Drug Safety 2015, 38, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wang, T. Risk assessment of upper tract urothelial carcinoma related to aristolochic acid. Cancer Epidemiol. Biomarkers Prev. 2013, 22, 812–820. [Google Scholar] [CrossRef]
- Chen, C.H.; Dickman, K.G.; Huang, C.Y.; Shun, C.T.; Tai, H.C.; Huang, K.H.; Wang, S.M.; Lee, Y.J.; Grollman, A.P.; Pu, Y.S. Recurrence pattern and TP53 mutation in upper urinary tract urothelial carcinoma. Oncotarget 2016, 7, 45225–45236. [Google Scholar] [CrossRef]
- Lane, B.R.; Smith, A.K.; Larson, B.T.; Gong, M.C.; Campbell, S.C.; Raghavan, D.; Dreicer, R.; Hansel, D.E.; Stephenson, A.J. Chronic kidney disease after nephroureterectomy for upper tract urothelial carcinoma and implications for the administration of perioperative chemotherapy. Cancer 2010, 116, 2967–2973. [Google Scholar] [CrossRef]
- Kaag, M.G.; O’Malley, R.L.; O’Malley, P.; Godoy, G.; Chen, M.; Smaldone, M.C.; Hrebinko, R.L.; Raman, J.D.; Bochner, B.; Dalbagni, G.; et al. Changes in renal function following nephroureterectomy may affect the use of perioperative chemotherapy. Eur. Urol. 2010, 58, 581–587. [Google Scholar] [CrossRef]
- Margulis, V.; Shariat, S.F.; Matin, S.F.; Kamat, A.M.; Zigeuner, R.; Kikuchi, E.; Lotan, Y.; Weizer, A.; Raman, J.D.; Wood, C.G.; et al. Outcomes of radical nephroureterectomy: A series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer 2009, 115, 1224–1233. [Google Scholar] [CrossRef]
- Jablonska-Trypuc, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzyme Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef]
- Shen, M.Y.; Liu, C.L.; Hsiao, G.; Liu, C.Y.; Lin, K.H.; Chou, D.S.; Sheu, J.R. Involvement of p38 MAPK phosphorylation and nitrate formation in aristolochic acid-mediated antiplatelet activity. Planta Med. 2008, 74, 1240–1245. [Google Scholar] [CrossRef]
- Chao, W.; Deng, J.S.; Huang, S.S.; Li, P.Y.; Liang, Y.C.; Huang, G.J. 3, 4-dihydroxybenzalacetone attenuates lipopolysaccharide-induced inflammation in acute lung injury via down-regulation of MMP-2 and MMP-9 activities through suppressing ROS-mediated MAPK and PI3K/AKT signaling pathways. Int. Immunopharmacol. 2017, 50, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, S.; Takeda, K. ERKsignalling as a regulator of cell motility. J. Biochem. 2017, 162, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mao, Y.; Mao, Q.; Fan, W.; Xu, L.; Chen, Y.; Xu, L.; Wang, J. FLOT1 promotes tumor development, induces epithelial-mesenchymal transition, and modulates the cell cycle by regulating the Erk/Akt signaling pathway in lung adenocarcinoma. Thoracic Canc. 2019, 10, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Reznikoff, C.A.; Loretz, L.J.; Christian, B.J.; Wu, S.Q.; Meisner, L.F. Neoplastic transformation of SV40-immortalized human urinary tract epithelial cells by in vitro exposure to 3-methylcholanthrene. Carcinogenesis 1988, 9, 1427–1436. [Google Scholar] [CrossRef]
- Liu, C.; Lin, J.J.; Yang, Z.Y.; Tsai, C.C.; Hsu, J.L.; Wu, Y.J. Proteomic study reveals a co-occurrence of gallic acid-induced apoptosis and glycolysis in B16F10 melanoma cells. J. Agric. Food Chem. 2014, 62, 11672–11680. [Google Scholar] [CrossRef]
- Lin, J.J.; Su, J.H.; Tsai, C.C.; Chen, Y.J.; Liao, M.H.; Wu, Y.J. 11-epi-Sinulariolide acetate reduces cell migration and invasion of human hepatocellular carcinoma by reducing the activation of ERK1/2, p38MAPK and FAK/PI3K/AKT/mTOR signaling pathways. Marine Drugs 2014, 12, 4783–4798. [Google Scholar] [CrossRef]
- Luo, H.L.; Chiang, P.H.; Huang, C.C.; Su, Y.L.; Sung, M.T.; Tsai, E.M.; Lin, C.S.; Chiang, P.H. Methylation of SPARCL1 Is Associated with Oncologic Outcome of Advanced Upper Urinary Tract Urothelial Carcinoma. Int. J. Mol. Sci. 2019, 20, 1653. [Google Scholar] [CrossRef]
- Huang, E.Y.; Chen, Y.F.; Chen, Y.M.; Lin, I.H.; Wang, C.C.; Su, W.H.; Chuang, P.C.; Yang, K.D. A novel radioresistant mechanism of galectin-1 mediated by H-Ras-dependent pathways in cervical cancer cells. Cell Death Disease 2012, 3, e251. [Google Scholar] [CrossRef]
- Yang, T.Y.; Wu, M.L.; Chang, C.I.; Liu, C.I.; Cheng, T.C.; Wu, Y.J. Bornyl cis-4-Hydroxycinnamate Suppresses Cell Metastasis of Melanoma through FAK/PI3K/Akt/mTOR and MAPK Signaling Pathways and Inhibition of the Epithelial-to-Mesenchymal Transition. Int. J. Mol. Sci. 2018, 19, 2152. [Google Scholar] [CrossRef]
- Lin, J.J.; Wang, R.Y.; Chen, J.C.; Chiu, C.C.; Liao, M.H.; Wu, Y.J. Cytotoxicity of 11-epi-Sinulariolide Acetate Isolated from Cultured Soft Corals on HA22T Cells through the Endoplasmic Reticulum Stress Pathway and Mitochondrial Dysfunction. Int. J. Mol. Sci. 2016, 17, 1787. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, I.-H.; Luo, H.-L.; Su, Y.-L.; Huang, C.-C.; Chiang, P.-H.; Yu, C.-C.; Lee, N.-L.; Lin, J.-J.; Sung, M.-T. Aristolochic Acid Affects Upper Tract Urothelial Cancer Behavior through the MAPK Pathway. Molecules 2019, 24, 3707. https://doi.org/10.3390/molecules24203707
Chen I-H, Luo H-L, Su Y-L, Huang C-C, Chiang P-H, Yu C-C, Lee N-L, Lin J-J, Sung M-T. Aristolochic Acid Affects Upper Tract Urothelial Cancer Behavior through the MAPK Pathway. Molecules. 2019; 24(20):3707. https://doi.org/10.3390/molecules24203707
Chicago/Turabian StyleChen, I-Hsuan, Hao-Lun Luo, Yu-Li Su, Chun-Chieh Huang, Po-Hui Chiang, Chia-Cheng Yu, Nai-Lun Lee, Jen-Jie Lin, and Ming-Tse Sung. 2019. "Aristolochic Acid Affects Upper Tract Urothelial Cancer Behavior through the MAPK Pathway" Molecules 24, no. 20: 3707. https://doi.org/10.3390/molecules24203707
APA StyleChen, I.-H., Luo, H.-L., Su, Y.-L., Huang, C.-C., Chiang, P.-H., Yu, C.-C., Lee, N.-L., Lin, J.-J., & Sung, M.-T. (2019). Aristolochic Acid Affects Upper Tract Urothelial Cancer Behavior through the MAPK Pathway. Molecules, 24(20), 3707. https://doi.org/10.3390/molecules24203707





