Synthesis of Samarium-Based Metal Organic Compound Nanoparticles with Polychromatic-Photoluminescence for Bio-Tissue Fluorescence Imaging
Abstract
1. Introduction
2. Results
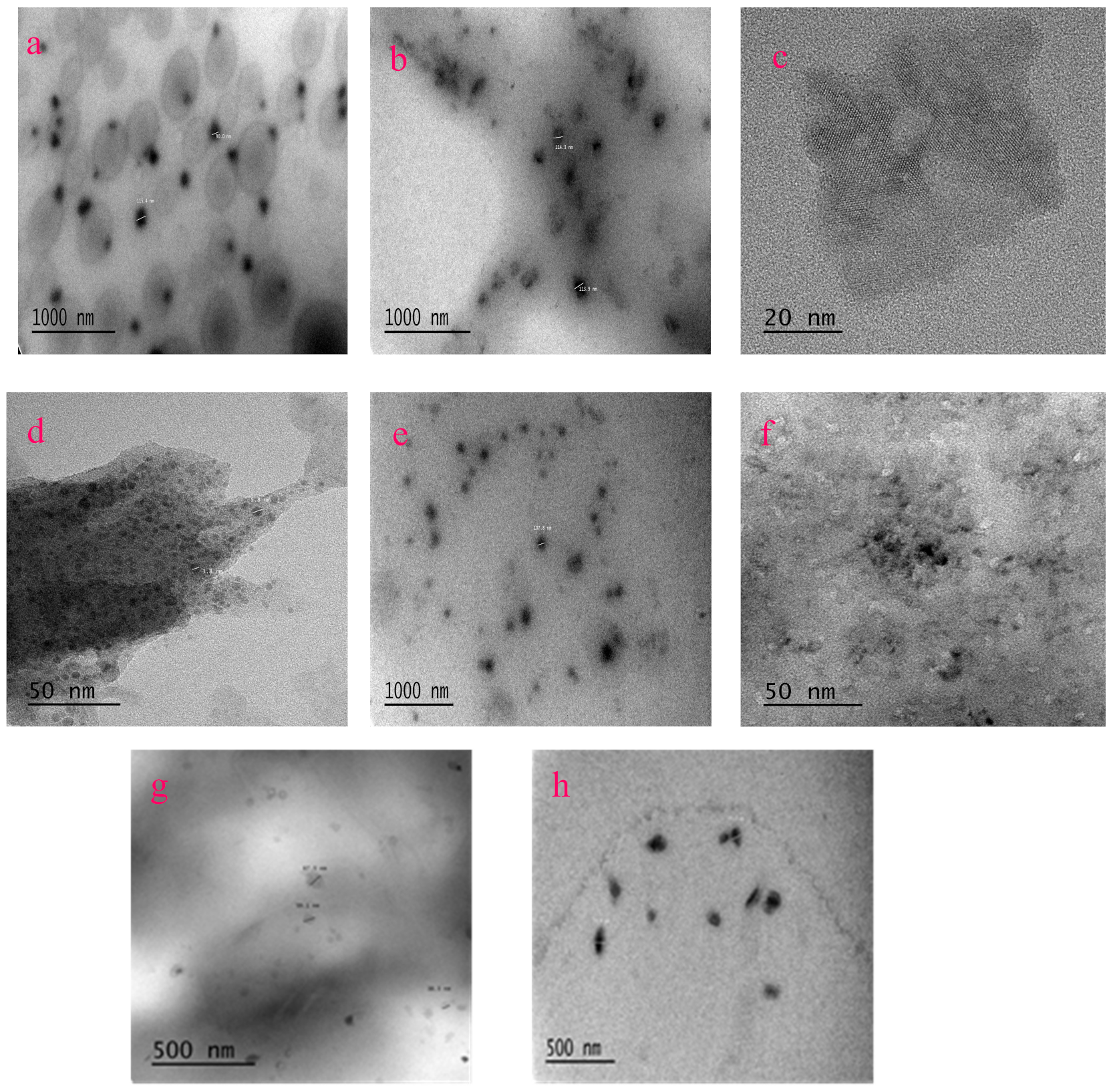
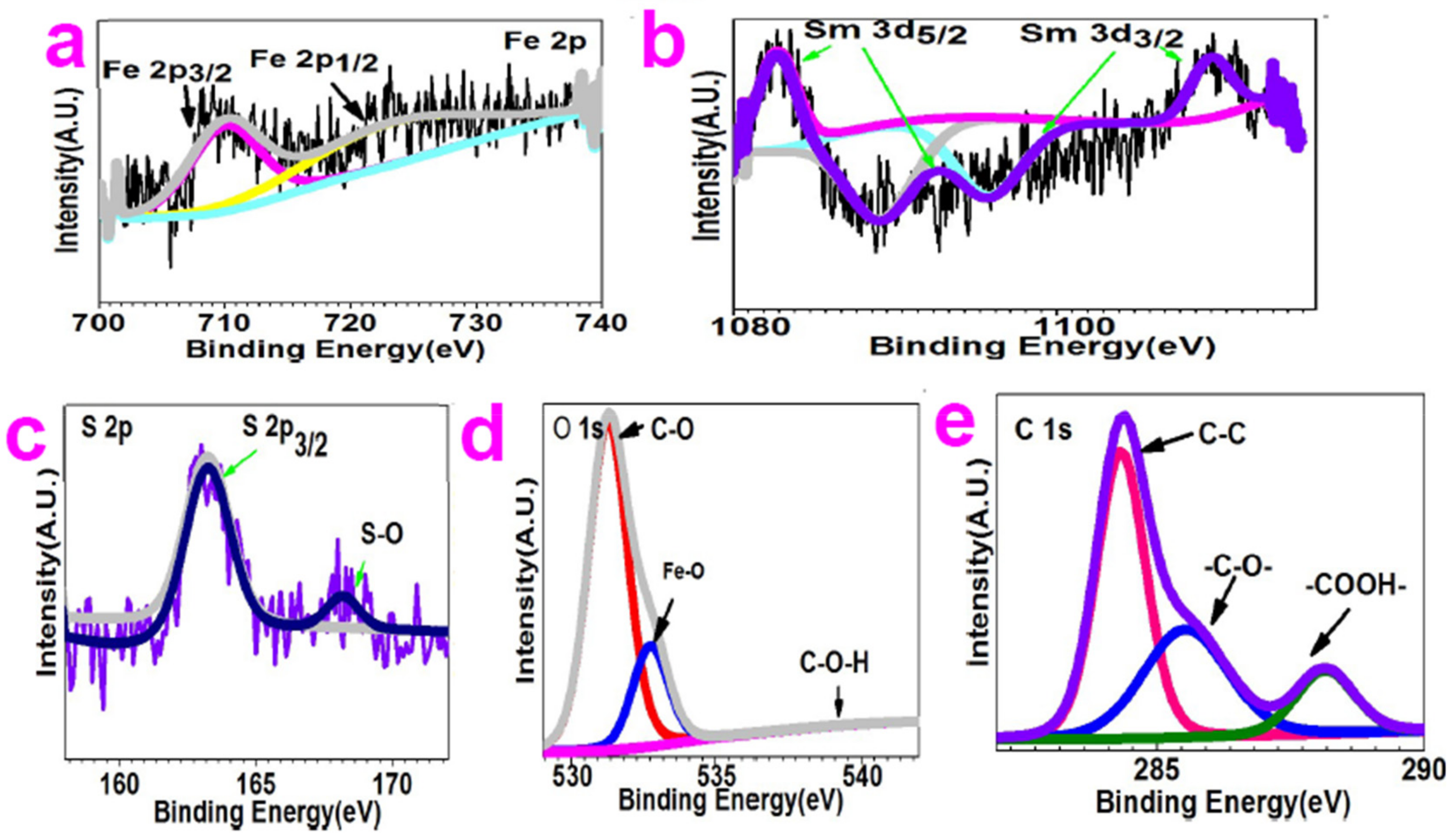
2.1. Characterization
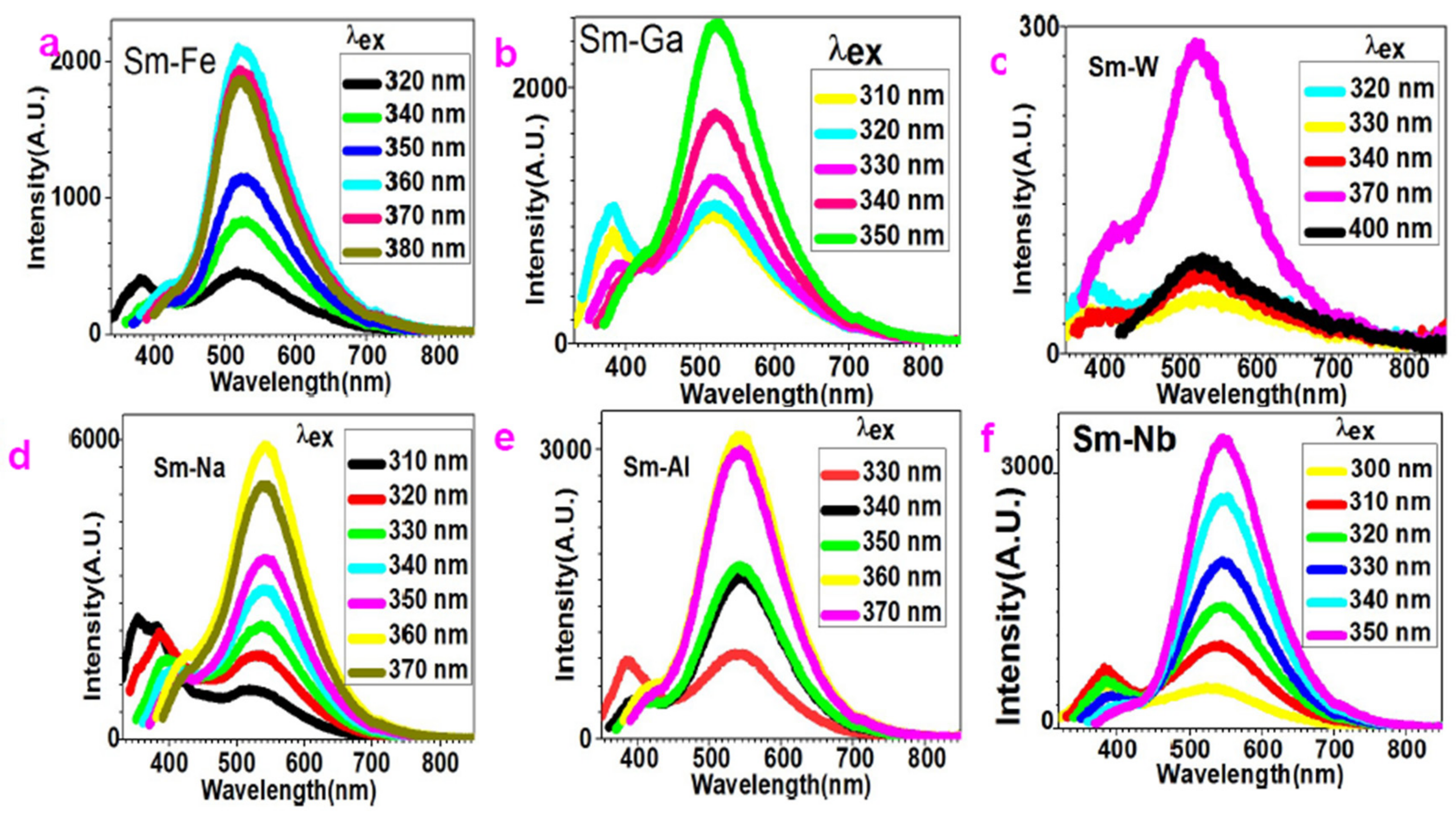
2.2. White Light Emission with Tunable White-to-Green Fluorescence by Variation of Excitation Light
2.3. Polychromatic-Photoluminescence
2.4. Near-Infrared Fluorescence
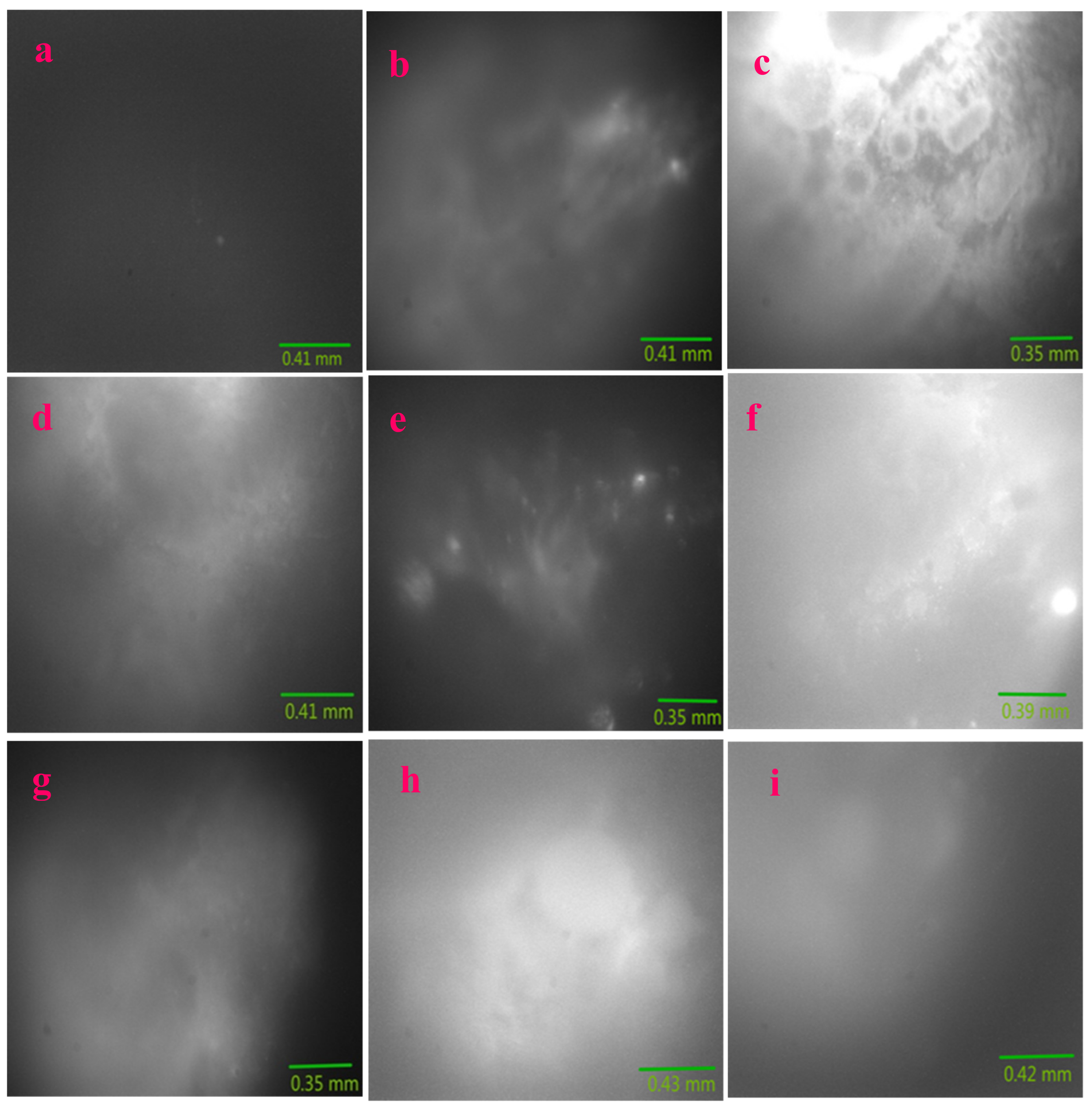
2.5. Fluorescence Imaging of Pig Kidney Tissues
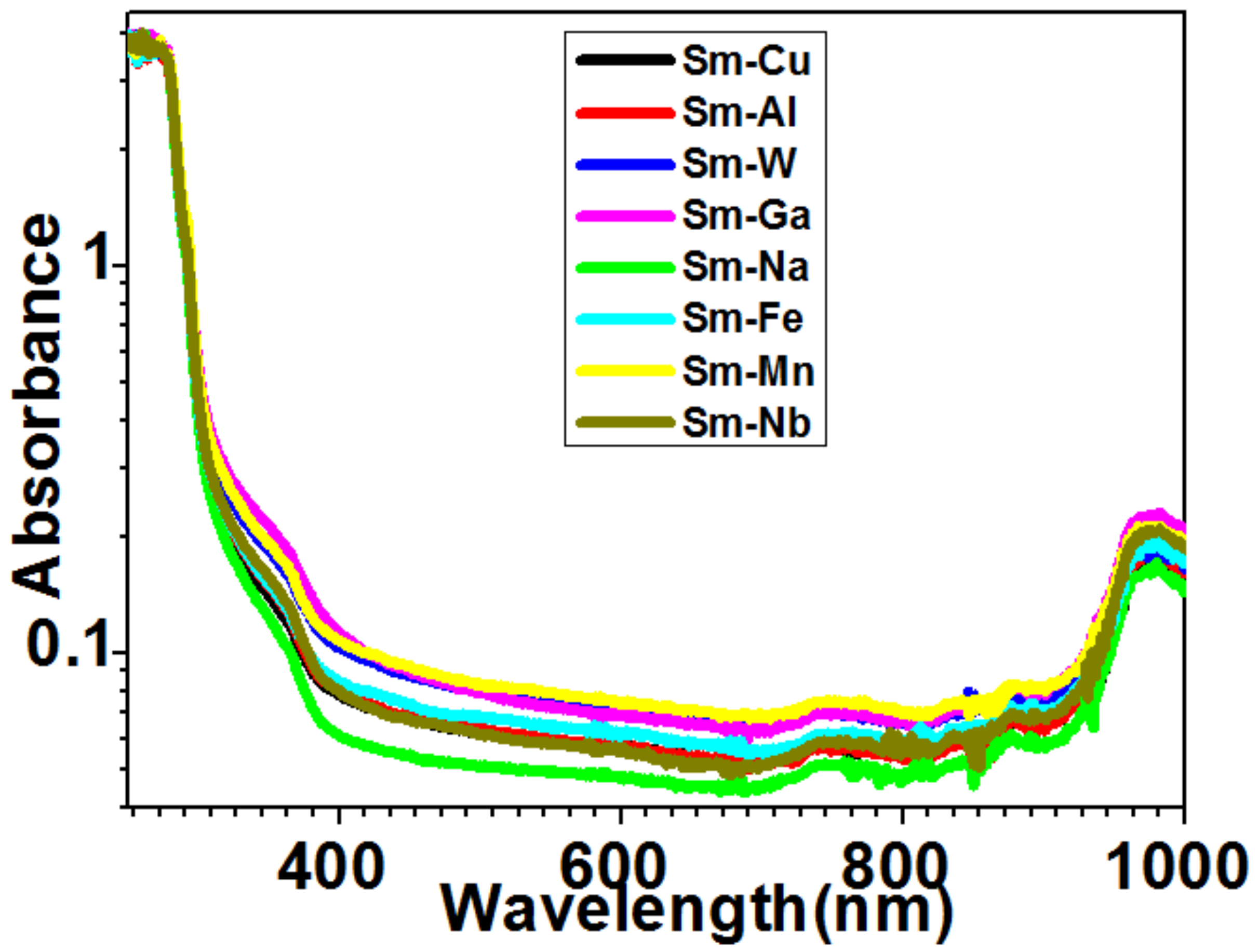
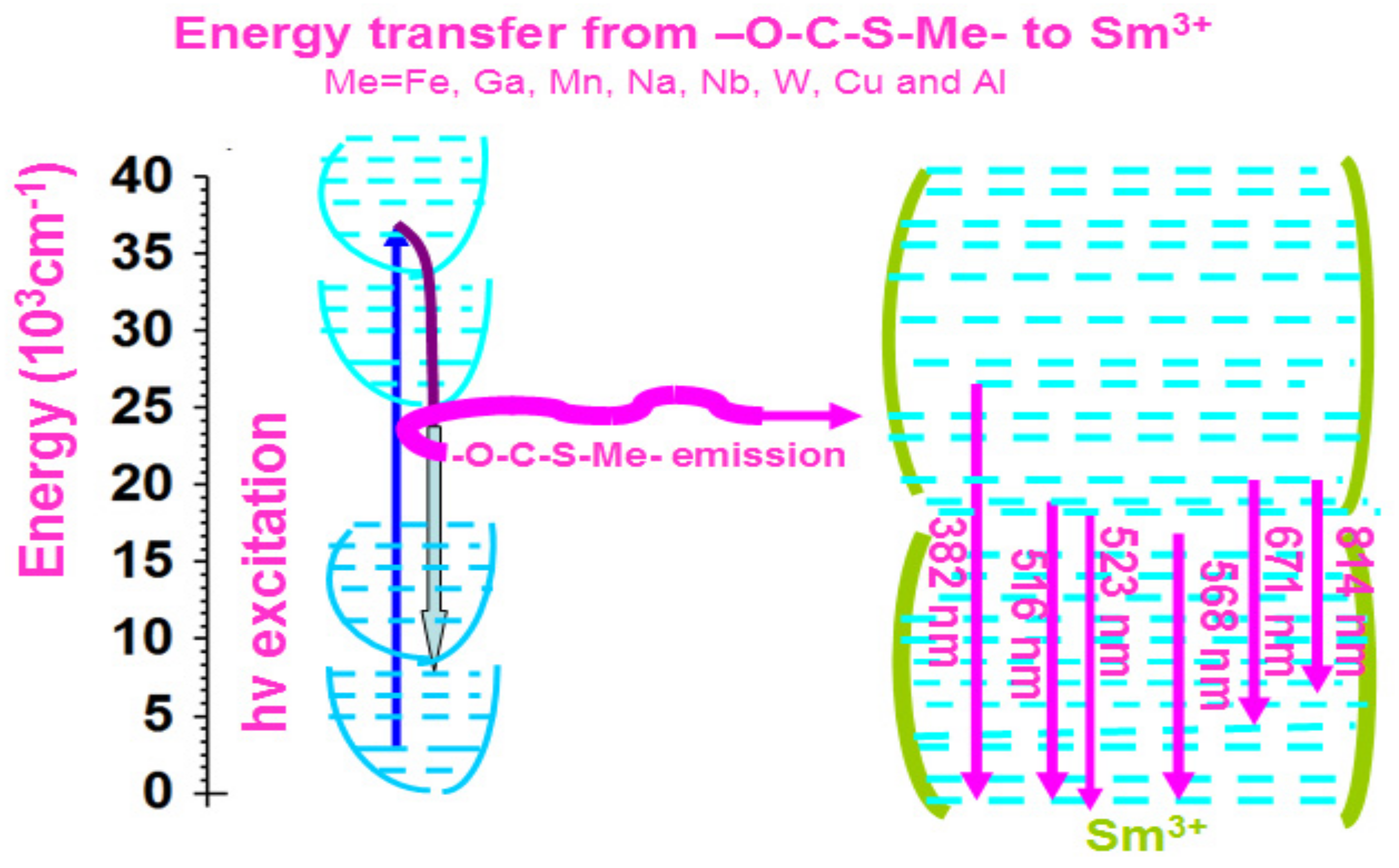
2.6. Optical Chemistry of Sm–Fe, Sm–Ga, Sm–Mn, Sm–Na, Sm–Nb, Sm–W, Sm–Cu, and Sm–Al Compounds
3. Discussion
4. Materials and Methods
4.1. Synthesis of Sm–Me (Me = Fe, Ga, Mn, Na, Nb, W, Cu, and Al) Nanoparticles
4.2. Instruments
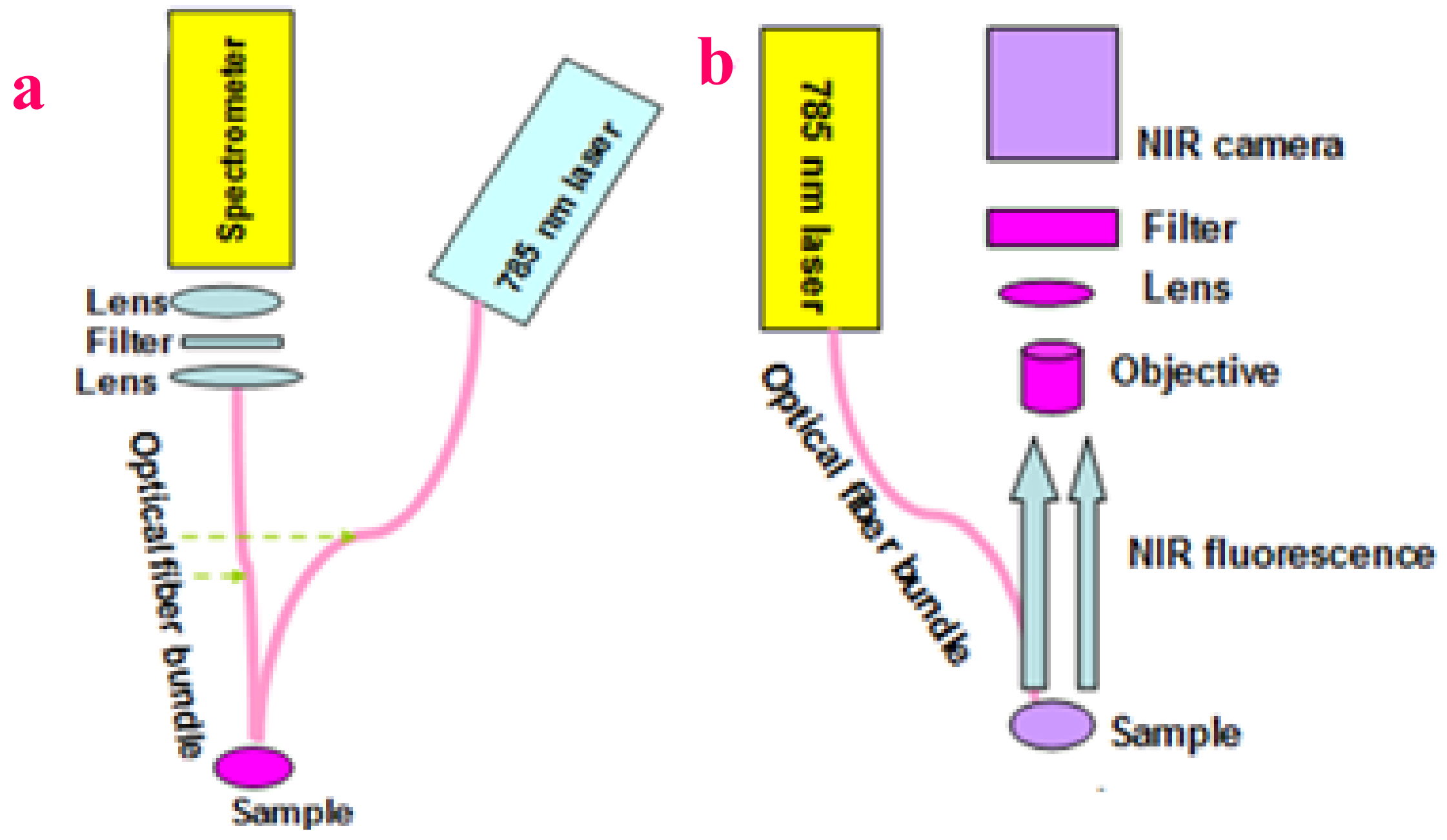
4.3. Experimental Setup of Fluorescence Spectroscopy and Fluorescence Imaging
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kamolz, L.P.; Andel, H.; Auer, T.; Meissl, G.; Frey, M. Evaluation of skin perfusion by use of indocyanine green video angiography: Rational design and planning of trauma surgery. J. Trauma 2006, 61, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Orth, D.H.; Patz, A.; Flower, R.W. Potential clinical applications of indocyanine green choroidal angiography-preliminary report. Eye Ear. Nose Throat Mon. 1976, 55, 15–28. [Google Scholar] [PubMed]
- Schaafsma, B.E.; Sven, D.; Mieog, J.; Hutteman, M.; Van Der Vorst, J.R.; Kuppen, P.J.K.; Lo Wik, C.W.G.M.; Frangioni, J.V.; Van De Velde, C.J.H.; Vahrmeijer, A.L. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J. Surg. Oncol. 2011, 104, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Troyan, S.L.; Kianzad, V.; Gibbs-Strauss, S.L.; Gioux, S.; Matsui, A.; Oketokoun, R.; Ngo, L.; Khamene, A.; Azar, F.; Frangioni, J.V. The FLARE intraoperative near-infrared fluorescence imaging system: A first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann. Surg. Oncol. 2009, 16, 2943–2952. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yao, S.; Xu, J.; Wu, Y.; Li, C.; He, Z. Endoscopic near-infrared dental imaging with indocyanine green: A pilot study. Ann. the New York Aca. Sci. 2018, 1421, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-S.; Zhao, B.; Zhai, B.; Shi, W.; Cheng, P.; Liao, D.-Z.; Yan, D.-Z. Syntheses, Structures, and Photoluminescence of One-Dimensional Lanthanide Coordination Polymers with 2,4,6-Pyridinetricarboxylic Acid. Cryst. Growth Des. 2007, 7, 1851. [Google Scholar] [CrossRef]
- Feng, X.; Wang, L.-Y.; Zhao, J.-S.; Wang, J.-G.; Weng, N.S.; Liu, B.; Shi, X.-G. Series of anion-directed lanthanide-rigid-flexible frameworks: Syntheses, structures, luminescence and magnetic properties. Cryst. Eng. Comm. 2010, 12, 774–783. [Google Scholar] [CrossRef]
- Liao, J.-H.; Tsai, C.-S.; Lin, T.-K. Syntheses, structural characterization and luminescent properties of M2(ATPA)3(DMF)2(H2O)2 (M = Nd, Sm, Eu, Gd, Tb, Dy; ATPA = 2-aminoterephthalate, DMF = N,N-dimethylformamide). Inorg. Chem. Commun. 2010, 13, 286–289. [Google Scholar] [CrossRef]
- Zhao, X.-Q.; Zhao, B.; Shi, W.; Cheng, P. Structures and luminescent properties of a series of Ln–Ag heterometallic coordination polymers. Cryst. Eng. Comm. 2009, 11, 1261–1269. [Google Scholar] [CrossRef]
- Bo, Q.-B.; Sun, G.-X.; Geng, D.-L. Novel Three-Dimensional Pillared-Layer Ln(III)−Cu(I) Coordination Polymers Featuring Spindle-Shaped Heterometallic Building Units. Inorg. Chem. 2010, 49, 561–571. [Google Scholar] [CrossRef]
- Liu, W.; Li, Z.; Wang, N.; Li, X.; Wei, Z.; Yue, S.; Y. Liu, Y. A new family of 3D heterometallic 3d–4f organodisulfonate complexes based on the linkages of 2D [Ln(nds)(H2O)]+ layers and [Cu(ina)2]− chains. Cryst. Eng.Comm. 2011, 13, 138–144. [Google Scholar] [CrossRef]
- Chandler, B.D.; Cramb, D.T.; Shimizu, G.K.H. Microporous Metal−OrganicFrameworks Formed in a Stepwise Manner from Luminescent Building Blocks. J. Am. Chem. Soc. 2006, 128, 10403–10412. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent Functional Metal–Organic Frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.P.; Bell, T.D.M.; Cox, R.P.; Daniels, D.P.; Deacon, G.B.; Jaroschik, F.; Junk, P.C.; Le Goff, X.F.; Lemercier, G.; Martinez, A.; et al. Divalent Tetra- Penta-phenylcyclopentadienyl Europium and Samarium Sandwich and Half-Sandwich Complexes: Synthesis, Characterization, and Remarkable Luminescence Properties. Organometallics 2015, 34, 5624–5636. [Google Scholar] [CrossRef]
- Melo, L.L.L.S.; Castro, G.P., Jr.; Gonçalves, S.M.C. Substantial Intensification of the Quantum Yield of Samarium (III) Complexes by Mixing Ligands: Microwave-Assisted Synthesis and Luminescence Properties. Inorg. Chem. 2019, 58, 3265–3270. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yang, M.; Che, Y.; Zhang, Z.; Ge, X.; Xiang, J.; Zhou, Y.; Xiong, K.; Yu, H. Structure−Optical Behavior Correlation, Optimized Photoluminescence, and DFT Calculation of La7O6(BO3)(PO4)2:Sm3+ Micropowder for Solid State Lighting. ACS Appl. Electron. Mater. 2019, 1, 1688–1697. [Google Scholar] [CrossRef]
- Knope, K.E.; de Lill, D.T.; Rowland, C.E.; Cantos, P.M.; de Bettencourt-Dias, A.; Cahill, C.L. Uranyl sensitization of samarium(III) luminescence in a two-dimensional coordination polymer. Inorg. Chem. 2012, 51, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Bui, A.T.; Grichine, A.; Brassele, S.; Duperray, A.; Andraud, C.; Maury, O. Unexpected Efficiency of a Luminescent Samarium(III) Complex for Combined Visible and Near-Infrared Biphotonic Microscopy. Chem. Eur. J. 2015, 21, 17757–17761. [Google Scholar] [CrossRef] [PubMed]
- Lunstroot, K.; Nockemann, P.; Van Hecke, K.; Van Meervelt, L.; Go rller-Walrand, C.; Binnemans, K.; Driesen, K. Visible and near-infrared emission by samarium (III)-containing ionic liquid mixtures. Inorg. Chem. 2009, 48, 3018–3026. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Suga, S.; Imada, S.; Ochiai, A.; Suzuki, T. XPS and BIS studies of electronic structures of Sm3Se4 and Sm4As3. Physica B 1993, 186, 59–62. [Google Scholar] [CrossRef]
- Hillebrecht, F.U.; Fuggle, J.C. Invalidity of 4f count determination and possibilities for determination of 4f hybridization in intermetallics of the light rare earths by core-level spectroscopy. Phys. Rev. B 1982, 25, 3550–3556. [Google Scholar] [CrossRef]
- Kelemen, S.R.; George, G.N.; Gorbaty, M.L. Direct determination and quantification of sulphur forms in heavy petroleum and coals: 1. The X-ray photoelectron spectroscopy (XPS) approach. Fuel 1990, 69, 939–944. [Google Scholar] [CrossRef]
- Smart, R.S.C.; Skinner, W.M.; Gerson, A.R. XPS of sulphide mineral surfaces: Metal-deficient, polysulphides, defects and elemental sulphur. Surf. Interface Anal. 1999, 28, 101–105. [Google Scholar] [CrossRef]
- Ganguly, A.; Sharma, S.; Papakonstantinou, P.; Hamilton, J. Probing the Thermal Deoxygenation of Graphene Oxide Using High-Resolution In Situ X-ray-Based Spectroscopies. J. Phys. Chem. C 2011, 115, 17009–17019. [Google Scholar] [CrossRef]
- Desimoni, E.; Casella, G.I.; Morone, A.; Salvi, A.M. XPS/SEM characterization of surface-treated carbon fibres. Surf. Inter. Anal. 1990, 16, 468–469. [Google Scholar] [CrossRef]
- Park, S.J.; Jang, Y.-S. Pore Structure and Surface Properties of Chemically Modified Activated Carbons for Adsorption Mechanism and Rate of Cr (VI). J. Colloid Inter. Sci. 2002, 249, 458–463. [Google Scholar] [CrossRef]
- Wang, M.-S.; Guo, S.-P.; Li, Y.; Cai, L.-Z.; Zou, J.-P.; Xu, G.; Zhou, W.-W.; Zheng, F.-K.; Guo, G.-C.A. A Direct White-Light-Emitting Metal−Organic Framework with Tunable Yellow-to-White Photoluminescence by Variation of Excitation Light. J. Am. Chem. Soc. 2009, 131, 13572–13573. [Google Scholar] [CrossRef]
- Biju, P.R.; Jose, G.; Thomas, V.; Nampoori, V.P.N.; Unnikrishnan, N.V. Energy transfer in Sm3+: Eu3+ system in zinc sodium phosphate glasses. Opt. Mat. 2004, 24, 671–677. [Google Scholar] [CrossRef]
- Jamalaiah, B.C.; Suresh Kumar, J.; Mohan Babu, A.; Suhasini, T.; Rama Moorthy, L. Photoluminescence properties of Sm3+ in LBTAF glasses. J. Luminescence 2009, 129, 363–369. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Yang, J.; Lin, Y.; Xu, J. Synthesis of Samarium-Based Metal Organic Compound Nanoparticles with Polychromatic-Photoluminescence for Bio-Tissue Fluorescence Imaging. Molecules 2019, 24, 3657. https://doi.org/10.3390/molecules24203657
Wu Y, Yang J, Lin Y, Xu J. Synthesis of Samarium-Based Metal Organic Compound Nanoparticles with Polychromatic-Photoluminescence for Bio-Tissue Fluorescence Imaging. Molecules. 2019; 24(20):3657. https://doi.org/10.3390/molecules24203657
Chicago/Turabian StyleWu, Ye, Jiquan Yang, Yingcheng Lin, and Jian Xu. 2019. "Synthesis of Samarium-Based Metal Organic Compound Nanoparticles with Polychromatic-Photoluminescence for Bio-Tissue Fluorescence Imaging" Molecules 24, no. 20: 3657. https://doi.org/10.3390/molecules24203657
APA StyleWu, Y., Yang, J., Lin, Y., & Xu, J. (2019). Synthesis of Samarium-Based Metal Organic Compound Nanoparticles with Polychromatic-Photoluminescence for Bio-Tissue Fluorescence Imaging. Molecules, 24(20), 3657. https://doi.org/10.3390/molecules24203657





