1. Introduction
With China’s high regard for Chinese medicines, the Chinese medicine industry has developed rapidly. Chinese herbal medicines and Chinese patent medicines have been widely accepted and used as the main drugs. However, during the process, large amounts of wastes from Chinese medicines has been produced every day. It is reported that the annual amount of drug residues in China was more than 30 million tons [
1]. Chinese medicine slag usually contains high moisture and certain nutrients, which is highly prone to be corrupted, consequently causing serious pollution to the environment. At present, some Chinese medicine slags have been recycled with being used as edible fungi cultivation bases, organic fertilizers, livestock and poultry feed, papermaking, etc. However, the additional values of these products is still low. Additionally, these products may cause secondary pollution to the environment [
2]. Therefore, it is of great significance to develop high value-added products of Chinese medicine slag, to establish a recycling industrial chain, to accomplish the benign recycling of ecological resources, to realize the sustainable development strategy of resources, and to solve the serious pollution problems.
Astragalus radix (
radix), a top grade herb, was first recorded in “Shen Nong’s Herbal Classic”. Its medicinal history has been recorded for more than 2000 years. In clinical application, 80% prescriptions of Chinese herbal medicines contain
A. radix. Due to its importance,
A. radix has been listed as the 60 strategic key varieties of the state and the 18 major varieties of Chinese medicinal materials in the Ministry of Commerce. Moreover,
A. radix has been included in the list of national drug and food homology in 2018. At present, the annual production of residues of
A.
radix in China exceeds tens of thousands of tons, which is still increasing year by year. Unlike the main components of cell wall hemicellulose of other higher plants, i.e., xyloglucan (no pharmacological activity), our previous studies [
3] showed that the main component of the cell wall hemicellulose of
radix herb residues is arabinoxylan. The main chain and the side chain of arabinoxylan (molecular weight distribution 3.323 × 10
5–7.256 × 10
6 Da) is composed of β-1,4-
d-xylopyranose and α-
l-arabinofuranose, respectively. Current studies have shown [
4] that arabinoxylan extracted from wheat bran has anti-tumor activity with significantly inhibiting the growth of tumor-bearing mice and the growth of S180 mouse transplantable tumors, as well as with remarkably promoting thymus and spleen indexes, splenocyte proliferation, natural killer cell and macrophage phagocytosis activity, interleukin 2 production, and delayed-type hypersensitivity reaction.
The amount of arabinoxylan accounts for about 10% of herb residues of A. radix, which is 1000 times higher than that of soluble arabinogalactan in the radix cytoplasm. The latter has been developed as A. radix polysaccharide for injection, which is a commercial drug. However, the extraction process of soluble arabinogalactan consumes a large number of crude herb materials and is costly. At present, the drug is mainly used in leukopenia, lung cancer and other diseases. Yet, it is still not clear whether arabinoxylan of A. radix herb residues has anti-lung cancer activity. Therefore, herein, we aimed to explore the mechanisms of immune regulation and promotion of tumor cell apoptosis of arabinoxylan by investigating its chemical structure including monosaccharide composition, junction site, etc. and its antitumor activity both in vitro and in vivo. We aimed to develop high value-added anti-tumor products of arabinoxylan, and to establish a recycling industry chain, as well as to lay a foundation for the benign recycling of ecological resources. Eventually, the findings of this study would contribute to the solution of the serious environmental pollution problems currently faced.
3. Conclusions and Discussion
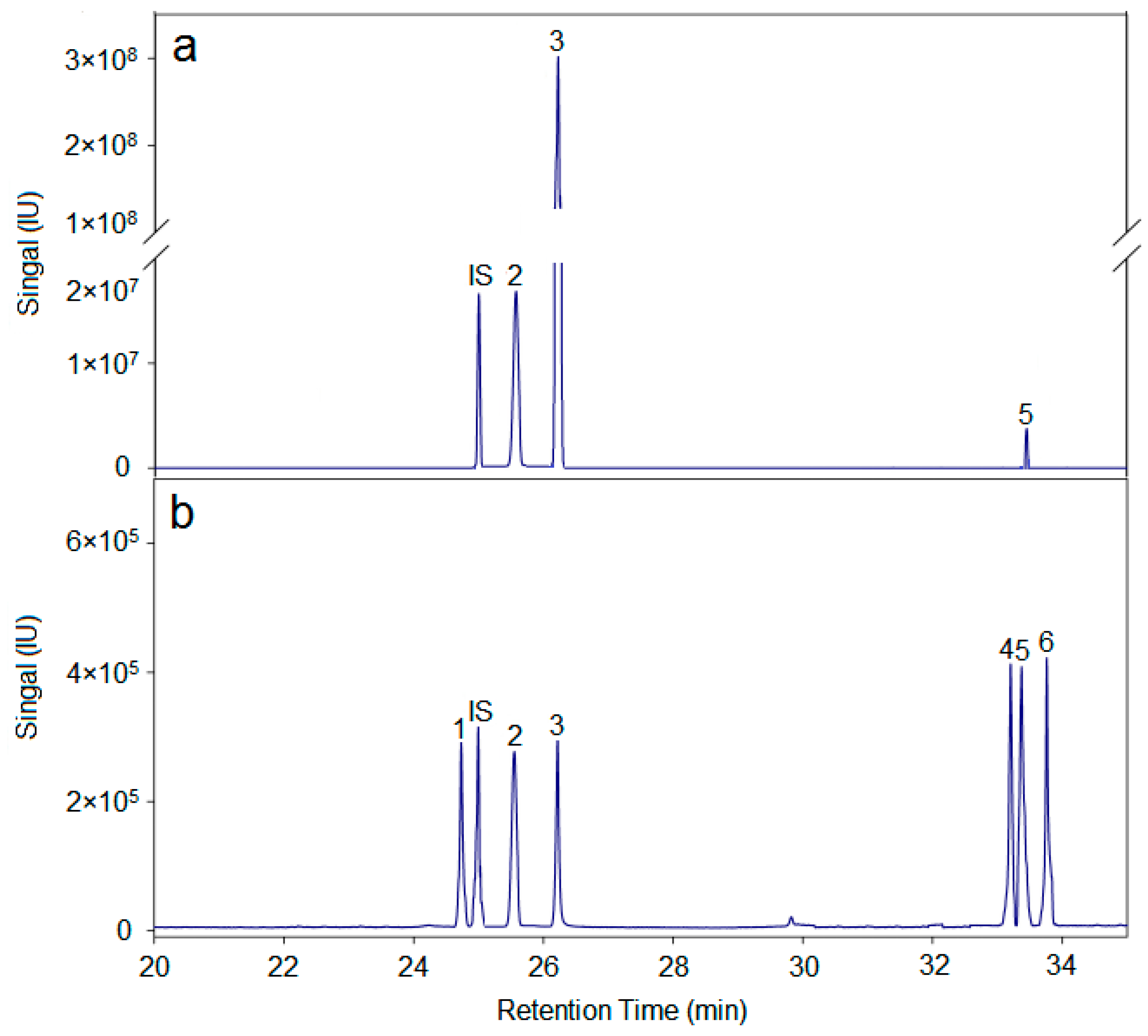
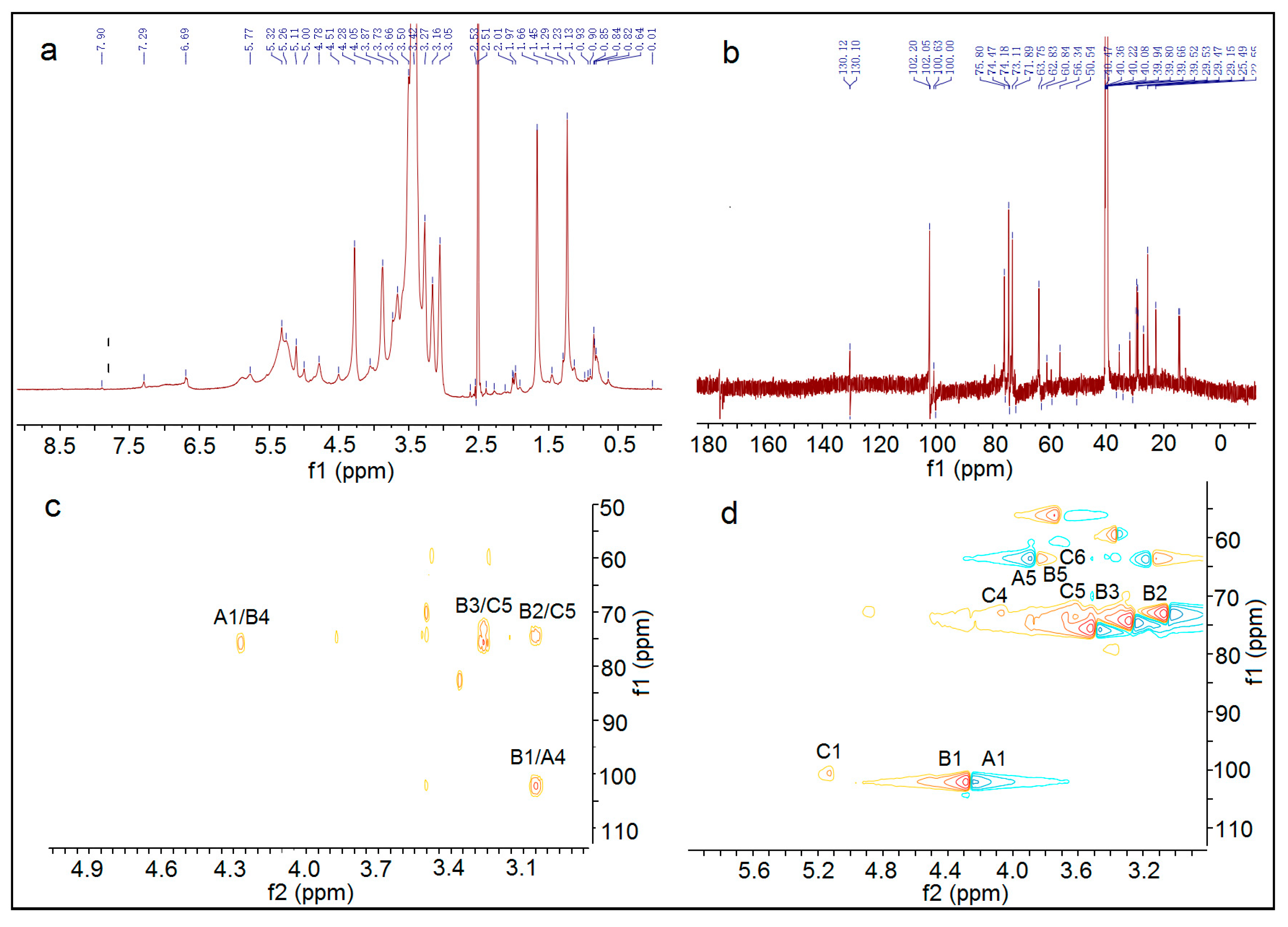
In summary, the hemicellulose polysaccharide was isolated from Astragalus Radix herb residue. The physicochemical properties, chemical structure, and antitumor activities of AX-I-3b were studied. Results showed that AX-I-3b was a heteropolysaccharide containing arabinose (tR = 25.51 min), xylose (tR = 26.25 min), and glucose (tR = 33.41 min) at a molar ratio of 10.4:79.3:1.1. The main chain of this component was a β-pyran type polysaccharide, and the connection manner of the monosaccharide residue was →4)-β-d-Arap-(1→, →2,3,4)-β-d-Xylp-(1→, →4)-β-d-Glcp-(1→.
Currently, chemotherapy remains the main treatment option for cancer patients. However, the severe clinical side effects limit its therapeutic use [
34,
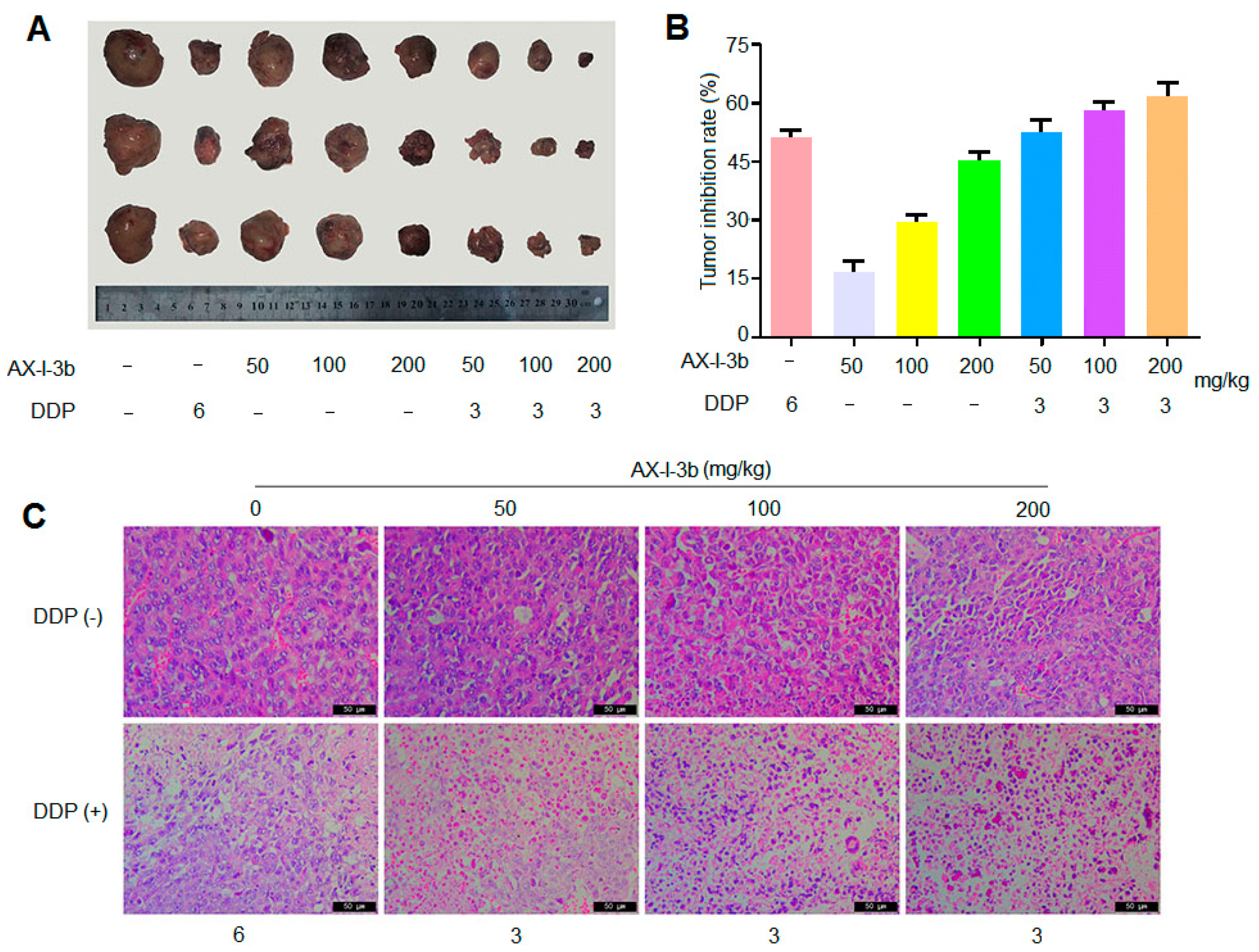
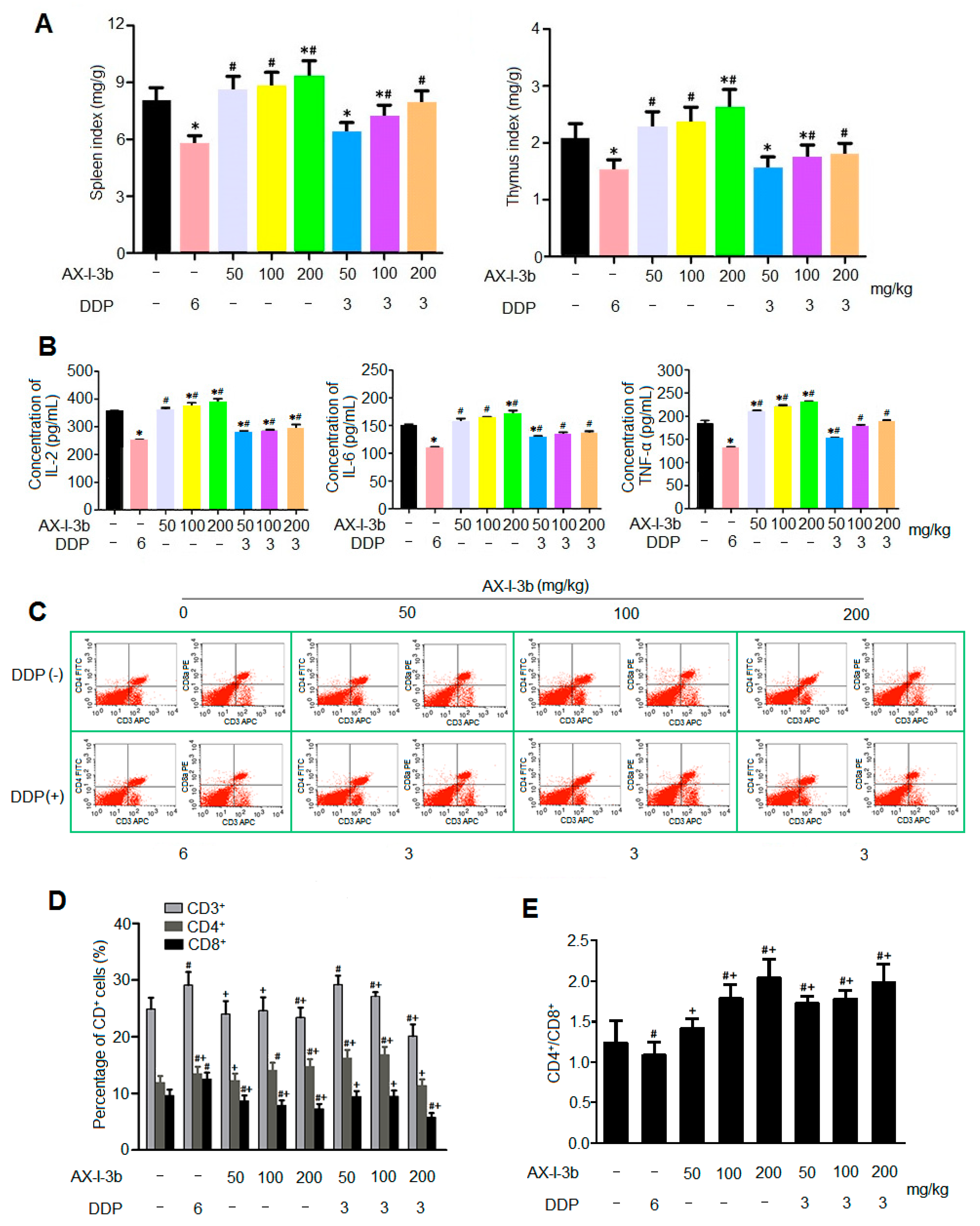
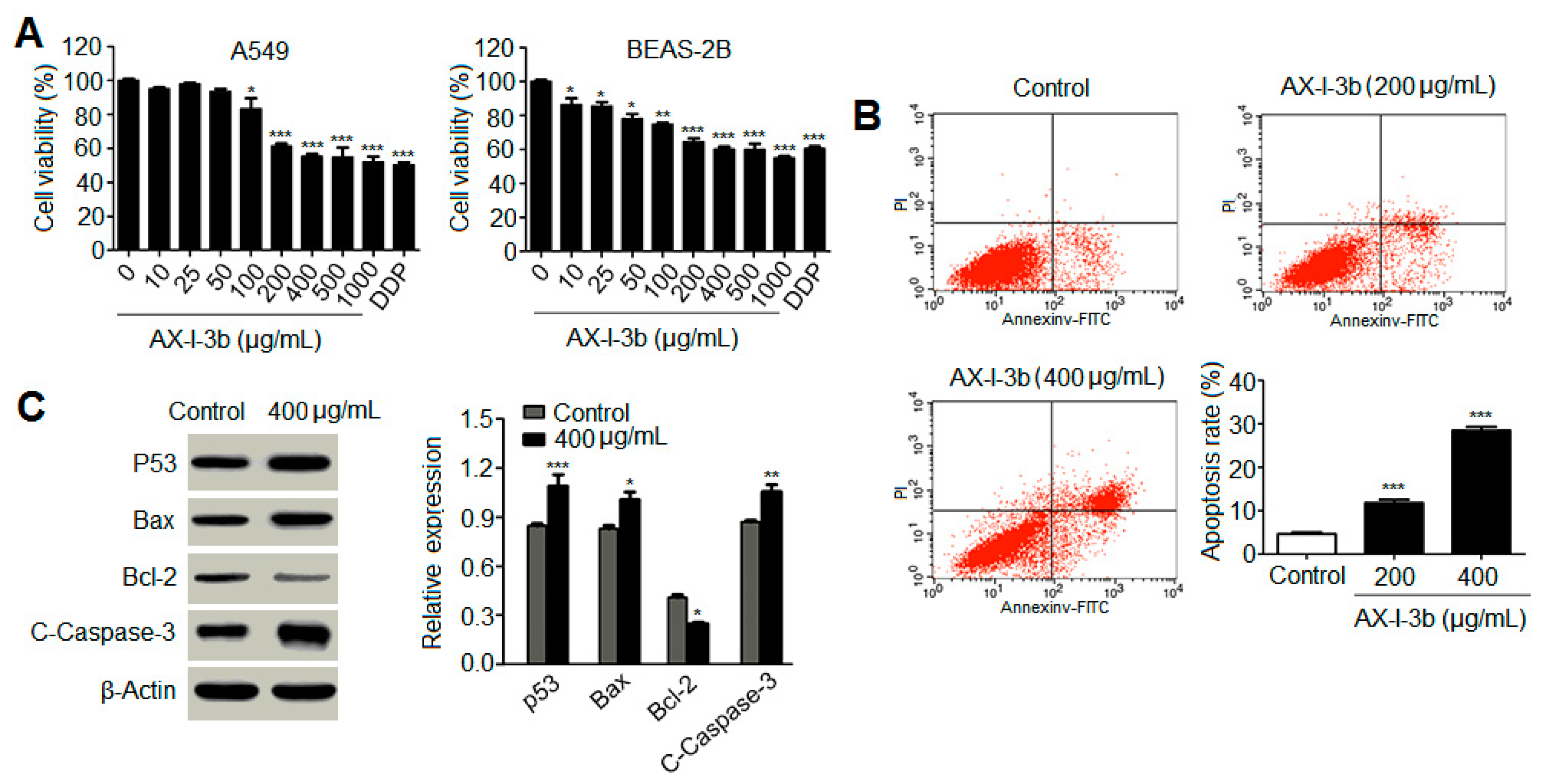
35]. In this study, the antitumor activity in vitro was studied. Results showed that AX-I-3b significantly inhibited the proliferation of human lung-adenocarcinoma A549 cells and induced apoptosis. In vivo anti-tumor experiments demonstrated that intraperitoneal injection of AX-I-3b significantly delayed the growth of transplanted tumors in Lewis lung cancer mice. DDP alone can inhibit the growth of transplanted tumors in Lewis lung cancer mice and high doses of AX-I-3b can greatly enhance this effect. On the other hand, DDP reduced the immune function of Lewis lung cancer mice, but this inhibition was largely offset by AX-I-3b.
In the blood, since most of the tumor cells can be cleared by the host’s immune surveillance function, only a very small number of tumor cells can form distant metastases. Therefore, tumor patients can only gradually recover if they regulate and improve immune function [
36]. Modern medicine also believes that the so-called "carcinogenic factors" are actually only a condition for tumorigenesis. The key factor for tumorigenesis and development is the low function of the immune system, and this view coincides with the theory of "positive virtual tumor" of Chinese medicine [
37]. It has been confirmed that during the four processes of tumor, i.e., occurrence, development, invasion and metastasis, many Chinese medicines can achieve the effect of inhibiting tumor by enhancing the body’s immune function [
38,
39]. T lymphocyte-mediated cellular immunity is the main factor in tumor immunity. Therefore, the level of T lymphocyte transformation rate (proliferation index) can be used as one of the indicators reflecting the immune function of the body. In the tumor-bearing state, the host’s T cells have changed in terms of number, function, and proportion of subpopulations. In the T lymphocyte subset, CD4
+ represents helper T cells (Th), which secrete IL-2 and IFN-γ, and cooperate with natural killer cells (NK) to kill tumor cells and assist immune response. CD8
+ represents inhibition T cells (Ts), which can inhibit the immune response of cells by inhibiting the helper function of Th cells, and can directly inhibit humoral immunity, which is opposite to that of CD4
+ cells. Regulatory T cells (Tregs) are a subset of T cells that have a negative regulatory role in suppressing immune function. Peripheral CD4
+ T cells express the IL-2 receptor alpha chain CD25, which lacks an autoimmune disease, CD4
+CD25
+ regulatory T cell Treg, an immunological marker that regulates its development and function [
40], therefore the number of Tregs is closely related to the prognosis of the tumor [
41].
Studies have shown that [
42] the use of formula containing
Astragalus radix can significantly increase the thymus and spleen index of tumor-bearing mice, increase the percentage of CD4, CD3, CD19 and CD8 lymphocytes, thereby inhibiting lung metastasis. In this study, the results of spleen T lymphocyte assay showed that AX-I-3b may improve the cellular immune function of mice by increasing the ratio of CD4
+ T cells and CD8
+ T cells.
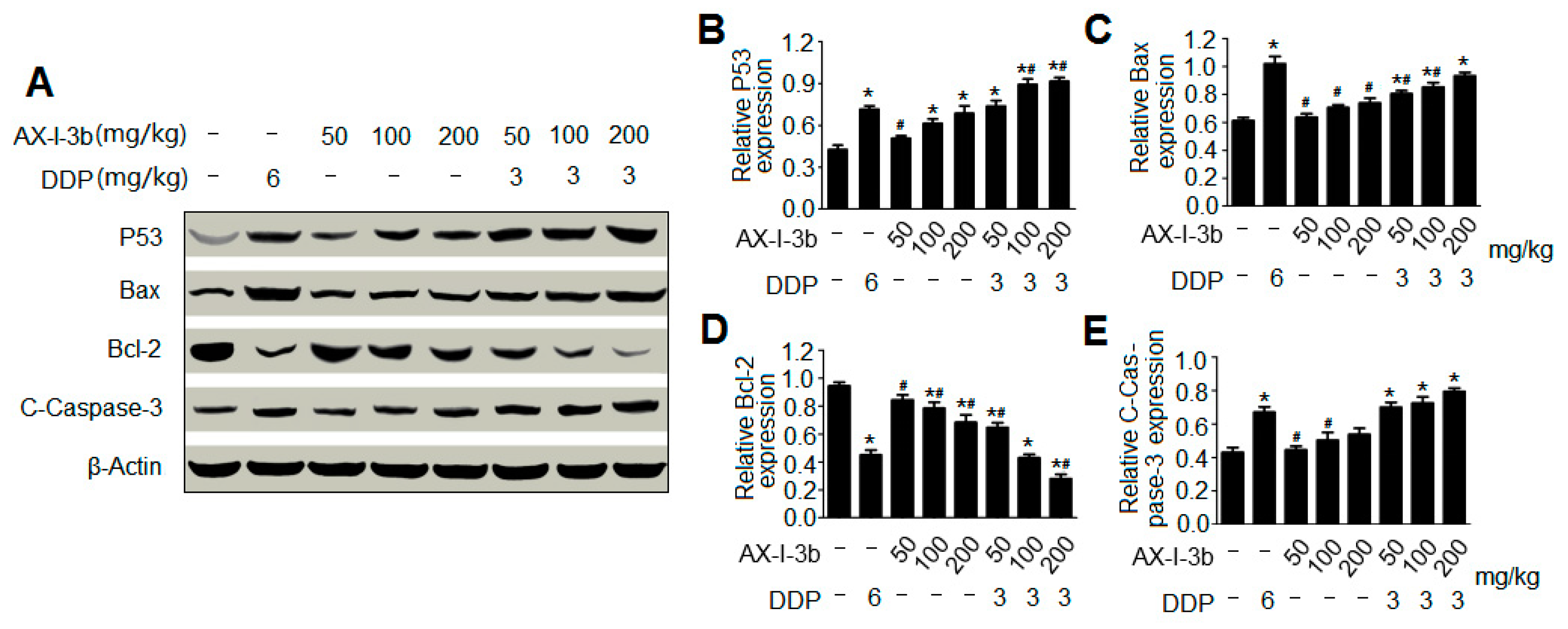
Western blot analysis showed that AX-I-3b treatment significant increased expression of p53 protein, increased expression of proapoptotic protein Bax and decreased the expression of anti-apoptotic protein Bcl-2. Cytochrome c was released from mitochondria into the cytosol, and then the expression of caspase-3 protein increased. Thus, this induces tumor cell apoptosis. In combination with a half dose of DDP, these effects can enhance the chemotherapeutic effect and reduce the toxicity of DDP.
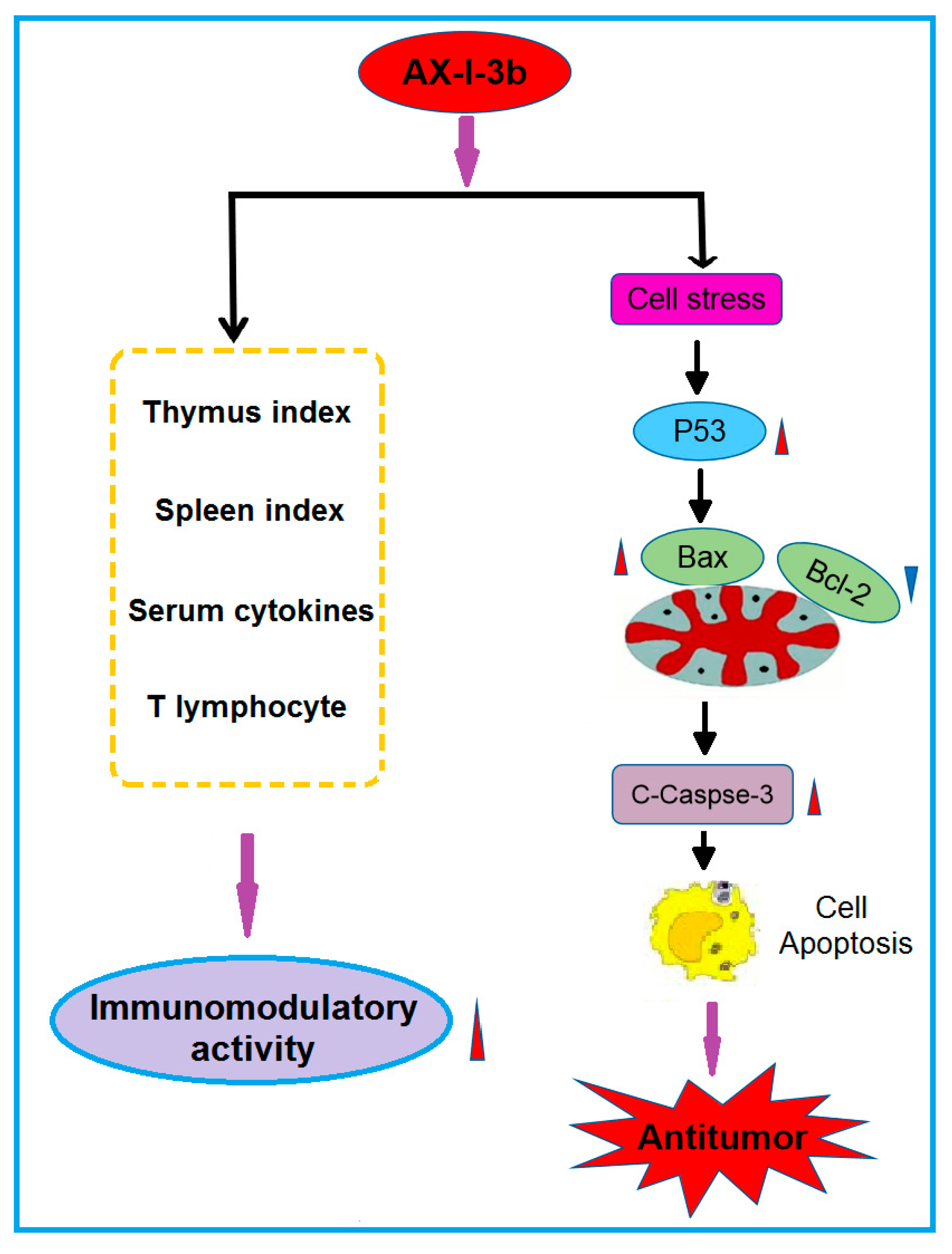
Collectively, the present findings suggest that AX-I-3b can enhance the body’s immunity and induce apoptosis through the caspase-3 dependent mitochondrial pathway, which has potential for chemoprevention and adjuvant therapy of lung cancer. Possible immune and antitumor mechanisms are shown in
Figure 11. Although the immunomodulatory and antitumor mechanisms of AX-I-3b remain to be further exploited, this research has reference value for the development of additional products of AR herb residues. The use of active ingredients in AR herb residue can not only reduce the consumption of AR resources, but also reduce pollution.
4. Materials and Methods
4.1. Materials and Chemicals
Astragalus radix was obtained from the wild A. membranaceus (Fisch.) Bge.var.mongholicus (Bge.) Hsiao in Hunyuan (Shanxi, China). It was identified as a dry root of the leguminous plant A. membranaceus (Fisch.) Bge. Var. mongholicus (Bge.) Hsiao by Professor Qin Xuemei of Shanxi University. The specimens were stored at the Modern Research Center of Traditional Chinese Medicine of Shanxi University. Diethylaminoethyl (DEAE)-cellulose 52 and Sephacryl S-400 HR were purchased from Beijing Solarbio Science & Technology Co., Ltd (Beijing, China). Anhydrous dimethyl sulfoxide (99.7% pure; passed through a molecular sieve) was purchased from the Beijing Bailingwei Technology Co., Ltd (Beijing, China). Sodium borohydride (NaBD4) and Deuterated dimethyl sulfoxide were bought from Shanghai Jianglai Biotechnology Co., Ltd (Shanghai, China). The dextran standards including Dextran 4320, Dextran 12600, Dextran 73800, Dextran 110000, Dextran 289000, and Dextran 496000 were obtained from China Institute of Metrology (Beijing, China). Ribitol, fucitol, arabitol, rhamnitol, mannitol, xylitol, sorbitol, and galactitol were bought from Sigma (St. Louis, MO, USA). All other reagents were of analytical grade. Cisplatin and double antibody (Hyclone penicillin-streptomycin) were purchased from Sigma. Phosphate-buffered saline (PBS), DMEM high-glucose medium, trypsin, fetal bovine serum (FBS), MTT reagent, and dimethylsulfoxide (DMSO) were purchased from Beijing Suo Laibao Technology Co., Ltd (Beijing, China). Antibodies against p53, Bcl-2, Bax, caspase-3, β actin and ELISA detection kits (IL-2, IL-6 and TNF-α) were purchased from the Wuhan Boster Bioengineering Co., Ltd (Wuhan, China).
4.2. Polysaccharide Extraction
Preparation of A. radix residue: A. radix material was pulverized and passed through a 100-mesh sieve. Then, 20 g powder was weighed into a 1000 mL flask to which 600 mL of water was added. After the mixture was heated in an electric heating jacket at 42 °C for 1 h and centrifuged (3000 r/min, 10 min), the supernatant was discarded. This extraction process was repeated for three times, and the resulting precipitate was dried at 30 °C to obtain a Scutellaria residue.
Pretreatment of
A. radix residue: To prepare alcohol-insoluble residue (AIR) powder, 20 g
A. radix residue powder was weighed according to a previous method [
43], and 600 mL of 80% ethanol solution was added. After the mixture was heated in a water bath at 60 °C for 1 h and centrifuged (3000 r/min, 10 min), the supernatant was discarded. The process was repeated for several times until the upper layer was clear and colorless. After centrifugation for 10 minutes at 3000 r/min, the precipitate was collected. The acetone solution was added to the above precipitate at a solid-to-liquid ratio of 1:10. Afterwards, the mixture was allowed to stand. After discarding the supernatant, the precipitate was dried in an oven at 45 °C. Porcine pancreatic α-amylase solution was added to the dried powder (material-to-liquid ratio = 1:100; 20 U/mL; concentration = 50 mmol/ L Tris-HCl buffer; pH 7.0). The mixture was then incubated for 12 h at 37 °C in a 200 r/min shaker and centrifuged (3000 r/min, 10 min). The precipitate was washed repeatedly with distilled water adding with acetone solution (feed ratio = 1:10), and then was allowed to stand still. After centrifugation at 3000 r/min for 5 min, the supernatant was discarded. The precipitate was dried in an oven at 45 °C to obtain AIR powder.
Extraction of hemicellulose (AX) from
A. radix residue: According to a previous method [
44], 20 g AIR powder was placed in a 1000 mL Erlenmeyer flask, adding 400 mL of sodium chlorite solution with a mass fraction of 0.6%, of which the pH was adjusted to 4.2 to 4.7 with glacial acetic acid. The flask was sealed and placed in a water bath at 75 °C, with shaking manually every 20 min. Sodium chlorate solution (0.6%) was added after 1 h. The pH was adjusted to 4.2–4.7 by adding glacial acetic acid. The above process was repeated for 1 h. After the completion of the reaction, the bottle wall was immediately rinsed with cold water to room temperature and centrifuged (3000 r/min, 10 min). And then the precipitate was collected. The precipitate was repeatedly washed with distilled water to neutrality, centrifuged at 3000 r/min for 10 min, and dried in an oven at 50 °C to obtain hemicellulose.
According to the previous method [
45], dried hemicellulose (10 g) was put in a 1000 mL Erlenmeyer flask, to which 500 mL pectinase (3 U/mL and sodium acetate 50 mmol/L; pH 5.0) was added. After being incubated at 24 °C in a 200 r/min shaker for 24 h, the mixture was centrifuged (3000 r/min, 10 min), and then the supernatant was discarded. This procedure was repeated for three times, and the pellet was washed for three times with 500 mL of distilled water. After centrifugation, 500 mL of a mixed solution of Na
2CO
3 (50 mmol/L) and EGTA (mmol/L) was added to the precipitate. The mixture was incubated at 24 °C in a 200 r/min shaker for 24 h and centrifuged, and then the supernatant was discarded. This step was repeated for three times, and the precipitate was washed three times with 500 mL of distilled water.
A 500 mL mixed solution of 1 mol/L KOH and 1% NaBH4 (liquid-to-liquid ratio = 1:50) was added to the obtained precipitate. The mixture was incubated for 12 h at 24 °C in a 200 r/min shaker and centrifuged. The supernatant was then collected. This step was repeated for three times. Afterwards, supernatant was combined and stored in a refrigerator at 4 °C to obtain the hemicellulose component I. To adjust the pH to 5.0, glacial acetic acid at certain amount was added to the above hemicellulose component solution. And then, dialysis against distilled water was performed four times for 12 h each time. The product was concentrated and lyophilized to obtain polysaccharide of hemicellulose component I of A. radix residue, labeled as AX-I.
4.3. Sevage Method for Protein Removal
The protein in AX-I was removed by using a previous method [
46]. A certain amount of
A. radix residue polysaccharide AX-I was weighed and reconstituted with water in a separatory funnel, after which one-fourth volume of Sevage reagent (CHCl
3:CH
3(CH
2)
3OH = 4:1) was added. The funnel was shaken for 15 min. After standing, the lower and middle layers were discarded. The Sevage reagent was further added to the upper solution and shaken for 15 min. This step was repeated for three to four times until the protein layer disappeared.
4.4. Separation and Purification of Hemicellulose Polysaccharide
According to a previous method [
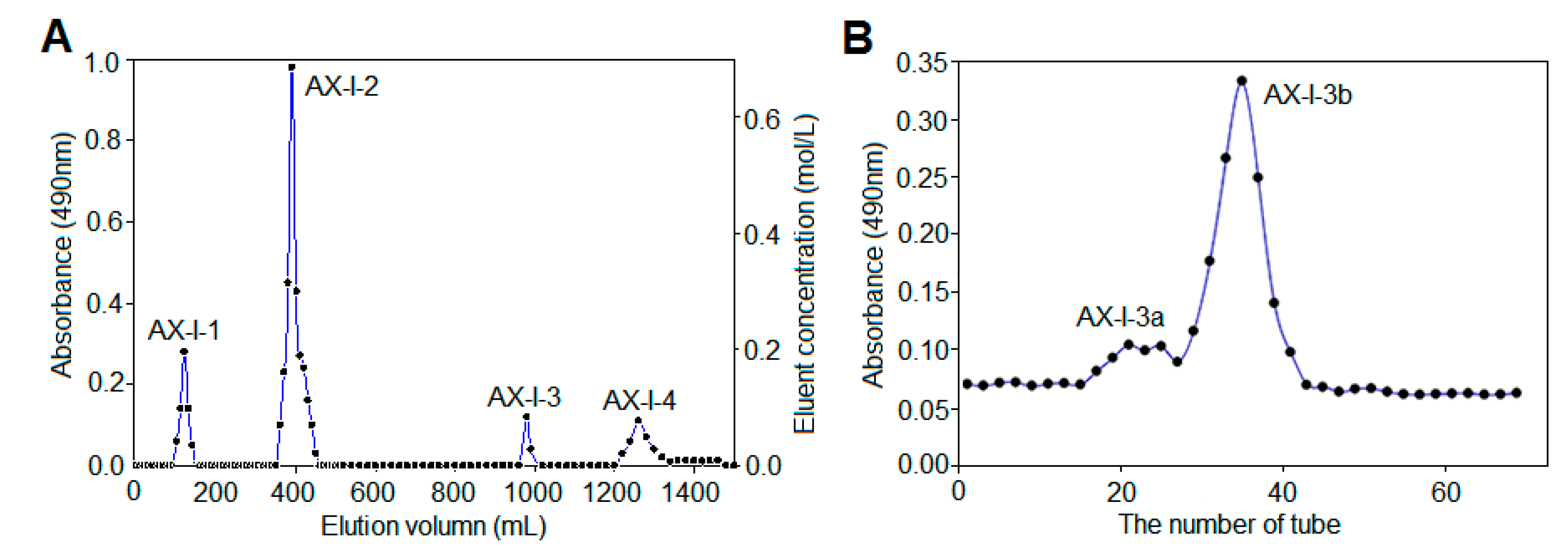
47], 0.2 g polysaccharide powder was weighed and dissolved in 2 mL distilled water for DEAE-cellulose 52 column chromatography. Each tube was collected every 10 min at a flow rate of 0.6 mL/min. First, it was eluted with distilled water until no sugar was detected. And then, it was eluted with 0.3 mol/L NaCl solution and 0.6 mol/L NaCl aqueous solution. Finally, it was eluted with 0.1 mol/L NaOH aqueous solution and 0.3 mol/L NaOH aqueous solution. The absorbance of each tube solution at 490 nm was determined by phenol-sulfuric acid method, according to which the elution curve was drawn. The polysaccharide fractions of each tube were collected according to the curve, concentrated, and dialyzed in a 3 kDa dialysis bag for 48 h, followed by reconcentration and lyophilization to obtain the separated polysaccharides AX-I-1-AX-I-4.
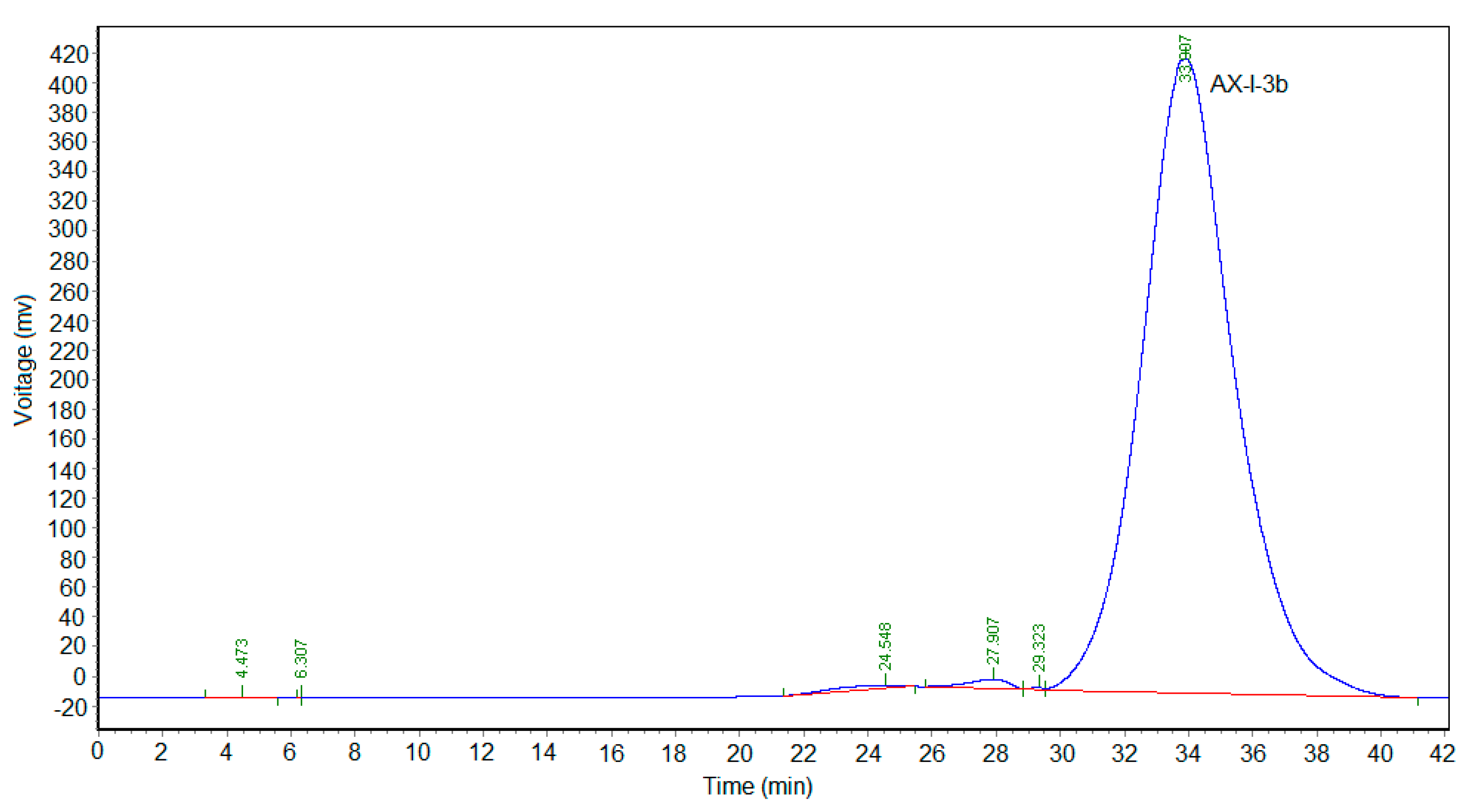
Approximately 0.1 g isolated polysaccharide powder was dissolved in 1 mL of distilled water. Further purification was performed using a Sephacryl S-400 HR gel column. Distilled water was used as the mobile phase at a flow rate of 0.5 mL/min. Each tube was collected every 10 min. The phenol-sulfuric acid method was used to detect and collect the polysaccharides of each component and then concentrate and freeze dry them to obtain a purified hemicellulose component.
4.5. Homogeneity and Molecular Weight
The homogeneity and average molecular weight of AX-I-3b were identified by high-performance gel-permeation chromatography (HPGPC). We accurately weighed 1 mg each dextran standard to prepare a 2 mg/mL solution. The mobile phase was pure water, the injection volume was 20 μL, and the flow rate was set at 0.3 mL/min. The gel column model was TSKgel G4000 PWxl (10) 7.8 × 300 mm2. The column temperature and the detector temperature was set to 35 °C and 34 °C, respectively. The retention time (tR) of each standard was plotted on the horizontal axis and lgMw was plotted on the vertical axis. The calibration was obtained and the linear equation was calculated. The polysaccharide AX-I-3b was formulated into a sample solution with a concentration of 2 mg/mL. The relative molecular mass was calculated by taking the measured tR into the equation.
4.6. FT-IR Spectroscopy Analysis
Based on the literature [
48], 1–2 mg sample was weighed. The IR of the polysaccharide was determined by KBr tableting. The scanning wavelength was ranged from 4000 cm
−1 to 400 cm
−1.
4.7. Monosaccharide-Composition Analysis
According to the literature [
49], 5 mg of polysaccharide AX-I-3b was weighed, 1.5 mL of TFA at a concentration of 2 mol/L was added. Hydrolysis for 3 h in an oil bath at 110 °C was performed after the reaction. After being dried with air, adding 1 mL of methanol, and re-drying in the air, the process was repeated for three times to obtain a complete acid hydrolysis product. This product was dissolved in a ribitol internal standard solution at a concentration of 0.1 mg/mL, and the mass fraction was added. About 2% NaBH
4 in DMSO was added, and the mixture was incubated at 40 °C in a 200 r/min shaker for 90 min. After the reaction, pH was adjusted to neutral with glacial acetic acid, in which 500 μL acetic anhydride and 50 μL 1-methylimidazole were then added. After reacting at 25 °C for 10 min and cooling to room temperature, the reaction was stopped by adding 5 mL of water, extracted three times with 1 mL of CH
2Cl
2, and the organic phase was combined. Extraction with 1 mL of water was performed for three times and retain. The organic phases were combined. The combined organic phase was dried in air, redissolved in CH
2Cl
2, and filtered through a 0.22 μm organic filter. Finally, 2 μL filtrate was injected into the GC-MS.
The monosaccharide standard was weighed and dissolved in dimethyl sulfoxide solution with 0.1 mg/mL ribitol as internal standard. Then, 200 μL of acetic anhydride and 50 μL of 1-methylimidazole were added. The mixture was vortex and let stand, react at 25 °C for 10–15 min. Afterwards, 500 μL distilled water was added to terminate the reaction, add 500 μL of two Methyl chloride, vortex, centrifugation, aspirate the lower layer, repeat the extraction twice, combine the organic phase, blow dry with air, reconstitute with methylene chloride, add anhydrous sodium sulfate to remove water, filter. 1 μL of filtrate was injected into GC-MS analysis to obtain gas chromatography-mass spectrometry of monosaccharide standards.
The monosaccharide composition in the sample was determined by comparing the GC-MS spectra of the monosaccharide standards and samples. The molar ratio of each monosaccharide in the sample was determined by calculating the peak area.
Gas chromatographic conditions: Hue chromatography column, DB-5MS capillary column (30 m × 0.5 cm × 0.25 μm); carrier gas, high purity helium (99.99%); injection volume 2 μL; carrier gas flow rate 1.0 mL/min; split ratio 10:1; inlet temperature 220 °C; temperature programming conditions, the starting temperature is 100 °C; rise to 180 °C at 5 °C /min for 1 min; 1 °C/min to 190 °C for 2 min; to 30 °C/min rises to 220 °C for 2 min; rises to 230 °C at 1 °C/min for 2 min; rises to 280 °C at 20 °C/min for 10 min.
Mass spectrometry conditions: Electron bombardment source (EI); ion source temperature, 220 °C; ion source, 70 eV; transmission line temperature, 250 °C; scan mode for full scan (Full Scan); mass scan range m/z: 30~550.
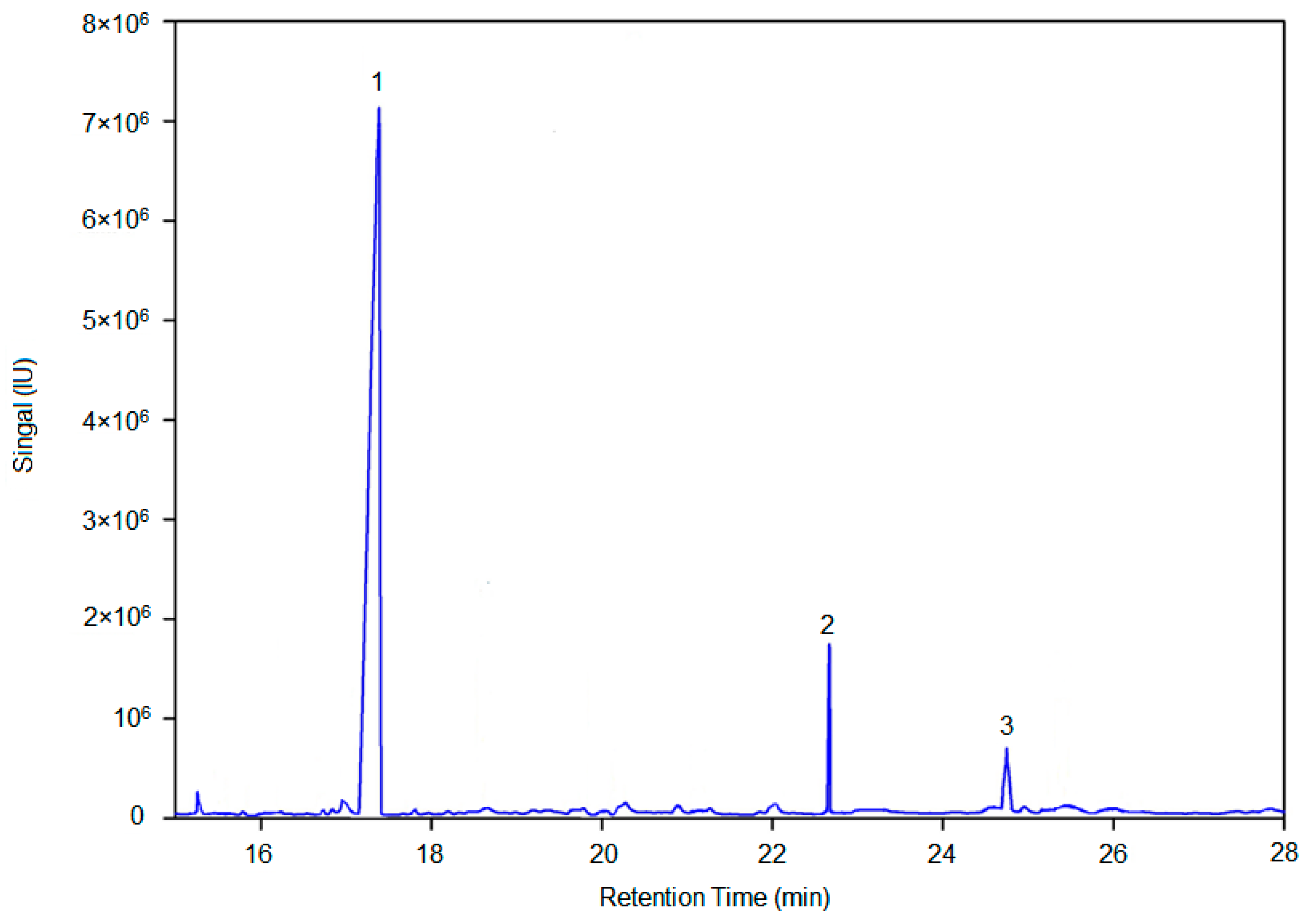
4.8. Methylation Analysis
According to the literature [
50], 2 mL of anhydrous DMSO was added to 5 mg polysaccharide AX-I-3b. Ultrasonication was performed in an argon atmosphere until completely dissolution. Then, 50 mg of NaH powder was weighed and placed in a flask, 1 mL of anhydrous DMSO was added, and argon was charged. At 60 °C and 500 r/min, magnetic stirring was performed for 1 h until no hydrogen was produced. The above two liquids were combined and ultrasonically reacted for 1 h under argon-filled conditions. After cooling in an ice bath, adding 1 mL of CH
3I in the dark, protecting with argon, and ultrasonicating at 20 °C for 30 min three times, 5 mL of 4 mM Na
2S
2O
3 aqueous solution and 5 mL of CHCl
3 were added to the reaction solution. The lower layer was extracted and aspirated. This process was repeated for five times. The lower-layer liquid was added with 5 mL of distilled water and extracted, and then the upper-layer liquid was discarded. This process was repeated for five times. Anhydrous Na
2SO
4 was added to the chloroform phase, which was filtered and dried with nitrogen. The methylation product was detected by infrared spectroscopy, and no absorption peak was detected at 3400 cm
-1, indicating the completion of the methylation reaction.
About 2 mL of 2 mol/L TFA was added, and the mixture was hydrolyzed at 120 °C for 90 min after sealing. The mixture was air dried, adding with 1 mL of methanol, and re-dried. The process was repeated for three times to obtain a hydrolyzate. And then, 2 mL of freshly prepared NaBD4 aqueous solution with a mass fraction of 2% was added, followed by incubation at 40 °C and 200 r/min for 90 min. After the reaction, pH was adjusted to neutral with glacial acetic acid and the solution was dried with nitrogen. We added 500 μL of acetic anhydride and 50 μL of 1-methylimidazole with setting the reaction temperature at 25 °C for 10 min. 1 mL of water was added to end the reaction, after which extraction with 1 mL of CH2Cl2 was performed for 3 times. The organic phase was combined and extracted with 1 mL of distilled water for three times. The organic phase was combined again, dried with nitrogen, dissolved in CH2Cl2. After filtration with a 0.22 μm organic filter, 2 μL of the solution was injected into the GC-MS. The conditions of gas chromatography and mass spectrometry were the same as described in 2.7.
4.9. NMR Spectroscopy
According to the literature [
51], 30 mg dry polysaccharide sample AX-I-3b was dissolved in 0.5 mL DMSO-d6.
1H-NMR,
13C-NMR, and
2D-NMR (HMBC and HSQC) spectra were obtained at 30 °C.
4.10. Animal Experiments
4.10.1. Animals and Experimental Design
In total, 72 male SPF-class C57BL/6 mice were used in the study. Except for 8 mice in the blank control group, the remaining 64 mice were injected with 0.2 mL 1 × 107 cells/mL LLC cells at a distance of 1 cm from the right axilla to establish a subcutaneous xenograft model. When the tumor long diameter (L) and short diameter (W) were both 5 mm, the model was considered to be successful, and the transplanted mice were subsequently grouped (n = 8).
The dosing regimen was set as follows: mice in the blank control group and model control group were intraperitoneally injected with normal saline once a day, 0.3 mL each time; mice in the DDP group (6 mg/kg) were intraperitoneally injected once a week, 0.3 mL each time; the low dose AX-I-3b group, middle dose AX-I-3b group and high dose AX-I-3b group (50, 100, 200) mg/kg, intraperitoneal injection of 0.3 mL per day; low dose combination group, middle dose combination group, high dose combination group: 1/2 DDP + AX-I-3b: (3 + 50, 3 + 100, 3 + 200) mg/kg, of which AX-I-3b was intraperitoneally injected 0.3 mL per day at the above doses. DDP was administered at a dose of 3 mg/kg once a week. For all drugs, the total volume was 0.3 mL. Each group started to take the drug on the first day after the successful modeling, lasting for 15 days.
4.10.2. Tumor Inhibition Rate and Immune Organ Index
24 h after the last administration, all mice were weighted and then sacrificed by cervical dislocation. The tumor, spleen and thymus of the mice were then carefully dissected and weighted. Immune organ indexes of the spleens and thymuses were used to express the effects of AX-I-3b on the immune organs, following the formula [
52]:
We measured the tumor inhibitory rate using the formula: inhibitory rate (%) = (the tumor weight of the model group − the tumor weight of the treatment group)/the tumor weight of the model group × 100% [
53].
4.10.3. Measurement of Cytokines
The serum levels of cytokines were determined by ELISA according to the manufacturer’s instructions. ELISA kits were employed to measure the levels of interleukin-2 (IL-2), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α).
4.10.4. Determination of Spleen T Lymphocyte Subsets
We analyzed the T lymphocyte subsets in the spleen using flow cytometry. The mice were sacrificed by cervical dislocation. And then, the spleen was weighted and placed in a 5 mL centrifuge tube, gently ground. The culture medium was added, filtered through a 200-mesh sieve, and the filtrate was collected with centrifugation at 1000 r/min for 5 min. After discarding the supernatant, red blood cell lysis was added. The solution was shaken and centrifuged. The precipitate was milky white. After washing twice with PBS, the cell concentration was adjusted to 1×107 cells/mL, and 10 μL of CD3-APC, CD4-FITC and CD8a-PE monoclonal antibody were added, respectively. The cells were incubated for 30 min at 4 °C in the dark, and then were detected by flow cytometry.
4.10.5. Detection of Tumor Tissue Lesions by HE Staining
The mice were sacrificed by cervical dislocation and the tumor was stripped and weighed. After weighing, a tumor block of about 5 mm thickness was cut and quickly placed in a 4% paraformaldehyde solution for fixation. The paraffin-embedded blocks were routinely prepared, sliced 5 μm, and routinely dewaxed to water. After conventional HE staining and sealing, the tumor tissue morphology was examined by light microscopy.
4.10.6. Western Blot Analyses
Proteins were extracted from tumor tissue using RIPA (strong) tissue cell lysate for Western blotting. A protein concentration assay kit was applied to measure the protein concentration. The samples were boiled in 5 x SDS loading buffer for 10 minutes and separated by SDS-polyacrylamide gel electrophoresis (PAGE). The isolated protein was transferred to a pure nitrocellulose blotting membrane. These membranes were incubated with 5% (w/v) skim milk powder dissolved in tris buffered saline containing 0.05% Tween-20 (TBST) for 2 hours at room temperature. Membranes were then incubated with antibodies p53, Bax, BCl-2 and Caspase-3 overnight at 4 °C. After being washed with PBST, the HRP-labeled secondary antibody was incubated with the membrane for 2 hours at room temperature. After re-washing with PBST, the membrane was scanned with a fully automated chemi-luminescence analyzer and the associated strip gray values were read by TANON GIS software.
4.11. In Vitro Antitumor Assay
4.11.1. Cell Culture
A549 cells were cultured in DMEM containing 10% fetal bovine serum, 100 g/L penicillin, and 100 g/L streptomycin at 37 °C under 5% CO2 saturated humidity until the cells were grown to log phase.
4.11.2. Cell-Growth Inhibition Assay (MTT Assay)
A cell suspension with a concentration of 1 × 105 cells/mL was adjusted in DMEM medium containing 10% FBS and inoculated into a 96-well plate (100 μL per well) at 37 °C in a 5% CO2 saturated humidity incubator. The cells were fully adhered for 24 h. The culture solution was aspirated, and the culture medium containing 25, 50, 100, 200, 400, 500, and 1000 μg/mL of AX-I-3b polysaccharide was added. A negative control well was added to 100 μL of serum-free DMEM medium, while the positive control group was added to 10 μg/mL of cisplatin. And six replicate wells per group were set. The culture was continued for 24 h. At the end of the reaction, the culture solution was aspirated. 90 μL of serum-free medium and 10 μL of 5 mg/mL MTT solution were added into each well. The culture was continued for 4 h. After completion of the culture, the liquid was aspirated, 150 μL of prewarmed DMSO at 37 °C was added to each well, and then shaking for 10 min. The absorbance (A) value of the microplate reader was measured at 490 nm, and the cell survival rate was calculated. The same assay was performed on BEAS-2B cells to obtain cytotoxicity results. Cell-proliferation inhibition rate (%) = (1 − A drug/A control group) × 100%.
4.11.3. Cell Cycle and Apoptosis Analysis by Flow Cytometry
A549 cells in the logarithmic growth phase were digested and collected, uniformly inoculated into a six-well plate, and the hemicellulose slag hemicellulose polysaccharide AX-I-3b (0, 200, and 400 μg/mL) was added. A negative control was set. There were three replicates for each concentration. After 24 h, the cells were trypsinized, collected, and washed once with prechilled PBS. Each sample was fixed with 4 °C precooled 70% ethanol for 20 min. Centrifugation (1000 rpm, 5 min) was conducted to remove the fixative. After washing once with PBS, re-suspending in 450 μL of PBS, adding 10 μL of 100 μg/mL RNase and 300 μL of 50 μg/mL PI buffer. Incubation was carried out for 20 min in the dark to protect cells from light. Cell-cycle distribution was measured by flow cytometry (BD, Franklin Lakes, NJ, USA). After treating the cells for 24 h in the same manner as described above, cells were collected, and 5 μL of Annexin V stain and 10 μL of PI stain were added to each sample. The samples were stained for 20 min at room temperature in the dark, and apoptosis rate was detected by flow cytometry. Each experiment was repeated for three times.
4.12. Data Processing and Statistical Analysis
All data were statistically analyzed through one-way variance analysis (ANOVA) and t-test using SPSS 16.0 software package (SPSS, Chicago, IL, USA) (company, City, state abbrev if USA, Country). The test level α = 0.05 and p < 0.05 were considered statistically significant.